Abstract
Whole tumor cell-based vaccines administered within the first 2 to 3 months after allogeneic stem cell transplantation stand out as a promising approach to enhance graft-vs.-leukemia responses. Herein, the implications of this finding for the development of strategies to improve the outcome of patients subjected to allogeneic stem cell transplantation are discussed.
Keywords: cancer vaccines, CLL, immune reconstitution, multiepitope vaccines, neoantigens, stem cell transplantation, whole tumor-cell vaccination
Graft-vs.-leukemia (GvL) activity is a crucial principle of allogeneic hematopoietic stem cell transplantation (allo-HSCT) and underlies curative responses observed in many patients with advanced hematologic malignancies who undergo this treatment modality.1 As a therapeutic strategy, allo-HSCT has the benefit of resulting in the engraftment of a normal donor immune system that has the potential to overcome host immune defects. In particular, donor-derived T cells are thought to be the primary cells responsible for the effectiveness of allo-HSCT as they may recognize host antigens including tumor-associated or -specific antigens (i.e., neoantigens, overexpressed antigens, antigens selectively expressed in malignant cells or viral antigens) and alloantigens, which arise from differences in genetic polymorphisms between donor and recipient (Fig. 1).1 The recognition of alloantigens by donor T cells has been thought to be the basis of the close association between GvL effects (i.e., the immune response against alloantigens expressed on hematopoietic cells) and graft-vs.-host disease (GvHD, i.e., the immune response against broadly expressed alloantigens) in allo-HSCT recipients.1
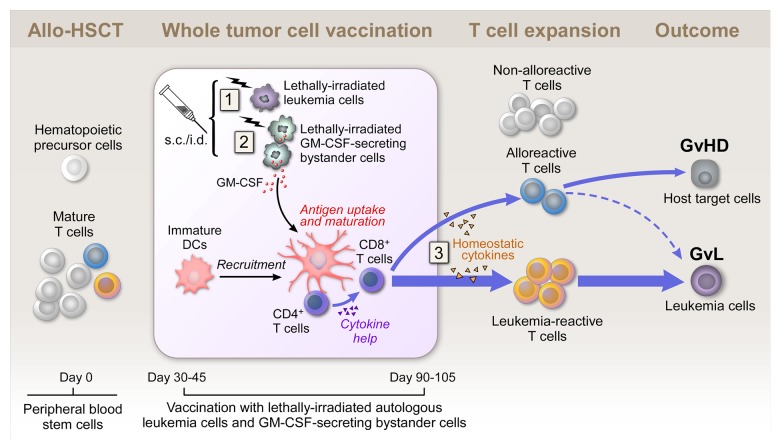
Figure 1. Whole tumor-cell vaccination early after allogeneic stem cell transplantation. Subcutaneously injected irradiated autologous cancer cells provide a source of tumor antigens at the vaccination site (1). Granulocyte macrophage colony-stimulating factor (GM-CSF) secreted by irradiated bystander cells stimulates the recruitment, maturation and immunostimulatory activity of dendritic cells (DCs) at the vaccination site (2). The allograft contains hematopoietic precursor cells and mature T cells, which might be tumor-reactive, alloreactive or non-alloreactive. Early after allogeneic stem cell transplantation (allo-HSCT), homeostatic cytokines support T cell expansion in the lymphopenic host (3). Autologous whole tumor cell-based vaccination may tip the balance between leukemia-specific and alloreactive T cell responses in favor of a graft-vs.-leukemia (GvL) effect. GvHD, graft-vs.-host disease; s.c., subcutaneous; i.d., intradermal.
With the development of reduced intensity conditioning (RIC) regimens, in which donor cell engraftment can be achieved with diminished morbidity and mortality as compared with conventional myleoablative regimens, the safety of the transplant procedure itself has improved, but the long-term control of leukemia remains a challenge.2,3 Thus, the development of strategies for inducing ever more robust GvL responses under conditions of minimal toxicity to achieve improved outcomes upon allo-HSCT remains a high priority.
One established approach to enhancing GvL in patients with relapsed hematologic malignancies is donor lymphocyte infusion (DLI). However, the clinical responses to DLI are not universal and the toxicities associated with this procedure are consistent with broad alloantigen stimulation.1 Alternatively, an appealing approach to stimulate GvL is to focus post-engraftment T cells on the tumor. An informative setting to implement and study strategies for enhancing GvL is allo-HSCT in patients with advanced chronic lymphocytic leukemia (CLL), for several reasons.
First, clinical remissions in transplanted CLL patients have been associated with the induction of GvHD or have been recorded upon the withdrawal of immunosuppressive GvHD-prophylactic medications, demonstrating that CLL is susceptible to immunological destruction and supporting the critical role of GvL responses in the control of CLL.4 Second, many CLL patients experience a relatively indolent disease course, which supplies the time that is required for the stimulation of GvL effects. Finally, malignant cells can be readily obtained from the peripheral blood of CLL patients, providing a reliable autologous source of tumor. The immunization of transplant recipients with CLL-associated antigens may boost antitumor immunity upon allo-HSCT, as the presentation of tumor antigens by stimulated dendritic cells may enhance donor T cell expansion and function and help focus immune responses toward leukemia cells.5
We recently tested this hypothesis in a Phase I clinical trial. In this setting, 18 patients with advanced CLL received up to six vaccine doses consisting of irradiated autologous leukemia cells combined with the adjuvant granulocyte macrophage colony-stimulating factor (GM-CSF) within the first 2–3 months following allo-HSCT.6 Vaccines were well tolerated, and the incidence of GvHD was similar to that observed in historical controls. Of note, we observed a rise in circulating CLL-specific (rather than alloreactive) CD8+ T cells and promising clinical activity in the study participants.6
Three features unique to our vaccination protocol may have been critical for its effectiveness (Fig. 1):
Antigen Source
We used CLL cells themselves as the source of CD4+ and CD8+ T cell antigens, since they are a reliable source of personal tumor antigens, including neoantigens. In contrast to vaccination with recombinant pre-defined antigens, our autologous whole tumor-cell vaccination approach has the advantage of harnessing the profile of immunogenic tumor antigens that is unique in each CLL patient.
Adjuvant
Our approach involved the combination of irradiated autologous leukemia cells with the continuous local production of GM-CSF. A reliable and standardized source of the adjuvant was provided by the HLA-negative human cell line K562, which was engineered to produce large amounts of GM-CSF. The paracrine production of GM-CSF at the vaccination site can stimulate antigen presentation.7 However, paradoxically, GM-CSF can also serve a regulatory role in dampening T cell responses and mediating immune homeostasis.7 Interestingly, we obtained evidence in support of both pro- and anti-inflammatory effects of GM-CSF in vaccinated patients, implying that in the early post-transplant setting, this cytokine may play a beneficial role in balancing the stimulation of antitumor immunity without exacerbating GvHD.6
Timing
Our vaccine was administered between days 30 and 100 after allo-HSCT, while patients were on stable doses of GvHD-prophylactic medication. Together with the results of preclinical studies, our findings suggest that the efficacy of vaccination may be improved by administration during the early post-transplant period. We think this is the case because (1) donor T cells are undergoing rapid homeostatic expansion in a lymphopenic setting (generated by the transplant conditioning regimen) and (2) are not functionally compromised by the immunosuppressive effects of a high tumor burden or chemotherapy (Fig. 1).6,8,9 Our findings argue against a more conventional point of view, in which the early post-transplant period is regarded as a poor platform for immunotherapy due to incomplete immune reconstitution and the immunosuppressive effects of GvHD prophylaxis.
Our pilot clinical study suggests that a multi-epitope vaccination strategy can tip the balance between leukemia-specific and alloantigen-reactive immunity in favor of GvL effects in transplant recipients. However, randomized studies with larger patient cohorts are required to determine whether our promising results translate into a definitive clinical benefit for patients.6
We propose that our findings provide the foundations for the development of new strategies that can be implemented to advance long-term leukemia control following HSCT. In particular, formulations based on neoantigens (identified by DNA and RNA sequencing) represent highly attractive next-generation post-transplant anticancer vaccines as they have the potential to induce T cell responses with exquisite specificity for malignant cells.10
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
U.E.B. acknowledges support from the German Research Foundation (BU 3028/1–1).
Citation: Burkhardt UE, Wu CJ. Boosting leukemia-specific T cell responses in patients following stem cell transplantation. OncoImmunology 2013; 2:e26587; 10.4161/onci.26587
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/26587
References
- 1.Wu CJ, Ritz J. Induction of tumor immunity following allogeneic stem cell transplantation. Adv Immunol. 2006;90:133–73. doi: 10.1016/S0065-2776(06)90004-2. [DOI] [PubMed] [Google Scholar]
- 2.Dreger P, Brand R, Milligan D, Corradini P, Finke J, Lambertenghi Deliliers G, Martino R, Russell N, van Biezen A, Michallet M, et al. Chronic Leukemia Working Party of the EBMT Reduced-intensity conditioning lowers treatment-related mortality of allogeneic stem cell transplantation for chronic lymphocytic leukemia: a population-matched analysis. Leukemia. 2005;19:1029–33. doi: 10.1038/sj.leu.2403745. [DOI] [PubMed] [Google Scholar]
- 3.Chakraverty R, Mackinnon S. Allogeneic transplantation for lymphoma. J Clin Oncol. 2011;29:1855–63. doi: 10.1200/JCO.2010.32.8419. [DOI] [PubMed] [Google Scholar]
- 4.Schetelig J, Thiede C, Bornhauser M, Schwerdtfeger R, Kiehl M, Beyer J, Sayer HG, Kroger N, Hensel M, Scheffold C, et al. Cooperative German Transplant Study Group Evidence of a graft-versus-leukemia effect in chronic lymphocytic leukemia after reduced-intensity conditioning and allogeneic stem-cell transplantation: the Cooperative German Transplant Study Group. J Clin Oncol. 2003;21:2747–53. doi: 10.1200/JCO.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 5.Ho VT, Vanneman M, Kim H, Sasada T, Kang YJ, Pasek M, Cutler C, Koreth J, Alyea E, Sarantopoulos S, et al. Biologic activity of irradiated, autologous, GM-CSF-secreting leukemia cell vaccines early after allogeneic stem cell transplantation. Proc Natl Acad Sci U S A. 2009;106:15825–30. doi: 10.1073/pnas.0908358106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burkhardt UE, Hainz U, Stevenson K, Goldstein NR, Pasek M, Naito M, Wu D, Ho VT, Alonso A, Hammond NN, et al. Autologous CLL cell vaccination early after transplant induces leukemia-specific T cells. J Clin Invest. 2013;123:3756–65. doi: 10.1172/JCI69098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jinushi M, Hodi FS, Dranoff G. Enhancing the clinical activity of granulocyte-macrophage colony-stimulating factor-secreting tumor cell vaccines. Immunol Rev. 2008;222:287–98. doi: 10.1111/j.1600-065X.2008.00618.x. [DOI] [PubMed] [Google Scholar]
- 8.Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, Hwang LN, Yu Z, Wrzesinski C, Heimann DM, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202:907–12. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teshima T, Mach N, Hill GR, Pan L, Gillessen S, Dranoff G, Ferrara JL. Tumor cell vaccine elicits potent antitumor immunity after allogeneic T-cell-depleted bone marrow transplantation. Cancer Res. 2001;61:162–71. [PubMed] [Google Scholar]
- 10.Hacohen N, Fritsch EF, Carter TA, Lander ES, Wu CJ. Getting personal with neoantigen-based therapeutic cancer vaccines. Cancer Immunol Res. 2013;1:11–5. doi: 10.1158/2326-6066.CIR-13-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]