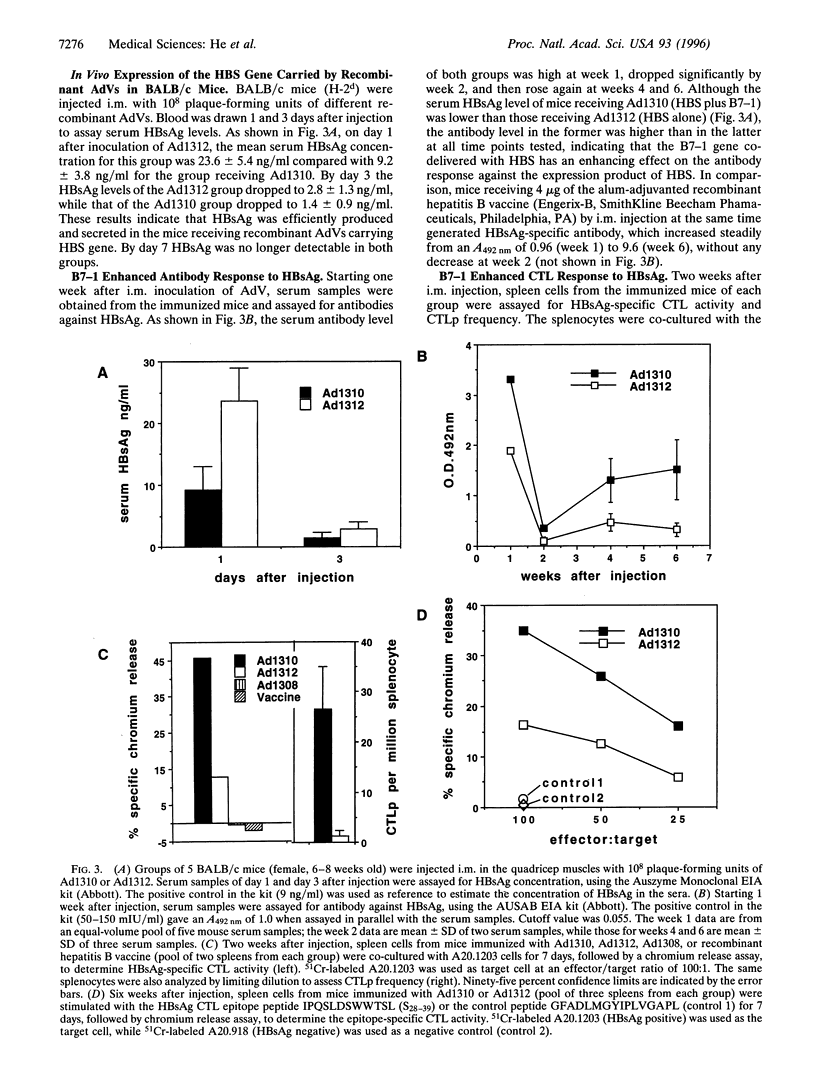
Abstract
There is a need for more effective therapy for chronic virus infections. A principle natural mechanism for elimination of virus-infected host cells is activation of viral antigen-specific cytotoxic T lymphocytes (CTL). In an effort to develop methods of inducing virus-specific CTL responses that might be utilized in therapy of virus infections, we have investigated the effect of B7, a costimulatory factor for T-cell activation. In this study we show that delivery of genes encoding human B7-1 and a viral antigen in the same recombinant viral vector to cells of mice induces a greater viral antigen-specific CTL response than does similar delivery of the viral antigen gene alone. Two recombinant adenovirus vectors were constructed with the foreign genes inserted in the early region 3. One of them (Ad1312) directed expression of the surface antigen gene of hepatitis B virus (HBS); the other (Ad1310) directed coexpression of HBS and human B7-1 (CD80) by means of an internal ribosomal entry site placed between the two coding sequences. When inoculated into BALB/c mice, both vectors induced a viral surface antigen-specific CTL response. The response induced by Ad1310 was stronger than that by Adl312 as measured by a chromium release assay for CTL activity and limiting dilution analysis for CTL precursor frequency, indicating that the B7-1 gene co-delivered with the HBS gene had an enhancing effect on the CTL response against surface antigen. Ad1310 also induced a higher titer of antibody against surface antigen than did Ad1312. This result suggests that expression of a costimulatory protein and a viral antigen in the same cells in vivo induces stronger immune responses than expression of the antigen alone. This could be a novel strategy for development of both preventive and therapeutic vaccines against infectious agents.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkhatib G., Briedis D. J. High-level eucaryotic in vivo expression of biologically active measles virus hemagglutinin by using an adenovirus type 5 helper-free vector system. J Virol. 1988 Aug;62(8):2718–2727. doi: 10.1128/jvi.62.8.2718-2727.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando K., Moriyama T., Guidotti L. G., Wirth S., Schreiber R. D., Schlicht H. J., Huang S. N., Chisari F. V. Mechanisms of class I restricted immunopathology. A transgenic mouse model of fulminant hepatitis. J Exp Med. 1993 Nov 1;178(5):1541–1554. doi: 10.1084/jem.178.5.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both G. W., Lockett L. J., Janardhana V., Edwards S. J., Bellamy A. R., Graham F. L., Prevec L., Andrew M. E. Protective immunity to rotavirus-induced diarrhoea is passively transferred to newborn mice from naive dams vaccinated with a single dose of a recombinant adenovirus expressing rotavirus VP7sc. Virology. 1993 Apr;193(2):940–950. doi: 10.1006/viro.1993.1203. [DOI] [PubMed] [Google Scholar]
- Burgert H. G., Maryanski J. L., Kvist S. "E3/19K" protein of adenovirus type 2 inhibits lysis of cytolytic T lymphocytes by blocking cell-surface expression of histocompatibility class I antigens. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1356–1360. doi: 10.1073/pnas.84.5.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S. M., Lee S. G., Ronchetti-Blume M., Virk K. P., Mizutani S., Eichberg J. W., Davis A., Hung P. P., Hirsch V. M., Chanock R. M. Coexpression of the simian immunodeficiency virus Env and Rev proteins by a recombinant human adenovirus host range mutant. J Virol. 1992 Nov;66(11):6721–6727. doi: 10.1128/jvi.66.11.6721-6727.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors M., Collins P. L., Firestone C. Y., Sotnikov A. V., Waitze A., Davis A. R., Hung P. P., Chanock R. M., Murphy B. R. Cotton rats previously immunized with a chimeric RSV FG glycoprotein develop enhanced pulmonary pathology when infected with RSV, a phenomenon not encountered following immunization with vaccinia--RSV recombinants or RSV. Vaccine. 1992;10(7):475–484. doi: 10.1016/0264-410x(92)90397-3. [DOI] [PubMed] [Google Scholar]
- Duncan S. J., Gordon F. C., Gregory D. W., McPhie J. L., Postlethwaite R., White R., Willcox H. N. Infection of mouse liver by human adenovirus type 5. J Gen Virol. 1978 Jul;40(1):45–61. doi: 10.1099/0022-1317-40-1-45. [DOI] [PubMed] [Google Scholar]
- Eloit M., Gilardi-Hebenstreit P., Toma B., Perricaudet M. Construction of a defective adenovirus vector expressing the pseudorabies virus glycoprotein gp50 and its use as a live vaccine. J Gen Virol. 1990 Oct;71(Pt 10):2425–2431. doi: 10.1099/0022-1317-71-10-2425. [DOI] [PubMed] [Google Scholar]
- Freeman G. J., Gray G. S., Gimmi C. D., Lombard D. B., Zhou L. J., White M., Fingeroth J. D., Gribben J. G., Nadler L. M. Structure, expression, and T cell costimulatory activity of the murine homologue of the human B lymphocyte activation antigen B7. J Exp Med. 1991 Sep 1;174(3):625–631. doi: 10.1084/jem.174.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimmi C. D., Freeman G. J., Gribben J. G., Sugita K., Freedman A. S., Morimoto C., Nadler L. M. B-cell surface antigen B7 provides a costimulatory signal that induces T cells to proliferate and secrete interleukin 2. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6575–6579. doi: 10.1073/pnas.88.15.6575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Gómez-Foix A. M., Coats W. S., Baqué S., Alam T., Gerard R. D., Newgard C. B. Adenovirus-mediated transfer of the muscle glycogen phosphorylase gene into hepatocytes confers altered regulation of glycogen metabolism. J Biol Chem. 1992 Dec 15;267(35):25129–25134. [PubMed] [Google Scholar]
- Harding F. A., Allison J. P. CD28-B7 interactions allow the induction of CD8+ cytotoxic T lymphocytes in the absence of exogenous help. J Exp Med. 1993 Jun 1;177(6):1791–1796. doi: 10.1084/jem.177.6.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding F. A., McArthur J. G., Gross J. A., Raulet D. H., Allison J. P. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992 Apr 16;356(6370):607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- Herz J., Gerard R. D. Adenovirus-mediated transfer of low density lipoprotein receptor gene acutely accelerates cholesterol clearance in normal mice. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2812–2816. doi: 10.1073/pnas.90.7.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Jang S. K., Kräusslich H. G., Nicklin M. J., Duke G. M., Palmenberg A. C., Wimmer E. A segment of the 5' nontranslated region of encephalomyocarditis virus RNA directs internal entry of ribosomes during in vitro translation. J Virol. 1988 Aug;62(8):2636–2643. doi: 10.1128/jvi.62.8.2636-2643.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins M. K., Ashwell J. D., Schwartz R. H. Allogeneic non-T spleen cells restore the responsiveness of normal T cell clones stimulated with antigen and chemically modified antigen-presenting cells. J Immunol. 1988 May 15;140(10):3324–3330. [PubMed] [Google Scholar]
- Jenkins M. K., Taylor P. S., Norton S. D., Urdahl K. B. CD28 delivers a costimulatory signal involved in antigen-specific IL-2 production by human T cells. J Immunol. 1991 Oct 15;147(8):2461–2466. [PubMed] [Google Scholar]
- Kirschmeier P. T., Housey G. M., Johnson M. D., Perkins A. S., Weinstein I. B. Construction and characterization of a retroviral vector demonstrating efficient expression of cloned cDNA sequences. DNA. 1988 Apr;7(3):219–225. doi: 10.1089/dna.1988.7.219. [DOI] [PubMed] [Google Scholar]
- Larsen C. P., Ritchie S. C., Pearson T. C., Linsley P. S., Lowry R. P. Functional expression of the costimulatory molecule, B7/BB1, on murine dendritic cell populations. J Exp Med. 1992 Oct 1;176(4):1215–1220. doi: 10.1084/jem.176.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P. S., Brady W., Grosmaire L., Aruffo A., Damle N. K., Ledbetter J. A. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med. 1991 Mar 1;173(3):721–730. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P. S., Brady W., Urnes M., Grosmaire L. S., Damle N. K., Ledbetter J. A. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991 Sep 1;174(3):561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P. S., Clark E. A., Ledbetter J. A. T-cell antigen CD28 mediates adhesion with B cells by interacting with activation antigen B7/BB-1. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5031–5035. doi: 10.1073/pnas.87.13.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Marshall G. S., Ricciardi R. P., Rando R. F., Puck J., Ge R. W., Plotkin S. A., Gönczöl E. An adenovirus recombinant that expresses the human cytomegalovirus major envelope glycoprotein and induces neutralizing antibodies. J Infect Dis. 1990 Nov;162(5):1177–1181. doi: 10.1093/infdis/162.5.1177. [DOI] [PubMed] [Google Scholar]
- McDermott M. R., Graham F. L., Hanke T., Johnson D. C. Protection of mice against lethal challenge with herpes simplex virus by vaccination with an adenovirus vector expressing HSV glycoprotein B. Virology. 1989 Mar;169(1):244–247. doi: 10.1016/0042-6822(89)90064-0. [DOI] [PubMed] [Google Scholar]
- Milich D. R. Genetic and molecular basis for T- and B-cell recognition of hepatitis B viral antigens. Immunol Rev. 1987 Oct;99:71–103. doi: 10.1111/j.1600-065x.1987.tb01173.x. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986 Aug;6(8):2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal S. K., Bett A. J., Prevec L., Graham F. L. Foreign gene expression by human adenovirus type 5-based vectors studied using firefly luciferase and bacterial beta-galactosidase genes as reporters. Virology. 1995 Jun 20;210(1):226–230. doi: 10.1006/viro.1995.1337. [DOI] [PubMed] [Google Scholar]
- Morin J. E., Lubeck M. D., Barton J. E., Conley A. J., Davis A. R., Hung P. P. Recombinant adenovirus induces antibody response to hepatitis B virus surface antigen in hamsters. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4626–4630. doi: 10.1073/pnas.84.13.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama T., Guilhot S., Klopchin K., Moss B., Pinkert C. A., Palmiter R. D., Brinster R. L., Kanagawa O., Chisari F. V. Immunobiology and pathogenesis of hepatocellular injury in hepatitis B virus transgenic mice. Science. 1990 Apr 20;248(4953):361–364. doi: 10.1126/science.1691527. [DOI] [PubMed] [Google Scholar]
- Prevec L., Campbell J. B., Christie B. S., Belbeck L., Graham F. L. A recombinant human adenovirus vaccine against rabies. J Infect Dis. 1990 Jan;161(1):27–30. doi: 10.1093/infdis/161.1.27. [DOI] [PubMed] [Google Scholar]
- Razi-Wolf Z., Freeman G. J., Galvin F., Benacerraf B., Nadler L., Reiser H. Expression and function of the murine B7 antigen, the major costimulatory molecule expressed by peritoneal exudate cells. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4210–4214. doi: 10.1073/pnas.89.9.4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmbeck R., Melber K., Mertens T., Reimann J. Antibody and cytotoxic T-cell responses to soluble hepatitis B virus (HBV) S antigen in mice: implication for the pathogenesis of HBV-induced hepatitis. J Virol. 1994 Mar;68(3):1418–1425. doi: 10.1128/jvi.68.3.1418-1425.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmbeck R., Melber K., Mertens T., Reimann J. Selective stimulation of murine cytotoxic T cell and antibody responses by particulate or monomeric hepatitis B virus surface (S) antigen. Eur J Immunol. 1994 May;24(5):1088–1096. doi: 10.1002/eji.1830240512. [DOI] [PubMed] [Google Scholar]
- Schirmbeck R., Melber K., Reimann J. Hepatitis B virus small surface antigen particles are processed in a novel endosomal pathway for major histocompatibility complex class I-restricted epitope presentation. Eur J Immunol. 1995 Apr;25(4):1063–1070. doi: 10.1002/eji.1830250431. [DOI] [PubMed] [Google Scholar]
- Sheay W., Nelson S., Martinez I., Chu T. H., Bhatia S., Dornburg R. Downstream insertion of the adenovirus tripartite leader sequence enhances expression in universal eukaryotic vectors. Biotechniques. 1993 Nov;15(5):856–862. [PubMed] [Google Scholar]
- Siddiqui A., Marion P. L., Robinson W. S. Ground squirrel hepatitis virus DNA: molecular cloning and comparison with hepatitis B virus DNA. J Virol. 1981 Apr;38(1):393–397. doi: 10.1128/jvi.38.1.393-397.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taswell C. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J Immunol. 1981 Apr;126(4):1614–1619. [PubMed] [Google Scholar]


