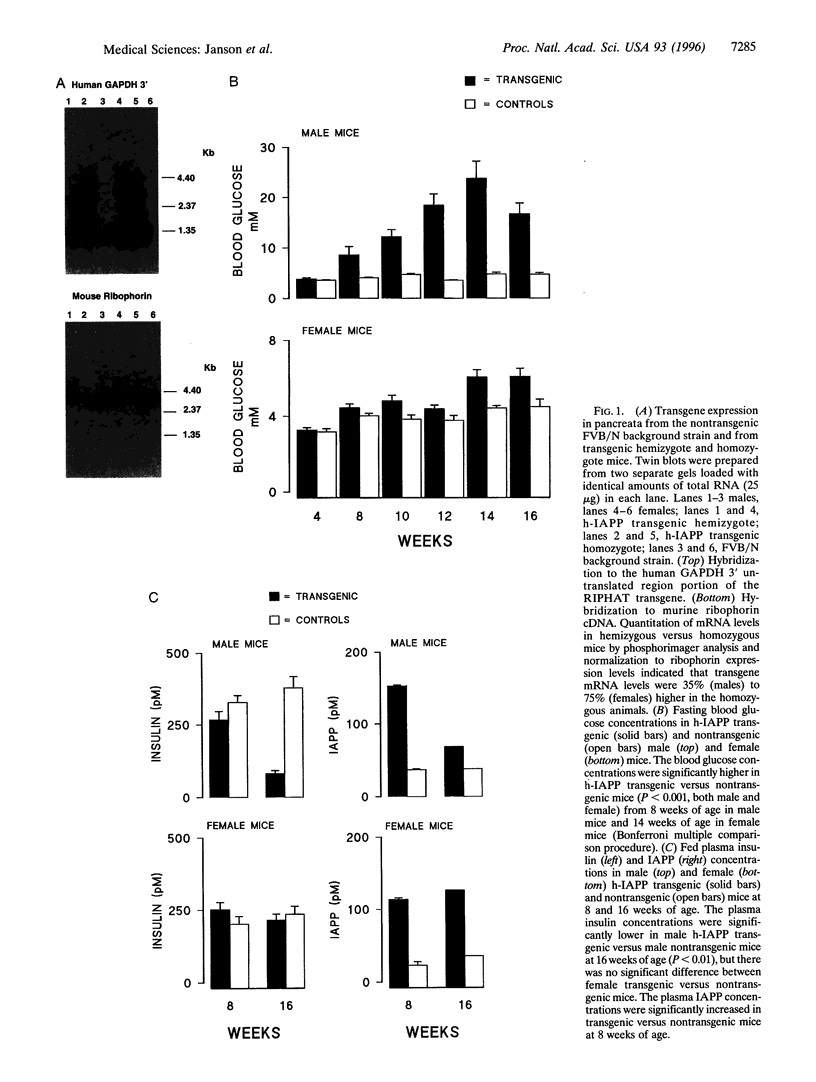
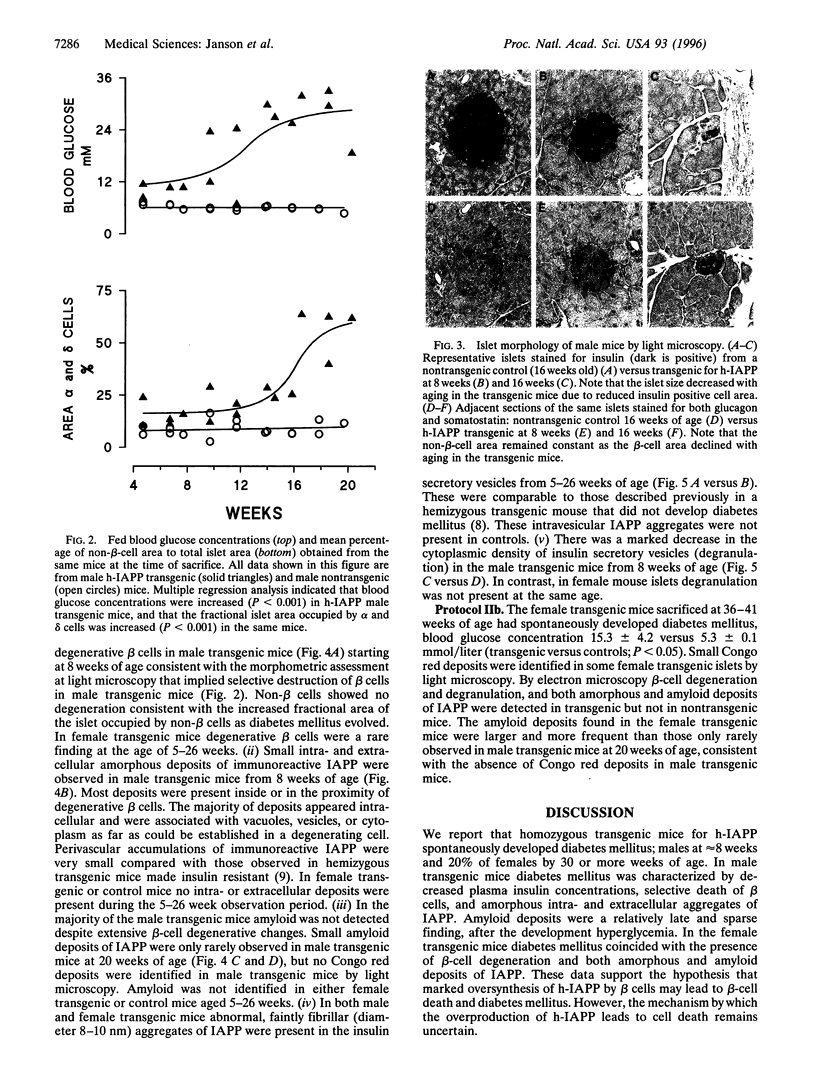
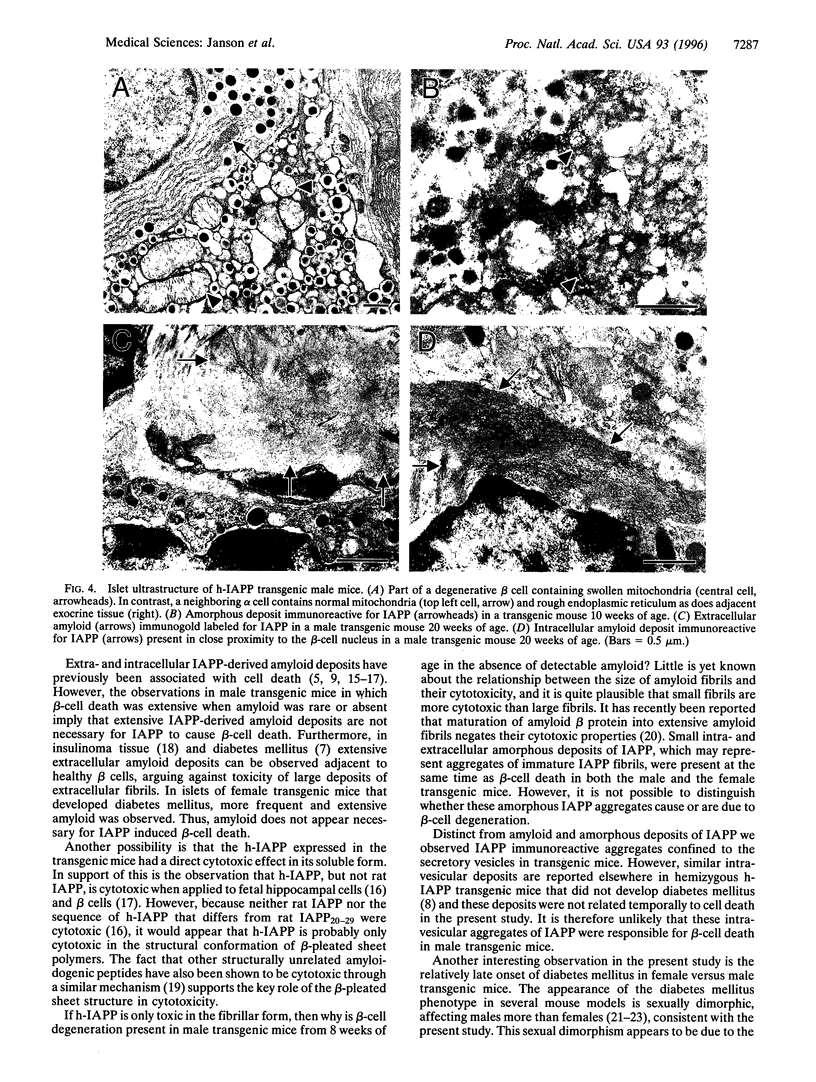
Abstract
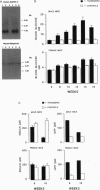
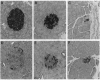
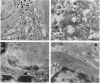
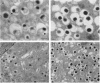
The islet in non-insulin-dependent diabetes mellitus (NIDDM) is characterized by loss of beta cells and large local deposits of amyloid derived from the 37-amino acid protein, islet amyloid polypeptide (IAPP). We have hypothesized that IAPP amyloid forms intracellularly causing beta-cell destruction under conditions of high rates of expression. To test this we developed a homozygous transgenic mouse model with high rates of expression of human IAPP. Male transgenic mice spontaneously developed diabetes mellitus by 8 weeks of age, which was associated with selective beta-cell death and impaired insulin secretion. Small intra- and extracellular amorphous IAPP aggregates were present in islets of transgenic mice during the development of diabetes mellitus. However, IAPP derived amyloid deposits were found in only a minority of islets at approximately 20 weeks of age, notably after development of diabetes mellitus in male transgenic mice. Approximately 20% of female transgenic mice spontaneously developed diabetes mellitus at 30+ weeks of age, when beta-cell degeneration and both amorphous and amyloid deposits of IAPP were present. We conclude that overexpression of human IAPP causes beta-cell death, impaired insulin secretion, and diabetes mellitus. Large deposits of IAPP derived amyloid do not appear to be important in this cytotoxicity, but early, small amorphous intra- and extracellular aggregates of human IAPP were consistently present at the time of beta-cell death and therefore may be the most cytotoxic form of IAPP.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison J., Malcolm L., Culvenor J., Bartholomeusz R. K., Holmberg K., Miller J. F. Overexpression of beta 2-microglobulin in transgenic mouse islet beta cells results in defective insulin secretion. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2070–2074. doi: 10.1073/pnas.88.6.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betsholtz C., Christmansson L., Engström U., Rorsman F., Svensson V., Johnson K. H., Westermark P. Sequence divergence in a specific region of islet amyloid polypeptide (IAPP) explains differences in islet amyloid formation between species. FEBS Lett. 1989 Jul 17;251(1-2):261–264. doi: 10.1016/0014-5793(89)81467-x. [DOI] [PubMed] [Google Scholar]
- Butler P. C., Chou J., Carter W. B., Wang Y. N., Bu B. H., Chang D., Chang J. K., Rizza R. A. Effects of meal ingestion on plasma amylin concentration in NIDDM and nondiabetic humans. Diabetes. 1990 Jun;39(6):752–756. doi: 10.2337/diab.39.6.752. [DOI] [PubMed] [Google Scholar]
- Couce M., O'Brien T. D., Moran A., Roche P. C., Butler P. C. Diabetes mellitus in cystic fibrosis is characterized by islet amyloidosis. J Clin Endocrinol Metab. 1996 Mar;81(3):1267–1272. doi: 10.1210/jcem.81.3.8772610. [DOI] [PubMed] [Google Scholar]
- Davis-Salinas J., Van Nostrand W. E. Amyloid beta-protein aggregation nullifies its pathologic properties in cultured cerebrovascular smooth muscle cells. J Biol Chem. 1995 Sep 8;270(36):20887–20890. doi: 10.1074/jbc.270.36.20887. [DOI] [PubMed] [Google Scholar]
- Efrat S., Fleischer N., Hanahan D. Diabetes induced in male transgenic mice by expression of human H-ras oncoprotein in pancreatic beta cells. Mol Cell Biol. 1990 Apr;10(4):1779–1783. doi: 10.1128/mcb.10.4.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox N., Schrementi J., Nishi M., Ohagi S., Chan S. J., Heisserman J. A., Westermark G. T., Leckström A., Westermark P., Steiner D. F. Human islet amyloid polypeptide transgenic mice as a model of non-insulin-dependent diabetes mellitus (NIDDM). FEBS Lett. 1993 May 24;323(1-2):40–44. doi: 10.1016/0014-5793(93)81444-5. [DOI] [PubMed] [Google Scholar]
- Gill A. M., Leiter E. H., Powell J. G., Chapman H. D., Yen T. T. Dexamethasone-induced hyperglycemia in obese Avy/a (viable yellow) female mice entails preferential induction of a hepatic estrogen sulfotransferase. Diabetes. 1994 Aug;43(8):999–1004. doi: 10.2337/diab.43.8.999. [DOI] [PubMed] [Google Scholar]
- Johnson K. H., O'Brien T. D., Betsholtz C., Westermark P. Islet amyloid polypeptide: mechanisms of amyloidogenesis in the pancreatic islets and potential roles in diabetes mellitus. Lab Invest. 1992 May;66(5):522–535. [PubMed] [Google Scholar]
- Johnson K. H., O'Brien T. D., Betsholtz C., Westermark P. Islet amyloid, islet-amyloid polypeptide, and diabetes mellitus. N Engl J Med. 1989 Aug 24;321(8):513–518. doi: 10.1056/NEJM198908243210806. [DOI] [PubMed] [Google Scholar]
- Lorenzo A., Razzaboni B., Weir G. C., Yankner B. A. Pancreatic islet cell toxicity of amylin associated with type-2 diabetes mellitus. Nature. 1994 Apr 21;368(6473):756–760. doi: 10.1038/368756a0. [DOI] [PubMed] [Google Scholar]
- Marshall B. A., Ren J. M., Johnson D. W., Gibbs E. M., Lillquist J. S., Soeller W. C., Holloszy J. O., Mueckler M. Germline manipulation of glucose homeostasis via alteration of glucose transporter levels in skeletal muscle. J Biol Chem. 1993 Sep 5;268(25):18442–18445. [PubMed] [Google Scholar]
- May P. C., Boggs L. N., Fuson K. S. Neurotoxicity of human amylin in rat primary hippocampal cultures: similarity to Alzheimer's disease amyloid-beta neurotoxicity. J Neurochem. 1993 Dec;61(6):2330–2333. doi: 10.1111/j.1471-4159.1993.tb07480.x. [DOI] [PubMed] [Google Scholar]
- O'Brien T. D., Butler A. E., Roche P. C., Johnson K. H., Butler P. C. Islet amyloid polypeptide in human insulinomas. Evidence for intracellular amyloidogenesis. Diabetes. 1994 Feb;43(2):329–336. doi: 10.2337/diab.43.2.329. [DOI] [PubMed] [Google Scholar]
- O'Brien T. D., Butler P. C., Kreutter D. K., Kane L. A., Eberhardt N. L. Human islet amyloid polypeptide expression in COS-1 cells. A model of intracellular amyloidogenesis. Am J Pathol. 1995 Sep;147(3):609–616. [PMC free article] [PubMed] [Google Scholar]
- Ohagi S., Nishi M., Bell G. I., Ensinck J. W., Steiner D. F. Sequences of islet amyloid polypeptide precursors of an Old World monkey, the pig-tailed macaque (Macaca nemestrina), and the dog (Canis familiaris). Diabetologia. 1991 Aug;34(8):555–558. doi: 10.1007/BF00400272. [DOI] [PubMed] [Google Scholar]
- Schubert D., Behl C., Lesley R., Brack A., Dargusch R., Sagara Y., Kimura H. Amyloid peptides are toxic via a common oxidative mechanism. Proc Natl Acad Sci U S A. 1995 Mar 14;92(6):1989–1993. doi: 10.1073/pnas.92.6.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermark P. Fine structure of islets of Langerhans in insular amyloidosis. Virchows Arch A Pathol Pathol Anat. 1973 Mar 20;359(1):1–18. doi: 10.1007/BF00549079. [DOI] [PubMed] [Google Scholar]
- Yagui K., Yamaguchi T., Kanatsuka A., Shimada F., Huang C. I., Tokuyama Y., Ohsawa H., Yamamura K., Miyazaki J., Mikata A. Formation of islet amyloid fibrils in beta-secretory granules of transgenic mice expressing human islet amyloid polypeptide/amylin. Eur J Endocrinol. 1995 Apr;132(4):487–496. doi: 10.1530/eje.0.1320487. [DOI] [PubMed] [Google Scholar]
- de Koning E. J., Höppener J. W., Verbeek J. S., Oosterwijk C., van Hulst K. L., Baker C. A., Lips C. J., Morris J. F., Clark A. Human islet amyloid polypeptide accumulates at similar sites in islets of transgenic mice and humans. Diabetes. 1994 May;43(5):640–644. doi: 10.2337/diab.43.5.640. [DOI] [PubMed] [Google Scholar]