Abstract
Geographic barriers and Quaternary climate changes are two major forces driving the evolution, speciation, and genetic structuring of extant organisms. In this study, we used Pinus armandii and eleven other Asian white pines (subsection Strobus, subgenus Pinus) to explore the influences of geographic factors and Pleistocene climatic oscillations on species in South China, a region known to be centers of plant endemism and biodiversity hotspots. Range-wide patterns of genetic variation were investigated using chloroplast and mitochondrial DNA markers, with extensive sampling throughout the entire range of P. armandii. Both cpDNA and mtDNA revealed that P. armandii exhibits high levels of genetic diversity and significant population differentiation. Three geographically distinct subdivisions corresponding to the Qinling-Daba Mountains (QDM), Himalaya-Hengduan Mountains (HHM) and Yungui Plateau (YGP) were revealed in mainland China by cpDNA. Their break zone was located in the southeastern margin of the Qinghai-Tibetan Plateau (QTP). A series of massive mountains, induced by the QTP uplift, imposed significant geographic barriers to genetic exchange. The disjunct distribution patterns of ancestral haplotypes suggest that a large continuous population of the white pines may have existed from southwest to subtropical China. Repeated range shifts in response to the Pleistocene glaciations led to the isolation and diversification of the subtropical species. The two Taiwanese white pines share a common ancestor with the species in mainland China and obtain their chloroplasts via long-distance pollen dispersal from North Asian pines. Distinct genetic patterns were detected in populations from the Qinling-Daba Mountains, Yungui Plateau, Himalaya-Hengduan Mountains, and subtropical China, indicating significant contributions of geographic factors to the genetic differentiation in white pines. Our study depicts a clear picture of the evolutionary history of Chinese white pines and highlights the heterogeneous contributions of geography and Pleistocene climatic fluctuations to the extremely high plant species diversity and endemism in South China.
Introduction
Geographic barriers and climate change are two major forces driving the evolution, speciation and genetic structuring of extant organisms [1]–[2]. The Cenozoic collision between India and Asia created a highly complex montane system in southwest China, including the highest mountain range on Earth (the Himalayas) and the largest “roof of the world”-the Qinghai-Tibet Plateau (QTP). The uplift of the QTP reshaped the landscape features of central and eastern Asia and the global climate system [3]–[6]. In conjunction with the dramatic cooling of the global climate in the past three million years, geologic changes induced by the recent uplift of the QTP would be expected to have contributed to the development of the extremely high levels of species diversity in South China, a large region known to be centers of plant endemism and biodiversity hotspots. In this vast mountainous area, the Hengduan Mountain range, Central China, and the Nanling Mountains, which span the three-tier terrain of China from the high elevation west to the low elevation east, were recognized as plant diversity and endemism hotspots [7]. In particular, the Himalaya-Hengduan Mountains (HHM) region, which extends along the southern frontier to the southeastern margin of the QTP, encompasses 2 of the 34 biodiversity hotspots of the world, i.e., ‘Mountains of Southwest China’ and ‘Himalaya’ [8]. Although there is widespread agreement that dramatic geomorphology changes induced by the QTP uplift and/or Pleistocene glaciations profoundly affected the biota in this region, their relative influences on speciation and genetic structuring are largely unknown.
The last decade has witnessed an exponential growth in phylogeographic studies on the diverse taxa of many geographic regions in China (e.g., [9]–[12]). Unfortunately, most studies sampled only a single or a few closely related species, and concentrated extensively on the effects of Quaternary glaciations on genetic structure and the locations of glacial refugia. Only recently, have a few studies begun to disentangle the relative influences of the uplift of the QTP and the Pleistocene climatic fluctuations on speciation and genetic structuring in the QTP region and adjacent areas [13]–[16]. Moreover, there are few large-scale phylogeographic studies with sufficient coverage of distinct bioregions (for instance, warm central China, subtropical southern China, and mountainous western China) to detect the diversity of responses among closely related species to the geologic changes induced by the uplift of the QTP and Pleistocene glaciations.
The Chinese white pines, Pinus armandii and its relatives (Pinus, subgenus Strobus) provide an efficient system to explore these historical processes due to their extensive geographic distributions and relatively recent diversification (10∼20 million years ago; Ma) [17]. Although there is no universal agreement on taxonomic relationships, most studies, including our own recent molecular phylogenies (unpublished), show a close relationship among the nine white pines distributed in the south part of mainland China and Taiwan, i.e., P. armandii and its variety P. armandii var. mastersiana, P. bhutanica, P. dabeshanensis, P. fenzeliana, P. kwantungensis, P. morrisonicola, P. wallichiana, and P. wangii [18]–[22]. All of these white pines are assigned to the subsection Strobus of Section Quinquefoliae [23]. Among them, P. armandii has the broadest geographic distribution. It is extensively and discontinuously distributed in central and southwest China, across a latitudinal range of 23°30′–36°31′N and a longitudinal range of 85°30′–113°E, with one variety, P. armandii var. mastersiana, extending to Taiwan [24]. P. wallichiana is a common conifer tree occurring throughout the temperate regions of the Himalayas, whereas another Himalayan pine, P. bhutanica, is only endemic to the eastern Himalayas. Three very closely related pines, P. fenzeliana, P. kwantungensis, and P. wangii, have localized and patchy distributions in the lower mountains and montane forests of southern China and possibly in adjacent northern Vietnam. P. dabeshanensis is a rare pine endemic to the Dabie Mountains, where it represents the easternmost distribution of soft pines in mainland China. In North China, three widespread north Asian white pines, P. sibirica, P. pumila and P. koraiensis, have a relatively limited distribution in the northwest and northeast (Fig. 1A).
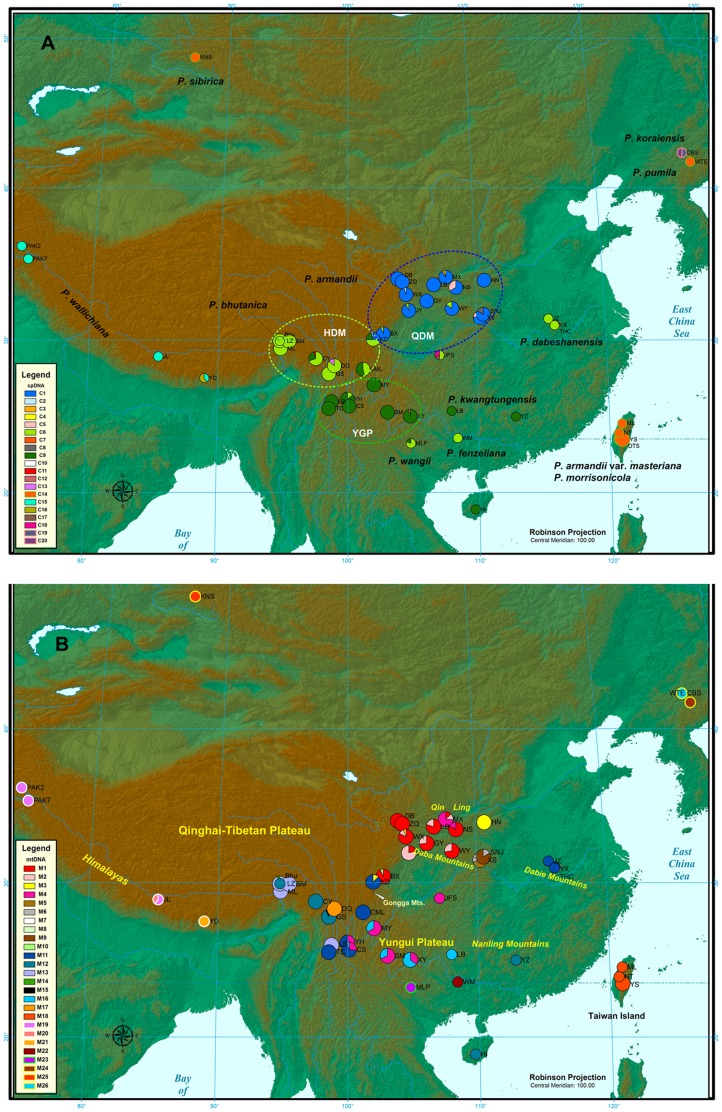
Figure 1. Geographic distribution of chlorotypes (A) and mitotypes (B).
Small circles in the pie charts indicate populations from the relatives of Pinus armandii. The three geographic subdivisions of P. armandii in mainland China are shown in A.
In this study, we used a combination of phylogeography, inter-specific phylogeny and molecular dating techniques with two paternally inherited chloroplast DNA fragments and three maternally inherited mitochondrial DNA markers to elucidate the effects of geography and Quaternary climatic oscillations on the genetic structure, speciation and evolution of the Chinese white pines. This study will shed further light on the mechanisms underlying plant speciation and the origin of the strikingly high plant diversity in southern China.
Materials and Methods
Ethics statement
No specific permits were required for the described locations in China because all researchers collecting the samples had introduction letters from the Institute of Botany, Chinese Academy of Sciences (IBCAS), Beijing. The samples from northern Pakistan were obtained from the international scientific research project of “Flora of Pan-Himalaya”. The field studies involved collection of endangered species.
Population sampling
A total of 415 individuals from 30 populations of P. armandii and P. armandii var. mastersiana, representing the entire geographic range of this species, were included in our analysis. Each population had 13–23 individuals except for six from mainland China (DB, ZQ, LB, NS, SNJ, and HN) and two from Taiwan (DTS and YS), which were represented by <10 individuals. Sixty-five additional trees from the other 10 white pines distributed in China and adjacent areas (i.e., P. wallichiana, P. kwangtungensis, P. fenzeliana, P. bhutanica, P. dabeshanensis, P. wangii, P. pumila, P. sibirica, P. morrisonicola, and P. koraiensis) were also sampled for phylogeographic analysis. The detailed sampling information is shown in Table 1.
Table 1. Geographic locations and sample sizes of the Asian white pines sampled in this study.
| Species | Subdivisions1 | Population name/location | Sample | Latitude | Longitude |
| size | (°N) | (°E) | |||
| Pinus armandii | QinLing-Daba Mts.(QDM) | DB/Diebu, Gansu | 3 | 34.01 | 103.92 |
| ZQ/Zhouqu, Gansu | 4 | 33.82 | 104.27 | ||
| WX/Wenxian, Gansu | 19 | 32.98 | 104.56 | ||
| MX/Meixian, Shanxi | 23 | 34.12 | 107.7 | ||
| LB/Liuba, Shanxi | 5 | 33.63 | 106.73 | ||
| NS/Ningshan, Shanxi | 6 | 33.45 | 108.47 | ||
| XS/Xingshan, Hubei | 17 | 31.47 | 110.3 | ||
| SNJ/Shennongjia, Hubei | 6 | 31.69 | 110.55 | ||
| HN/Lushi, Henan | 5 | 33.92 | 110.7 | ||
| WY/Wanyuan, Sichuan | 13 | 32.07 | 108.12 | ||
| GY/Guangyuan, Sichuan | 15 | 32.56 | 106.16 | ||
| JY/Jiangyou, Sichuan | 14 | 31.94 | 104.76 | ||
| BX/Baoxing, Sichuan | 15 | 30.41 | 102.76 | ||
| Himalaya-HengDuan Mts.(HHM) | KD/Kangding, Sichuan | 23 | 30.05 | 101.96 | |
| CML/Muli, Sichuan | 15 | 28.8 | 101.18 | ||
| CY/Chayu, Xizang(Tibet) | 23 | 28.78 | 97.53 | ||
| ML/Milin, Xizang(Tibet) | 15 | 29.44 | 94.81 | ||
| LZ/Linzhi, Xizang(Tibet) | 17 | 29.94 | 94.75 | ||
| BM/Bomi, Xizang(Tibet) | 14 | 29.92 | 95.46 | ||
| GS/Gongshan, Yunnan | 15 | 27.78 | 98.55 | ||
| DQ/Deqin,Yunnan | 15 | 28.31 | 98.97 | ||
| Yungui Plateau (YGP) | YH/Yuhu, Yunnan | 21 | 26.12 | 99.97 | |
| LS/Lushui, Yunnan | 15 | 25.96 | 98.75 | ||
| TC/Tengchong, Yunnan | 15 | 25.5 | 98.55 | ||
| CS/Cangshan, Yunnan | 15 | 25.66 | 100.12 | ||
| MY/Miyi, Sichuan | 15 | 27.05 | 102.01 | ||
| SM/Songming, Yunnan | 16 | 25.26 | 103.04 | ||
| XY/Xingyi, Guizhou | 20 | 25 | 104.79 | ||
| P. armandii var. masteriana | Taiwan Island | DTS/Datashan, Taiwan | 9 | 23.53 | 120.82 |
| YS/Yushan, Taiwan | 7 | 23.47 | 120.94 | ||
| P. wallichiana | JL/Jilong, Xizang(Tibet) | 5 | 28.92 | 85.38 | |
| YD/Yadong,Xizang(Tibet) | 5 | 27.5 | 88.97 | ||
| PAK2/Naltar Bala, Pakistan | 5 | 36.17 | 74.18 | ||
| PAK7/Rama Lake, Pakistan | 5 | 35.33 | 74.79 | ||
| P. kwangtungensis | LB/Libo, Guizhou | 3 | 25.35 | 107.95 | |
| YZ/Yizhang, Hunan | 2 | 24.98 | 112.85 | ||
| WM/Wuming Mts, Guangxi | 2 | 23.57 | 108.38 | ||
| P. fenzeliana | HI/Hainan Island | 2 | 18.88 | 109.66 | |
| JFS/Nanchuan, Chongqing | 6 | 29 | 107.09 | ||
| P. bhutanica | LZ/Linzhi, Xizang(Tibet) | 5 | 29.94 | 94.75 | |
| P. dabeshanensis | YX/Yuexi, Anhui | 2 | 31.01 | 116.07 | |
| JZ/Jinzhai, Anhui | 1 | 31.4 | 115.6 | ||
| THC/Taohuachong, Hubei | 1 | 30.97 | 116.06 | ||
| P. wangii | MLP/Molipo, Yunnan | 5 | 23.22 | 104.81 | |
| P. pumila | WTE/Changbai, Jilin | 5 | 41.73 | 127.86 | |
| P. sibirica | KNS/Kanasi, Xinjiang | 5 | 48.71 | 87.07 | |
| P. morrisonicola | NT/Nantou, Taiwan | 1 | 23.91 | 120.68 | |
| ML/Miaoli, Taiwan | 1 | 24.53 | 120.94 | ||
| P. koraiensis | CBS/Changbai Mts., Jilin | 4 | 42.33 | 127.27 |
DNA extraction, PCR amplification, and sequencing
Total genomic DNA was extracted from silica gel-dried leaves using the modified CTAB method [25] or DNAsecure Plant Kit (Tiangen). We initially screened 22 primer pairs for polymorphism by using a discovery panel composed of 20 individuals that represent all major geographic subdivisions of P. armandii. Two chloroplast fragments, atpH-atpI and trnC-trnD, and three mitochondrial fragments, cox1, nad5 intron 1, and nad7 intron1 were finally chosen for subsequent analysis in all sampled individuals. The five DNA segments were amplified with the primers used in previous studies [13], [26]–[29]. The PCR mixture and amplification program followed the protocols of Wei et al. [30], except that an annealing temperature of 50–55°C was used and the elongation time was adjusted according to the different markers. The PCR products were purified with PEG8000. Both the cox1 and nad5 intron 1 strands were sequenced using the amplification primers in all samples. The atpH-atpI gene and nad7 intron1 were sequenced by using a forward primer and trnC-trnD by using a reverse primer because all the informative polymorphisms could be covered by single primer sequencing. Sequencing was conducted with an ABI Prism 3730xl sequencer (PE Applied Biosystems). The sequences reported in this study were deposited in GenBank under accession numbers KF800948 - KF801058.
Gene genealogy
Nucleotide sequences were aligned using CLUSTAL X [31] and manually adjusted in BioEdit v7.05.3 [32]. Since organellar genomes in plants generally do not recombine and are uniparentally inherited, we treated the two cpDNA markers and the three mtDNA markers as a single locus each. A polymorphic site caused by polyG (9–12 bp) in nad5 was excluded due to some low quality reads. All phylogenetically informative gaps (indels) were coded as single mutation events for analyses. Unique haplotypes were determined using the program DnaSP v5 [33]. All the rare haplotypes were verified by re-extracting DNA, re-amplifying and re-sequencing both strands.
NETWORK 4.611 (http://www.fluxus-engineering.com) was used for generating haplotype networks by the median-joining network algorithm. P. koraiensis was used as the outgroup because of its relatively distant relationship with the southern white pines (unpublished). Substitutions and indels were considered to evolve with equal possibility.
Population genetic analyses
The spatial variance in haplotype distributions was analyzed using SAMOVA 1.0 (http://web.unife.it/progetti/genetica/Isabelle/samova.html) [34]. It implements a simulated annealing approach to define groups of populations (K) that are geographically homogenous and maximally differentiated from each other. The K values ranging from 2 to 9 were tested to search for the K that gave the highest F CT.
Nuclotide diversity (π) and Watterson's parameter (θW) were estimated using DnaSP v5 [33]. The program PERMUTE [35] (http://www.pierroton.inra.fr/genetics/labo/software) was used for calculating the average gene diversity within populations (H S), total gene diversity (H T), and two measures of population differentiation (G ST, N ST). The two estimates of population divergence were compared using a permutation test with 1000 permutations. Genetic differentiation among populations and groups (identified by SAMOVA) was estimated by the analysis of molecular variance (AMOVA) with significance tests based on 10, 000 permutations using Arlequin 3.11 [36]. Population expansion was examined using the mismatch distribution analysis (MDA). For each model, goodness-of-fit, based on the sum of squared deviations (SSD), was tested using parametric demographic expansion bootstrapping with 1000 replicates. In addition, we also calculated Tajima's D [37] and Fu's F S [38] to infer potential expansions. All the demographic tests were performed using Arlequin 3.11. When sudden expansions were detected, the MDA-derived expansion parameter (τ) was converted to absolute expansion time (t, in number of generations) according to the equation τ = 2ut [39], where u is the mutation rate per generation for the entire analyzed sequence. We calculated u according to u = μkg, where μ is the substitution rate per nucleotide site per year, k is the average sequence length of the DNA region under study, and g is the generation time in years. We assumed that pines take 25–100 years to reach full seed production [40]. Substitution rate was obtained from the clock-calibrated BEAST (see the Results section).
Phylogenetic analyses and estimates of divergence time
The evolutionary relationships of the chlorotypes and mitotypes were reconstructed with Maximum parsimony (MP), Maximum likelihood (ML), and Bayesian inference (BI) using PAUP*4.0b10 [41], PHYML2.4.4 [42] and MrBayes 3.1 [43], respectively. All phylogenetically informative gaps (indels) were coded as single mutation events. The final alignment was 1373 bp in length. The evolutionary models of GTR+I and GTR+I+G for cpDNA and mtDNA phylogenetic analyses, respectively, were determined by the Akaike information criterion (AIC) implemented in MrModeltest v2.3 [44]–[45]. All characters were treated as unordered and equally weighted in the MP analysis. A heuristic search strategy with 1000 replicates of random addition of sequences, tree-bisection-reconnection (TBR) branch-swapping and MULTREES options was involved in the MP and ML analyses. To assess the relative support for monophyletic groups, bootstrap analysis was conducted with 100 replicates using the same heuristic search settings as descripted above. In the Bayesian analysis, one cold and three incrementally heated Markov chain Monte Carlo (MCMC) chains were run simultaneously for 1,000,000 generations. Trees were sampled every 100 generations with the first 2500 samples discarded as burn-in. To establish the divergence time of the Asian white pines, a subset of our initial cpDNA data and five additional GenBank sequences from P. monophylla (NC_011158.4) and P. maximartinezii (JN854184.1) in Section Parrya, P. bungeana (JN854223.1) and P. gerardiana (NC_011154.4) in Subsection Gerardianae, and a North American white pine, P. lambertiana (FJ899577.1), were combined and reanalyzed with the aim of obtaining a useful calibration point. Phylogenetic trees were reconstructed with MP, ML, and BI by using the methods mentioned above. In this phylogenetic analysis, the two species from Section Parrya were used as outgroups due to the sister relationship between section Quinquefolia and section Parrya [23].
A Bayesian approach was employed to estimate the divergence time of each major group in the chlorotype phylogeny with BEAST v1.6.1 [46], using a relaxed uncorrelated lognormal clock model and the Yule model of speciation. Due to the lack of credible fossils from P. armandii or closely related species, we employed the crown node age of the two monophyletic sections Quinquefolia and Parrya (subgen. Strobus) reported by Willyard et al. [17]. We used a normal distribution centered at 35 Ma with a standard deviation of 1.0 Ma. This gave a central 95% range of 33–37 Ma, which is within the ranges reported by the two studies [17], [47] from independent fossil calibrations. The MCMC analysis was run for 30,000,000 generations. We sampled 1000 trees with the first 50% treated as burn-in. The output file was analyzed using TRACER 1.5 (http://beast.bio.ed.ac.uk/Tracer). The measures of effective sample sizes (ESS) were used to determine the Bayesian statistical significance of each parameter.
Results
Chlorotype distribution and genealogy
The lengths of the two cpDNA regions (atpH-atpI and trnC-trnD) in the white pines were found to be 674–686 bp and 893–954 bp, respectively. The combined cpDNA dataset is 1565 bp in length. Six indels and 13 substitutions were detected and resulted in 20 chlorotypes (C1–C20) among the trees surveyed. Of those, C1, C6 and C9 were the most common chlorotypes. P. armandii harbored 13 chlorotypes C1–C13. C14 is unique to the two Taiwanese white pines, P. armandii var. masteriana and P. morrisonicola, as well as the two north Asian species P. pumila and P. sibirica. C15 and C16 were fixed in P. wallichiana. C17 and C18 are rare chlorotypes, unique to JFS of P. fenzeliana. P. koraiensis harbored C19 and C20.
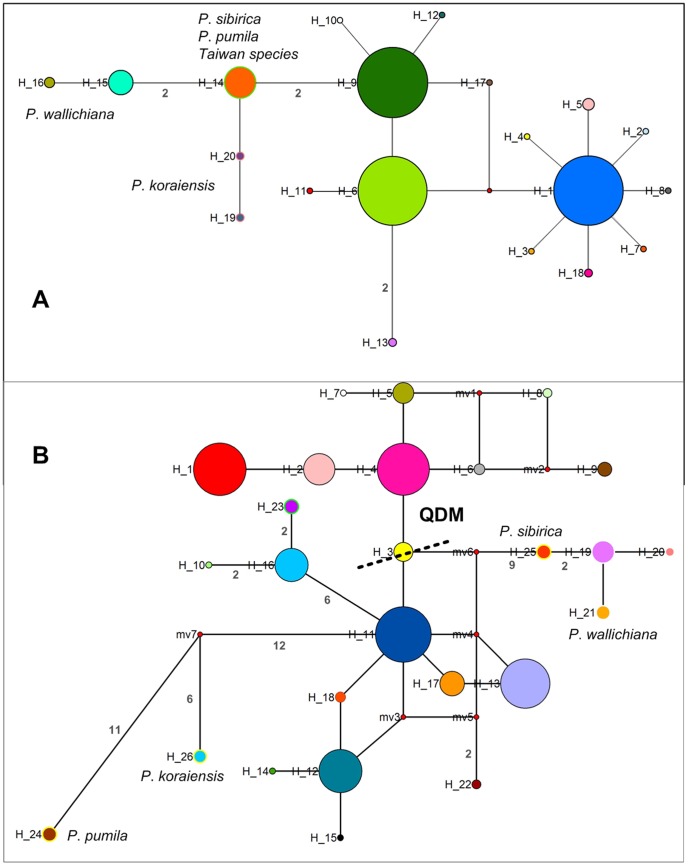
A strong geographic pattern of chlorotype variation was observed in P. armandii. SAMOVA showed that the value of F CT reached a plateau at 0.87 for four groups (K = 4). The two populations (DTS and YS) of P. armandii var. masteriana from Taiwan were separated as a distinct group. The other three groups from mainland China also had a strong geographic basis, generally corresponding to the Qinling-Daba Mountains (DB, ZQ, WX, MX, LB, NS, XS, SNJ, HN, WY, GY, JY, and BX), Himalaya-Hengduan Mountains (KD, CML, DQ, CY, ML, LZ, BM, and GS) and Yungui Plateau (YH, LS, TC, CS, MY, SM, and XY). Hereafter, we designate these three groups as QDM, HHM, and YGP, respectively. Each group harbored a dominant chlorotype (Fig. 1A). C1 was unique to QDM. C6 and C9 dominated in HHM and YGP, respectively. In addition, C6 occurred at a low frequency in several QDM populations (BX, JY, WY, and XS) and P. bhutanica, P. dabeshanensis, P. fenzeliana, and P. wangii. C9 only fixed in southern populations, including the three subtropical white pines, P. kwangtungensis, P. wangii, and P. fenzeliana. It is notable that all the three common chlorotypes were present in the KD population with high frequency (Fig. 1A). The chlorotype network delineated three major clades of P. armandii populations in mainland China (Fig. 2A), which broadly correspond to the three geographic subdivisions (QDM, HHM, and YGP).
Figure 2. NETWORK-derived genealogical relationship of chlorotypes (A) and mitotypes (B).
The sizes of the circles in the network are proportional to the observed frequencies of the haplotypes. The number of mutational steps larger than one is shown next to the solid line between haplotypes.
Mitotype distribution and genealogy
The sizes of the three mtDNA markers, cox1, nad5 intron 1, and nad7 intron 1, are 1225 bp, 1035–1042 bp and 809–1244 bp, respectively. Five individuals from populations WX, WY, and BX of P. armandii were excluded due to a polymorphic nucleotide site in the nad5 intron 1 and 15 individuals from P. armandii var. masteriana because of failed amplification of the nad7 intron 1. A total of 24 indels and 28 substitutions were identified for the concatenated mitochondrial DNA sequence which yielded 26 mitotypes (M1–M26). Of those, P. armandii harbored 17 (M1–M17), and four of them (M4, M11, M12, and M16) are shared across species. M18 was fixed in the two Taiwanese white pines (P. armandii var. masteriana and P. morrisonicola). M19–M26 were restricted to the remaining white pines. No more than three mitotypes were found in a single population, with the exception of XS, which harbored five (M4–M8).
QDM possessed ten mitotypes (M1–M10), and eight (M1–M2, M5–M10) were unique. Of these, M1 occurred in all populations except three in the east. M3 was only found in nine individuals from three populations (WX, HN, and KD) across a large geographic range. M4 dominated in eastern YGP, but it was also present in eastern QDM (MX, XS, NS) and in the JFS population of P. fenzeliana. M12 and M13 predominated in HHM. M16 occurred at high frequency in eastern YGP and shared with its adjacent population (LB) from P. kwangtungensis. M11 had a disjunct distribution between the southeastern margin of the QTP (P. armandii) and Dabie Mountains in eastern China (P. dabeshanensis).
Unlike the chlorotypes, the mitotype network did not show clear geographic relationships among QDM, HHM and YGP (Fig. 2B). It is compatible with the SAMOVA of mtDNA, in which no plateau value of F CT was obtained. Nevertheless, two clusters linked by M3 were broadly delineated in P. armandii, one comprised of all but one (M10) mitotype from the QDM lineage and the other included all the mitotypes from the HHM and YGP lineages except M4, which nested in the QDM cluster. In the latter cluster, M11 (KD, CML, YH, CS, and TC) was placed in the interior position, directly linked by M3 (KD and HN), M16 (CS, MY, SM, XY, and LB), M17 (DQ), and M18 (Taiwan) (Fig. 2B). In theory, haplotypes with an interior position in a network are often older than haplotypes on the tips [48]. In addition, the close relationship of M11 with P. koraiensis (M26), which was used as an outgroup in the network analysis, also suggests that it is old.
Genetic diversity and population differentiation of P. armandii
Both cpDNA and mtDNA showed high levels of total genetic diversity (H T) over populations of P. armandii from mainland China (0.692 for cpDNA, 0.886 for mtDNA), but the levels of average within-population diversity (H S) were much lower (0.157 and 0.239 for cpDNA and mtDNA, respectively; Table 2). Population differentiation was remarkably high, with G ST values being 0.773 and 0.730 for cpDNA and mtDNA, respectively (Table 2). The permutation tests of cpDNA showed that N ST was significantly higher than G ST (P<0.01), suggesting a strong phylogeographic structure in P. armandii. However, N ST was not significantly greater than G ST at mtDNA loci. AMOVA showed that 83.14% and 82.31% of the total cpDNA and mtDNA variation respectively resided among populations (Table 3).
Table 2. Total genetic diversity, average genetic diversity within populations and population differentiation estimates in Pinus armandii.
| Region | cpDNA | mtDNA | ||||||
| H T | H S | N ST | G ST | H T | H S | N ST | G ST | |
| QDM | 0.171 | 0.163 | 0.053 | 0.048 | 0.689 | 0.290 | 0.737* | 0.579 |
| HHM | 0.334 | 0.241 | 0.231 | 0.278 | 0.833 | 0.061 | 0.955 | 0.927 |
| YGP | 0.055 | 0.051 | 0.074 | 0.068 | 0.788 | 0.349 | 0.430 | 0.557 |
| Mainland China | 0.692 | 0.157 | 0.860** | 0.773 | 0.886 | 0.239 | 0.790 | 0.730 |
| Entire range | 0.730 | 0.147 | 0.889** | 0.799 | nc | nc | nc | nc |
extremely significant (Nst>Gst, P<0.01);
significant (Nst>Gst, P<0.05); nc, not be calculated due to single individual in YS population.
Table 3. Analysis of molecular variance (AMOVA) of Pinus armandii based on cpDNA and mtDNA haplotypes.
| Source of variation | d.f. | Sum of squares | Variance | Percentage | Fixation indices | |||||
| components | of variation | |||||||||
| cpDNA | mtDNA | cpDNA | mtDNA | cpDNA | mtDNA | cpDNA | mtDNA | cpDNA | mtDNA | |
| Mainland China | ||||||||||
| Among populations | 27 | 27 | 242.95 | 410.83 | 0.63 | 1.07 | 83.14 | 82.31 | ||
| Within populations | 371 | 366 | 47.10 | 84.30 | 0.13 | 0.23 | 16.86 | 17.69 | F ST = 0.83 | F ST = 0.82 |
| Total | 398 | 393 | 290.04 | 495.13 | 0.75 | 1.30 | ||||
| Divided into three regions | ||||||||||
| (QDM, HHM, and YGP) | ||||||||||
| Among regions | 2 | 2 | 233.13 | 195.78 | 0.88 | 0.67 | 85.73 | 44.45 | F SC = 0.13 | F SC = 0.72 |
| Among populations within | 25 | 25 | 9.81 | 215.05 | 0.02 | 0.61 | 1.86 | 40.27 | F ST = 0.88 | F ST = 0.85 |
| regions | F CT = 0.86 | F CT = 0.44 | ||||||||
| Within populations | 371 | 366 | 47.10 | 84.30 | 0.13 | 0.23 | 12.42 | 15.28 | ||
| Total | 398 | 393 | 290.04 | 495.13 | 1.02 | 1.51 | ||||
| Divided into two regions | ||||||||||
| (North and South) | ||||||||||
| Among regions | 1 | 1 | 189.55 | 159.46 | 1.01 | 0.82 | 79.38 | 47.60 | F SC = 0.68 | F SC = 0.75 |
| Among populations within | 26 | 26 | 53.39 | 251.37 | 0.14 | 0.68 | 10.69 | 39.10 | F ST = 0.82 | F ST = 0.87 |
| regions | F CT = 0.44 | F CT = 0.48 | ||||||||
| Within populations | 371 | 366 | 47.10 | 84.30 | 0.13 | 0.23 | 9.93 | 13.30 | ||
| Total | 398 | 393 | 290.04 | 495.13 | 1.28 | 1.73 | ||||
At the regional level, contrasting patterns of genetic diversity and population differentiation were observed at cpDNA and mtDNA loci (Table 2, 3). For cpDNA, the level of total genetic diversity (H T = 0.055–0.334) and of population differentiation (G ST = 0.048–0.278) were relatively low in each of the three subdivisions (QDM, HHM, and YGP). Accordingly, the hierarchical analysis of molecular variance (AMOVA) showed a high level of variation among regions (85.73%) and extremely low level of variation (1.86%) among populations within regions. Taiwan populations were monomorphic. In contrast, the total genetic diversity (H T = 0.689–0.833) and population differentiation (G ST = 0.557–0.927) were very high in mtDNA. The partitioning of the mtDNA variation among regions and among populations within regions were 44.45% and 40.27%, respectively. Among the three geographic subdivisions, HHM had the highest level of genetic diversity and population differentiation at both cpDNA and mtDNA (Table 2).
Phylogenetic analyses and divergence time estimate
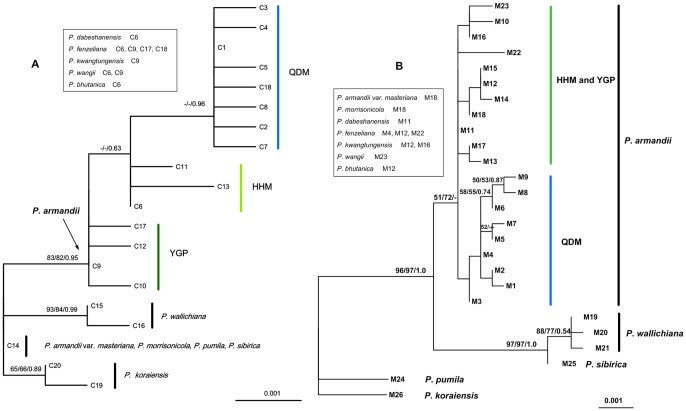
Both cpDNA and mtDNA phylogenies (Fig. 3A, B) indicated that the Himalayan pine, P. wallichiana, was distantly related to the southern pines in mainland China, i.e., P. armandii, P. bhutanica, P. dabeshanensis, P. fenzeliana, P. kwangtungensis, and P. wangii. In the cpDNA tree, three lineages corresponding to QDM, HHM, and YGP, were resolved within the clade including P. armandii. The two Taiwanese white pines clustered together with two North Asian pines, P. sibirica and P. pumila, and showed a sister relationship to P. armandii. In the mtDNA tree, however, the two white pines in Taiwan were included in the P. armandii clade.
Figure 3. Phylogenetic topography obtained by analysis of chlorotypes (A) and mitotypes (B).
Numbers above the branches are bootstrap values greater than 50% for ML (left) and MP (middle) analyses, and Bayesian posterior probabilities (right). A dash indicates a bootstrap value lower than 50%. The other species included in the Pinus armandii clade are shown in the box.
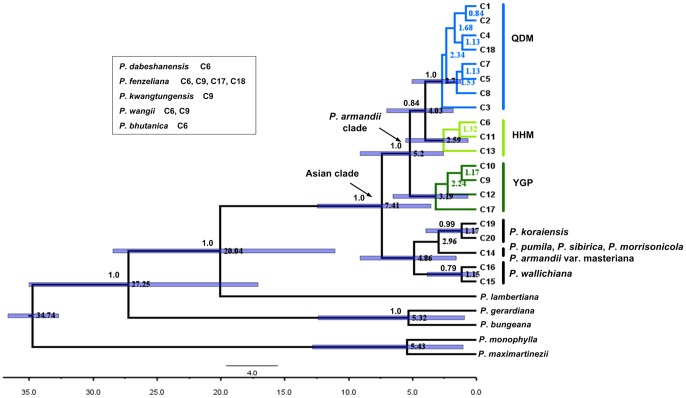
Molecular dating showed that the age of the split between the Asian and North American white pines was about 20.04 Ma (95% highest posterior density (HPD) interval, 28.46 Ma – 11.09 Ma). The Asian white pines were divided into two lineages around 7.41 Ma (95% HPD interval, 12.44 Ma – 3.57 Ma). One lineage was composed of the widespread Himalayan pine P. wallichiana, three northern pines, P. koraiensis, P. sibirica, P. pumila, and two from Taiwan, P. morrisonicola and P. armandii var. masteriana, while the other was comprised of P. armandii and the other white pines in southern China. Within the P. armandii clade, YGP diverged at about 5.2 Ma (95% HPD interval, 9.09 Ma – 2.58 Ma). QDM and HHM were separated 4.03 Ma (95% HPD interval, 7.01 Ma – 1.84 Ma). All these estimates are shown in Fig. 4. The mean nucleotide substitution rate for the combined cpDNA region was estimated to be 0.47×10−9 per site per year.
Figure 4. Phylogenetic chronogram of the chlorotypes generated from BEAST.
Numbers above the branches indicate the Bayesian posterior probabilities greater than 0.5. Median ages of nodes are shown, with horizontal bars indicating the 95% highest posterior density intervals. The other species included in the Pinus armandii clade are shown in the box.
Demographic analyses
Under a model of sudden population expansion (P>0.05), for cpDNA, each of the three regions, QDM, HHM and YGP, showed a strongly unimodal mismatch distribution and nonsignificant SSD index values (Fig. S1), indicating a historical population expansion. However, Fu's Fs and Tajima D tests suggest only QDM had significantly negative Fu's F S and Tajima D values (F S = −8.23581, P<0.001; D = −1.88930, P<0.05) (Table S1). Therefore, we can say with confidence that QDM experienced expansion. The expansion of QDM was estimated at 93 ka (a generation time of 25 y applied) and 23.2 ka (a generation time of 100 y applied). For mtDNA, none of them showed strongly unimodal mismatch distribution and significantly negative values of Tajima D or Fu's F S.
Discussion
Strong genetic differentiation of P. armandii and uplift of the QTP
Compared with the average genetic differentiation of conifers (cpDNA = 0.165; mtDNA = 0.764) [49] and those reported recently for other Pinaceae species (e.g., [50]–[55]), P. armandii exhibits high levels of total genetic diversity and significant population differentiation at both cpDNA (H T = 0.692, F ST = 0.83, G ST = 0.773) and mtDNA loci (H T = 0.886, F ST = 0.82, G ST = 0.730) (Table 2, 3). As a conifer with the capacity for long-distance pollen dispersal, the high level of the total cpDNA variation among regions (85.73%), and extremely low level among populations within regions (1.86%; Table 3), suggested that geography might play an important role in driving the genetic structure of P. armandii population.
The chlorotypes revealed three lineages of P. armandii in mainland China that geographically corresponding to the Qinling-Daba Mountains (QDM), Himalaya-Hengduan Mountains (HHM), and Yungui Plateau (YGP). Their break zone was observed along the southeastern margin of the QTP. The QDM lineage was separated from the two south lineages (HHM and YGP) at Kangding (KD) in western Sichuan, which is consistent with a previous provenance study [56]. Numerous high mountains and plateaus over 6,000 m above the sea level (a.s.l.), including the highest Gongga Mountain (7,556 m a.s.l.) (Fig. 1B), are located nearby and divide the Hengduan Mountains into northern and southern floristic sub-regions [57]. The genetic isolation of the HHM and YGP populations was consistent with the geographic transition of the Hengduan Mountains and the Yungui Plateau, two large natural geographic regions characterized by a series of parallel massive mountains and deeply carved valleys running roughly north to south and mid-elevation mountains oriented from west to east, respectively. It is likely that these massive mountains at the southeastern margin of the QTP imposed significant geographic barriers to the genetic exchange among different lineages of P. armandii. Active mountain building, induced by the recent uplift of the southeastern margin of the QTP, might strongly affect the genetic structure of P. armandii.
A close link was detected between the recent rapid uplift of the QTP and the differentiation of P. armandii. Based on our analysis of molecular dating, Pinus armandii split from the Himalayan pine P. wallichiana around 7.41 Ma (95% HPD interval, 12.44 Ma – 3.57 Ma). The subsequent divergence of P. armandii was dated to early Pliocene (5.2–4.03 Ma; Fig. 4). Although we need to be cautious with these estimates due to the limited data, they are concordant with a Miocene origin (10–20 Ma) of the two subsections Strobus and Cembroides that harbor the greatest number of soft pines [58]. Several lines of evidence suggest that the rapid uplift of the eastern part of the QTP took place around 13 to 5 Ma [59]–[60]. Dramatic geological changes induced by the QTP uplift contributed to the extremely complicated landscape in the eastern fringe, which likely acted as a major driving force for the separation of the three lineages of P. armandii. Divergent selection between populations in contrasting environments would accelerate their differentiation, and eventually promote allopatric speciation.
Recent diversification of the subtropical white pines and Taiwan species
In wind-pollinated conifers with contrasting modes of inheritance, weaker population differentiation is often observed in pollen-mediated cpDNA than seed-mediated mtDNA (e.g., [30], [49]–[50], [61]). In P. armandii, such asymmetric differentiation between cpDNA and mtDNA was indeed observed at the regional scale. However, across its entire range, the cpDNA differentiation was surprisingly much more highly structured geographically than the mtDNA. The hierarchical AMOVA also showed that 85.73% and 44.45% of the cpDNA and mtDNA variation respectively resided among regions (Table 3), indicating more restricted pollen-mediated gene flow than seed-mediated gene flow among the three lineages. This unique pattern detected in P. armandii can likely be attributed to its evolutionary history and considerable gene flow via seed migration in the past.
Several lines of evidence, including the chlorotype composition of the KD population, a large number of common mitotypes and the dominant presence of the putative ancestral mitotype (M11), indicate that P. armandii originated in southwest China and probably in the eastern part of the QTP (Fig. 1). It is surprising that M11 and C6, which dominated in the southeastern margin of the QTP, were also found in eastern China (P. dabeshanensis). A similar disjunct distribution pattern was also observed in M12 and C6, between the HHM lineage of P. armandii and some populations of P. fenzeliana (HI), P. kwantungensis (YZ), and P. wangii (MLP) in southern China. It is likely that a large continuous population of white pines once existed from southwest to subtropical China, but now only persists in isolated geographic aggregates. Range disjunctions for plant species are often a consequence of Quaternary climate changes [2]. A scenario could be inferred that in tracking repeated Pleistocene glaciations, these montane white pines may have migrated up and down elevation gradients. During these necessary movements into glacial refugia, they experienced severe fragmentation and isolation. In addition, population bottlenecks might have occurred in P. dabeshanensis and led to its extremely small population size and low genetic diversity. This scenario was supported by the phylogeographic studies of P. kwantungensis [54]–[55], as well as their current distributions and morphological similarities. These white pines are scattered in the broadleaved evergreen forest region in subtropical China, a vast region where well-known centers of plant diversity and endemism exist [7], and can also often be found on isolated mountaintops, cliffs, or slopes. Some areas, such as the Dabie Mountains, Jinfo Mountains, Hainan Island, Nanling Mountains, and eastern Yungui Plateau are often postulated as glacial refugia for warm-temperate evergreen forest species [54]–[55], [62]–[64]. Long-term persistence in isolated refugia would promote their divergence and eventual speciation.
The origin of the florist affinity between Taiwan and southwestern China/Himalayas is a fascinating question regarding the biogeography of eastern Asia [30], [65]. P. armandii var. masteriana and P. morrisonicola are endemic to Taiwan. Their primary difference is that P. armandii var. masteriana (like P. armandii in mainland China) has wingless seeds whereas P. morrisonicola has winged seeds. In addition, P. armandii var. masteriana generally occurs at higher altitudes (1800–3300 m) than P. morrisonicola (300–2300 m) [18]. It is interesting that the two species share a single mitotype (M18) which is closely related to M11, suggesting they evolved from a common ancestor in mainland China. The ancestor was probably in eastern China due to the presence of M11 in the Dabie Mountains and the short distance from Taiwan. The Taiwanese populations were genetically less diverse in both cpDNA and mtDNA markers, which might correspond to a rather recent colonization. Unexpectedly, the two Taiwanese white pines shared the single chlorotype C14 with two North Asian white pines, P. pumila and P. sibirica, indicating a North Asian origin of their chloroplasts. The inconsistency between the cpDNA and mtDNA markers was observed in the cytoplasmic phylogenies (Fig. 3). Introgression via long-distance pollen dispersal may be the most probable cause of the incongruence. Furthermore, a single chloroplast introgression event might occur in the two white pines followed by a loss of genetic diversity through drift effects in an island population. One may argue that the long-distance dispersal is inconsistent with the idea of limited pollen dispersal via geographic barriers among the three lineages of P. armandii in southwest China. However, the complicated landscapes in southwest China, particularly numerous massive mountains, likely imposed more significant barriers to pollen dispersal than the Pacific islands for wind-pollinated trees. The current distribution of P. pumila in Japan provides further support for this supposition. Frequent introgression and hybridization is well documented in pine species [66]. Our results suggested that interspecific introgression might also occur between the eastern YGP populations of P. armandii and LB population of P. kwantungensis, as well as between the HHM populations of P. armandii and P. bhutanica. Our study demonstrated the importance of including more closely related species in phylogeographic research and the value of a clear evolutionary background for resolving the taxonomic uncertainties in the problematic white pines.
Heterogeneous contributions of geography and climate changes to the genetic differentiation of Chinese white pines
Quaternary climate changes produced great changes in species distribution and population structure [2]. Nevertheless, the diversification patterns may be spatially varied. In the case of Chinese white pines, different genetic patterns were observed among populations from the Qinling-Daba Mountains, Himalaya-Hengduan Mountains, Yungui Plateau and southern China, which are diverse in geographic, climatologic and topographic features. The north facing Qinling Mountains oriented from east to west in central China form a natural dividing line between Northern and subtropical (Central/East/South) China and serve as a major geographic barrier to the cold, dry air coming in from the north. Thus, this region might be subjected to relatively stronger influences of Pleistocene glaciations. As expected, mismatch analysis and the indices of Fu's F* and Tajima D suggested that only the QDM lineage of P. armandii has undergone a sudden population expansion which was estimated to have occurred around 23–93 ka, close to the last glacial maximum (18–24 ka BP). The large number of private haplotypes and high levels of nucleotide diversity of the SNJ and XS populations (Table S1, S2, S3) suggest that the eastern Daba Mountains likely served as a main refugium for the QDM lineage. Among the three lineages of P. armandii, the HHM lineage exhibits the highest level of genetic diversity and population differentiation (Table 2). This could be attributed to the extremely dissected topography of the HHM, which acted as significant barriers to gene flow.
It is noteworthy that disjunct mitotypes (e.g., M11, M12) found in the eastern margin of the QTP and the subtropical China are missing in the eastern Yungui Plateau. Instead, the eastern YGP populations (MY, SM, and XY) of P. armandii are dominated by M4 and M16. It is likely that the relatively less complex geomorphological configuration of the YGP not only contributed to the low genetic diversity and population differentiation in that region (Table 2), but also promoted the expansion of some putative adaptive alleles. In contrast, subtropical China is characterized by extremely rugged low mountains and relatively stable environments, which may have favored the preservation of relict lineages. Phylogeographic studies of P. kwantungensis have revealed many glacial refugia in this region and high levels of population differentiation at both cpDNA (Gst = 0.63 vs. 0.068 in YGP) and mtDNA loci (Gst = 0.75 vs. 0.557 in YGP) [54]–[55]. These findings highlighted the significance of both Quaternary climate changes and geographic factors in shaping the current genetic structures of Chinese white pines.
Supporting Information
Mismatch distributions for the different subdivisions of Pinus armandii . Capital and lower case letters indicated the distributions of chlorotypes and mitotypes, and the black and gray lines represent the expected and observed mismatch distributions, repectively. Aa: mainland China; Bb: QDM; Cc: YGP; Dd: HDM.
(TIF)
Tajima's D and Fu's FS neutrality tests and nucleotide diversity of each population and geographic subdivision of Pinus armandii at cpDNA and mt DNA loci.
(DOC)
Chlorotype distribution in each population of Pinus armandii and the other white pines in this study.
(DOC)
Mitotype distribution in each population of Pinus armandii and the other white pines in this study.
(DOC)
Acknowledgments
We thank two anonymous reviewers for their insightful and valuable comments and suggestions on the manuscript, and Dr. Anna W. Schoettle (USDA Forest Service, Rocky Mountain Research Station) for reading over the manuscript. We are grateful to Professor Hong Deyuan, Chen Zhiduan and Li Liangqian, Drs. Ma Xintang, Yang Fusheng, Wang Mingming, Qin Aili, Wang Qiang and Wang Hongjuan (Institute of Botany, CAS), Liu Zhanlin and Wang Xinsha (Northwest University), Kan Xianzhao, Zhang Zhongxin and Xiang Xiaoyan (Anhui Normal University) for their help in the sample collections; Miss Jin Wanqing for her assistance in the DNA sequencing.
Funding Statement
This study was supported by the National Natural Science Foundation of China (Grant nos. 31270422, 30730010, 31160047). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Mayr E (1942) Systematics and the origin of species. New York: Columbia University Press. [Google Scholar]
- 2. Hewitt GM (2000) The genetic legacy of the Quaternary ice ages. Nature 405: 907–913. [DOI] [PubMed] [Google Scholar]
- 3. An ZS, Kutzbach JE, Prell WL, Porter SC (2001) Evolution of Asian monsoons and phased uplift of the Himalaya-Tibetan plateau since Late Miocene times. Nature 41: 62–66. [DOI] [PubMed] [Google Scholar]
- 4. Guo ZT, Ruddiman WF, Hao QZ, Wu HB, Qiao YS, et al. (2002) Onset of Asian desertification by 22 Myr ago inferred from loess deposits in China. Nature 416: 159–163. [DOI] [PubMed] [Google Scholar]
- 5. Jia GD, Peng PA, Zhao QH, Jian ZM (2003) Changes in terrestrial ecosystem since 30 Ma in East Asia: stable isotope evidence from black carbon in the South China Sea. Geology 31: 1093–1096. [Google Scholar]
- 6. Sun XJ, Wang PX (2005) How old is the Asian monsoon system?-Palaeobotanical records from China. Palaeogeo Palaeoclim Palaeoecol 222: 181–222. [Google Scholar]
- 7. Ying TS (2001) Species diversity and distribution pattern of seed plants in China. Biodivers Sci 9: 393–398. [Google Scholar]
- 8.Mittermeier RA, Gil PR, Hoffman M, Pilgrim J, Brooks T, et al.. (2005) Hotspots Revisited: Earth's Biologically Richest and Most Endangered Terrestrial Ecoregions. Cemex, Conservation International and Agrupación Sierra Madre, Monterrey, Mexico.
- 9. Yang SJ, Dong HL, Lei FM (2009) Phylogeography of regional fauna on the Tibetan Plateau: a review. Prog Nat Sci 19: 789–799. [Google Scholar]
- 10. Qiu YX, Fu CX, Comes HP (2011) Plant molecular phylogeography in China and adjacent regions: tracing the genetic imprints of Quaternary climate and environmental change in the world's most diverse temperate flora. Mol Phylogenet Evol 59: 225–244. [DOI] [PubMed] [Google Scholar]
- 11. Yang FS, Qin AL, Li YF, Wang XQ (2012) Great genetic differentiation among populations of Meconopsis integrifolia and its implication for plant speciation in the Qinghai-Tibetan Plateau. PLoS ONE 7: e37196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Qin AL, Wang MM, Cun YZ, Yang FS, Wang SS, et al. (2013) Phylogeographic evidence for a link of species divergence of Ephedra in the Qinghai-Tibetan Plateau and adjacent regions to the Miocene Asian aridification. PLoS ONE 8: e56243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cun YZ, Wang XQ (2010) Plant recolonization in the Himalaya from the southeastern Qinghai-Tibetan Plateau: Geographical isolation contributed to high population differentiation. Mol Phylogenet Evol 56: 972–982. [DOI] [PubMed] [Google Scholar]
- 14. Xu TT, Abbott RJ, Milne RI, Mao KS, Du FK, et al. (2010) Phylogeography and allopatric divergence of cypress species (Cupressus L.) in the Qinghai-Tibetan Plateau and adjacent regions. BMC Evol Biol 10: 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu SJ, Luo J, Li QQ, Wang YQ, Murphy RW, et al. (2013) Ecological genetics of Chinese Rhesus Macaque in response to mountain building: all things are not equal. PLoS ONE 8: e55315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yan F, Zhou WW, Zhao HT, Yuan ZY, Wang YY, et al. (2013) Geological events play a larger role than Pleistocene climatic fluctuations in driving the genetic structure of Quasipaa boulengeri (Anura: Dicroglossidae). Mol Ecol 22: 1120–1133. [DOI] [PubMed] [Google Scholar]
- 17. Willyard A, Syring J, Gernandt DS, Liston A, Cronn R (2007) Fossil calibration of molecular divergence infers a moderate mutation rate and recent radiations for Pinus . Mol Biol Evol 24: 90–101. [DOI] [PubMed] [Google Scholar]
- 18.Fu LG, Li N, Mill RR (1999) Flora of China 4. In: Wu CY, Raven PH, editors. Beijing & Missouri: Science Press & Missouri Botanical Garden Press.
- 19.Farjon A (2001) World checklist and bibliography of conifers, 2nd edn. Royal Botanic Gardens, Kew.
- 20. Businský R (2004) A revision of the Asian Pinus subsection Strobus (Pinaceae). Willdenowia 34: 209–257. [Google Scholar]
- 21. Syring J, Farrell K, Businský R, Cronn R, Liston A, et al. (2007) Widespread genealogical nonmonophyly in species of Pinus subgenus Strobus . Syst Biol 56: 163–181. [DOI] [PubMed] [Google Scholar]
- 22. Tsutsui K, Suwa A, Sawada K, Kato T, Ohsawa TA, et al. (2009) Incongruence among mitochondrial, chloroplast and nuclear gene trees in Pinus subgenus Strobus (Pinaceae). J Plant Res 122: 509–521. [DOI] [PubMed] [Google Scholar]
- 23. Gernandt DS, Lopez GG, Garcia SO, Liston A (2005) Phylogeny and classification of Pinus . Taxon 54: 29–42. [Google Scholar]
- 24. Wang HR, Hong JS (2004) Genetic resources, tree improvement and gene conservation of five-needle pines in East Asia. USDA Forest Service Proceedings RMRS-P 32: 73–78. [Google Scholar]
- 25. Rogers SO, Bendich AJ (1988) Extraction of DNA from plant tissues. Plant Mol Biol Manual A6: 1–10. [DOI] [PubMed] [Google Scholar]
- 26. Ran JH, Wei XX, Wang XQ (2006) Molecular phylogeny and biogeography of Picea (Pinaceae): Implications for phylogeographical studies using cytoplasmic haplotypes. Mol Phylogenet Evol 41: 405–419. [DOI] [PubMed] [Google Scholar]
- 27. Wang XQ, Tank DC, Sang T (2000) Phylogeny and divergence times in Pinaceae: Evidence from three genomes. Mol Biol Evol 17: 773–781. [DOI] [PubMed] [Google Scholar]
- 28. Duminil J, Pemonge MH, Petit RJ (2002) A set of 35 consensus primer pairs amplifying genes and introns of plant mitochondrial DNA. Mol Ecol Notes 2: 428–430. [Google Scholar]
- 29. Jaramillo-Correa JP, Beaulieu J, Bousquet J (2004) Variation in mitochondrialDNA reveals multiple distant glacial refugia in black spruce (Picea mariana), a transcontinental North American conifer. Mol Ecol 13: 2735–2747. [DOI] [PubMed] [Google Scholar]
- 30. Wei XX, Beaulieu J, Khasa DP, Vargas-Hernández J, López-Upton J, et al. (2011) Range-wide chloroplast and mitochondrial DNA imprints reveal multiple lineages and complex biogeographic history for Douglas-fir. Tree Genet Genomes 7: 1025–1040. [Google Scholar]
- 31. Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The clustal X Windons interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25: 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41: 95–98. [Google Scholar]
- 33. Librado P, Rozas J (2009) DnaSP v.5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25: 1451–1452. [DOI] [PubMed] [Google Scholar]
- 34. Dupanloup I, Schneider S, Excoffier L (2002) A simulated annealing approach to define the genetic structure of populations. Mol Ecol 11: 2571–581. [DOI] [PubMed] [Google Scholar]
- 35. Pons O, Petit RJ (1996) Measuring and testing genetic differentiation with ordered versus unordered haplotypes. Genetics 144: 1237–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Excoffier L, Laval G, Schneider S (2005) Arlequin version 3.0: an integrated software package for population genetics data analysis. Evol Bioinform Online 1: 47–50. [PMC free article] [PubMed] [Google Scholar]
- 37. Tajima F (1989) Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123: 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fu YX (1997) Statistical tests of neutrality of mutations against population growth, hitchhiking and backgroud selection. Genetics 147: 915–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rogers AR, Harpending H (1992) Population growth makes waves in the distribution of pairwise genetic differences. Mol Biol Evol 9: 552–569. [DOI] [PubMed] [Google Scholar]
- 40. Ma XF, Szmidt AE, Wang XR (2006) Genetic structure and evolutionary history of a diploid hybrid pine Pinus densata inferred from the Nucleotide variation at seven gene loci. Mol Biol Evol 23: 807–816. [DOI] [PubMed] [Google Scholar]
- 41.Swofford DL (2002) PAUP*. Phylogenetic Analysis Using Parsimony (*and other methods). Version 4. Sinauer, Sunderland, MA.
- 42. Guindon S, Gascuel O (2003) A simple, fast, and accurate algorithm to estimatelarge phylogenies by maximum likelihood. Syst Biol 52: 696–704. [DOI] [PubMed] [Google Scholar]
- 43. Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. [DOI] [PubMed] [Google Scholar]
- 44. Posada D, Crandall KA (1998) Modeltest: testing the model of DNA substitution. Bioinformatics 14: 917–918. [DOI] [PubMed] [Google Scholar]
- 45.Nylander JAA (2004) MrModeltest v2. Program distributed by the author. Evolutionary Biology Centre, Uppsala University.
- 46. Drummond AJ, Rambaut A (2007) BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol 7: 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gernandt DS, Magallon S, Lopez GG, Flores OZ, Willyard A, et al. (2008) Use of simultaneous analyses to guide fossil-based calibrations of Pinaceae phylogeny. Int J Plant Sci 169: 1086–1099. [Google Scholar]
- 48. Posada D, Crandall KA (2001) Intraspecific phylogenetics: trees grafting into networks. Trends Ecol Evol 16: 37–45. [DOI] [PubMed] [Google Scholar]
- 49. Petit RJ, Duminil J, Fineschi S, Hampe A, Salvini D, et al. (2005) Comparative organization of chloroplast, mitochondrial and nuclear diversity in plant populations. Mol Ecol 14: 689–701. [DOI] [PubMed] [Google Scholar]
- 50. Jaramillo-Correa JP, Beaulieu J, Ledig FT, Bousquet J (2006) Decoupled mitochondrial and chloroplast DNA population structure reveals Holocene collapse and population isolation in a threatened Mexican-endemic conifer. Mol Ecol 15: 2787–2800. [DOI] [PubMed] [Google Scholar]
- 51. Aizawa M, Yoshimaru H, Saito H, Katsuki T, Kawahara T, et al. (2007) Phylogeography of a northeast Asian spruce, Picea jezoensis, inferred from genetic variation observed in organelle DNA markers. Mol Ecol 16: 3393–3405. [DOI] [PubMed] [Google Scholar]
- 52. Meng LH, Yang R, Abbott RJ, Miehe G, Hu TH, et al. (2007) Mitochondrial and chloroplast phylogeography of Picea crassifolia Kom. (Pinaceae) in the Qinghai-Tibetan Plateau and adjacent highlands. Mol Ecol 16: 4128–4137. [DOI] [PubMed] [Google Scholar]
- 53. Chen KM, Abbott RJ, Milne RI, Tian XM, Liu JQ (2008) Phylogeography of Pinus tabulaeformis Carr. (Pinaceae), a dominant species of coniferous forest in northern China. Mol Ecol 17: 4276–4288. [DOI] [PubMed] [Google Scholar]
- 54. Tian S, Luo LC, Ge S, Zhang ZY (2008) Clear genetic structure of Pinus kwangtungensis (Pinaceae) revealed by a plastid DNA fragment with a novel minisatellite. Ann Bot 102: 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tian S, López-Pujol J, Wang HW, Ge S, Zhang ZY (2010) Molecular evidence for glacial expansion and interglacial retreat during Quaternary climatic changes in a montane temperate pine (Pinus kwangtungensis Chun ex. Tsiang) in southern China. Plant Syst Evol 284: 219–229. [Google Scholar]
- 56.Ma CG (1992) Symposium on selection of optimum seed sources for plantations of Pinus armandii Franch. Beijing: Beijing Agricultural University press. [Google Scholar]
- 57. Zhang DC, Boufford DE, Ree RH, Sun H (2009) The 29°N latitudinal line: an important division in the Hengduan Moutains, a biodiversity hotspot in southwest China. Nordic J Bot 27: 405–412. [Google Scholar]
- 58. Willyard A, Syring J, Gernandt D, Liston A, Cronn R (2006) Molecular evolutionary rates indicate a recent and rapid diversification for modern pine lineages. Mol Biol Evol 23: 1–12.16151190 [Google Scholar]
- 59. Harris N (2006) The elevation history of the Tibetan Plateau and its implications for the Asian monsoon. Palaeogeogr Palaeoclimatol Palaeoecol 241: 4–15. [Google Scholar]
- 60. Clark MA, House MA, Royden LH, Whipple KX, Burchfiel BC, et al. (2005) Late Cenozoic uplift of southeastern Tibet. Geology 33: 525–528. [Google Scholar]
- 61. Wang B, Mao JF, Gao J, Zhao W, Wang XR (2011) Colonization of the Tibetan Plateau by the homoploid hybrid pine Pinus densata . Mol Ecol 20: 3796–3811. [DOI] [PubMed] [Google Scholar]
- 62. Wang HW, Ge S (2006) Phylogeography of the endangered Cathaya argyrophylla (Pinaceae) inferred from sequence variation of mitochondrial and nuclear DNA. Mol Ecol 15: 4109–4122. [DOI] [PubMed] [Google Scholar]
- 63. Gao LM, Moeller M, Zhang XM, Hollingsworth ML, Liu J, et al. (2007) High variation and strong phylogeographic pattern among cpDNA haplotypes in Taxus wallichiana (Taxaceae) in China and North Vietnam. Mol Ecol 16: 4684–4698. [DOI] [PubMed] [Google Scholar]
- 64. Wang J, Gao P, Kang M, Lowe A J, Huang H W (2009) Refugia within refugia: the case study of a canopy tree (Eurycorymbus cavaleriei) in subtropical China. J Biogeogr 36: 2156–2164. [Google Scholar]
- 65. Chen ZD, Ying JS, Lu AM (2012) Disjunct distribution of seed plants between Southwestern China and Taiwan Island of China. Chinese Bull Bot 47: 551–570. [Google Scholar]
- 66.Critchdield WB (1984) Impact of the Pleistocene on the genetic structure of North American conifers. In: Lanner RM, editor. Proceedings of the 8th North America forest biology workshop. Logan, Utah. pp. 70–118. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mismatch distributions for the different subdivisions of Pinus armandii . Capital and lower case letters indicated the distributions of chlorotypes and mitotypes, and the black and gray lines represent the expected and observed mismatch distributions, repectively. Aa: mainland China; Bb: QDM; Cc: YGP; Dd: HDM.
(TIF)
Tajima's D and Fu's FS neutrality tests and nucleotide diversity of each population and geographic subdivision of Pinus armandii at cpDNA and mt DNA loci.
(DOC)
Chlorotype distribution in each population of Pinus armandii and the other white pines in this study.
(DOC)
Mitotype distribution in each population of Pinus armandii and the other white pines in this study.
(DOC)