Abstract
As skin cancer is one of the most costly health issues in many countries, particularly in Australia, the possibility that vitamin D compounds might contribute to prevention of this disease is becoming increasingly more attractive to researchers and health communities. In this article, important epidemiologic, mechanistic and experimental data supporting the chemopreventive potential of several vitamin D-related compounds are explored. Evidence of photoprotection by the active hormone, 1α,25dihydroxyvitamin D3, as well as a derivative of an over-irradiation product, lumisterol, a fluorinated analog and bufalin, a potential vitamin D-like compound, are provided. The aim of this article is to understand how vitamin D compounds contribute to UV adaptation and potentially, skin cancer prevention.
Keywords: 1α,25-dihydroxyvitaminD3; DNA damage; bufalin; photocarcinogenesis; photoimmune suppression; photoprotection; skin cancer prevention; vitamin D compounds; vitamin D photoproducts
Introduction
UVB irradiation from sunlight is the primary source for vitamin D synthesis in the epidermis. However UV can also cause skin cancer when excessive radiation is received. The same UVB that produces vitamin D also causes DNA and other damage that eventually results in skin cancers for large numbers of people. There is level 1 evidence from meta-analyses of randomized, controlled trials that adequate vitamin D status reduces parathyroid hormone levels, falls, fractures and overall mortality.1 There is also increasing evidence for a range of other health benefits of adequate vitamin D status.1,2 Considering the increase in skin cancer incidence worldwide, with up to approximately 450,000 new cases diagnosed per year in Australia alone, as reported by the Australian Institute for Health and Welfare,3 it is vital to find a balance between vitamin D sufficiency and preventing the hazardous effects of UV that lead to skin cancers and photoaging.
There is compelling evidence that because vitamin D and its metabolites are made in skin, the damage from UV exposure is less than it would be otherwise.4,5 Vitamin D compounds that do not cause hypercalcemia, are not photolabile but also protect skin cells from the hazardous effect of UV are promising alternatives to 1α,25dihydroxyvitamin D3 (1,25(OH)2D3) for therapeutic applications. The focus of this review is on the photoprotective actions and mechanisms of a number of natural and synthetic vitamin D compounds and vitamin D-like compounds, which might be used for protection from the hazardous effects of UV and for skin cancer prevention.
Structure of Vitamin D
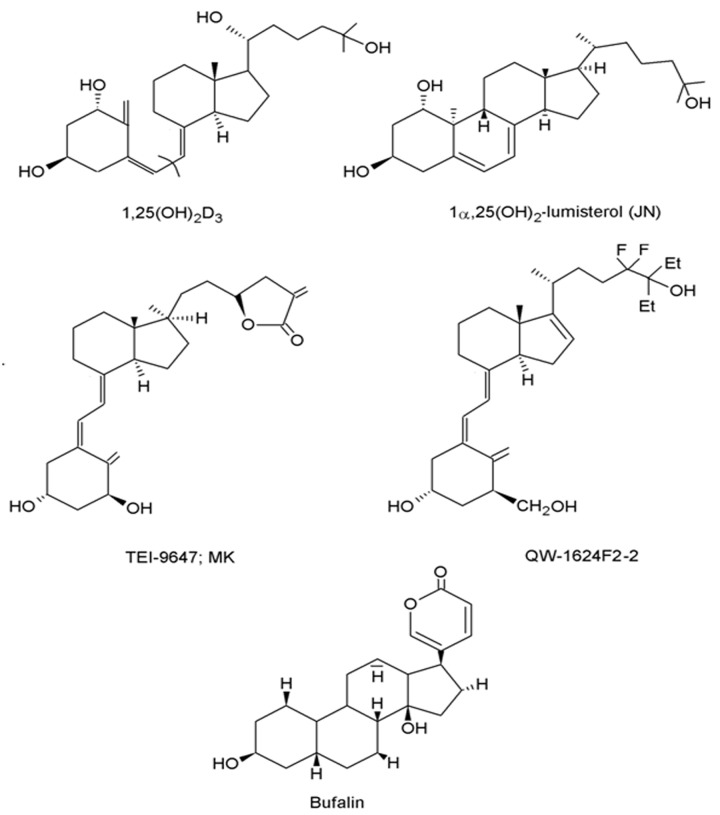
Technically speaking, vitamin D3 is not a true vitamin but a seco-steroid, synthesized in skin.6 The molecular structure of the vitamin D3 compound family, including the active hormone 1,25(OH)2D3, is chemically related to that of classical steroid hormones through a steroid hormone specific cyclopentanoperhydrophenanthrene 4 ring carbon skeleton.7 However, unlike its steroidal relatives, the 9-10 carbon-carbon bond of ring B is broken, making vitamin D3 a seco-steroid (Fig. 1).
Figure 1. The chemical structures of the various D compounds.
In comparison with their classical steroid counterparts, molecules of the vitamin D3 superfamily are particularly conformationally flexible, due to three major structural regions of their carbon skeleton. First, simple rotation of the 8-carbon side chain about the 5 carbon-carbon single bonds allows for a plethora of differing molecular shapes. Additionally, as a result of the 9-10 carbon-carbon breakage, the cyclohexane-like A-ring is liberated from the B-ring and undergoes rapid interconversion between chair-chair conformers. The mobility of the A-ring results in orientation of the 1α-hydroxyl and 3α-hydroxyl to move between axial and equatorial planes. Finally, the seco B-ring rotates freely around its 6-7 single carbon bond, producing 6-s-cis and 6-s-trans variates of conformation, which may be locked or flexible.7,8 It is the conformational flexibility of the vitamin D backbone, which allows for the generation of a vast number of ligand shapes that are able to generate different biological responses through the vitamin D receptor (VDR).
Photobiology and Metabolism of Vitamin D
Vitamin D is produced in skin by the photochemical conversion of 7-dehydrocholesterol in epidermis to pre-vitamin D. The energy for this reaction is provided by UV B radiation (UVB, 290–315 nm) which is the only part of the solar UV radiation (UVR, 290–400 nm) that can split the B-ring of the precursor. Thermal conversion at body temperature forms the stable form, vitamin D.9 Continued exposure of pre-vitamin D or vitamin D to UVB results in the formation of the so called “over-irradiation products” including lumisterol, tachysterol and suprasterols.9 Vitamin D made in skin of animals is cholecalciferol or vitamin D3, while vitamin D made from the plant precursor, ergosterol is vitamin D2 or ergocalciferol. The metabolism and actions of D2 or D3 compounds are fairly, though not entirely, similar.1 Subsequent to cutaneous production or intestinal absorption from food or supplements, vitamin D is transported in the circulation by serum vitamin D binding protein to the hepatocytes of the liver where the first hydroxylation occurs at the 25 position. As a result of this, 25(OH)D, the major circulating form of vitamin D is synthesized. This compound has a 15–50 day half-life and is widely used as the biological marker for vitamin D status in humans. This metabolite has little biological activity and needs to be hydroxylated again, in the proximal convoluted tubule cells of the kidney at the 1α-position, in order to yield the hormonally active metabolite, 1,25(OH)2D3 or calcitriol, which enters the blood.21,25(OH)2D3 is also able to be locally produced in many tissues including skin, which expresses both 25-hydroxylase and 1α-hydroxylase (CYP27B1) activity.10,11 Local production of the hormone probably restricts its effects to those immediate areas.
Transduction Pathways
1,25(OH)2D3 is the predominant structural ligand of the vitamin D endocrine system. It exerts its biological effects via two well-known pathways; a genomic pathway mediated by the well-known nuclear vitamin D receptor (VDR), and a non-genomic/rapid response pathway via a receptor that has not yet been clearly characterized.7,12-15
Genomic Pathway
A nuclear receptor of the large steroid receptor family, the VDR is found in nucleated cells in most tissues of the human body including the intestine, kidney, bone, skin, parathyroid gland, pancreas, pituitary and cells of the immune and reproductive systems, among others.16,17 The VDR is made up of six primary domains each designated a different functionality, specifically, a variable domain, a DNA binding domain, a hinge, a ligand binding domain and a transcriptional activation domain.16
The biological responses of 1,25(OH)2D3 are mediated by the classic genomic pathway through stereospecific ligand binding to the nuclear vitamin D receptor (VDR). The lipophilic 1,25(OH)2D3 molecule passes through the lipid bilayer of the plasma membrane and binds to the hydrophobic pocket in the ligand binding domain of the VDR. Upon activation, the VDR heterodimerizes with a retinoid X receptor (RXR) forming a VDR-RXR complex. Zinc fingers of the DNA binding domain recognize the VDR-RXR complex, allowing docking with vitamin D response elements (VDREs) in the DNA sequences of vitamin D target genes. These may be in the promoter region of target genes or at distant enhancer or repressor sites. Subsequent recruitment of protein co-modulators assists interaction with the general transcription apparatus wherein gene transcription is promoted or repressed.18,19
Non-Genomic Pathway
The non genomic pathway activated by 1,25(OH)2D3 generates a biological response within seconds to minutes by triggering a number of intracellular signaling pathways.7,16,18 These include the opening of chloride and calcium channels, mitogen activated protein kinases (MAPKs), protein kinase C, phosphatidylinositol 3-kinase (PI3K), phospholipase C and subsequent G-protein-coupled second messenger systems such as cyclic adenosine monophosphate (cAMP). Activated messenger systems may cross talk with the nucleus to contribute to gene transcription.16
The location of a binding protein/receptor through which 1,25(OH)2D3 exerts its non-genomic effects is not yet well established. There is evidence that this is the classic vitamin D receptor, which is located in lipid rafts in the cell membrane.12,20 There is also evidence for a rapid response binding protein for 1,25(OH)2D3, first identified in the basolateral membrane of chick epithelium, involved in rapid calcium absorption from the duodenum.13 Subsequent purification of the protein led to its definitive identification as the 66 kDa membrane-associated rapid response steroid binding (MARRS) protein, identical to ERp57/PDIA3.21
In the last decade, it has been proposed that the rapid actions of 1,25(OH)2D3 may occur via a putative alternative ligand binding pocket (AP) within the ligand binding domain of the VDR.22 Through molecular modeling, the natural 6 sec-cis locked analog of 1,25(OH)2D3, 1α,25 dihydroxylumisterol3 (JN) (Fig. 1), which has weak genomic activity but equivalent potency to 1,25(OH)2D3 in non-genomic signaling, was shown to dock with the classic VDR through a pocket other than the genomic pocket (GP) to elicit cellular responses. The flexible 1,25(OH)2D3 molecule was found to have a high affinity for both pockets, with differing modes of binding. It is thought that vitamin D sterol binding at the VDR occurs kinetically through the AP, while in a thermodynamic manner at the GP, and thus subsequent responses are highly dependent on the conformation and orientation of the ligand present.15,22 The AP prefers the planar 6-s-cis locked non-genomic agonist vitamin D sterols, such as JN, while the bowl shaped GP binds 6-s-trans conformed isomers of 1,25(OH)2D3. The conformationally flexible 1,25(OH)2D3 molecule can take up both structures, thus making it the most potent agonist for both genomic and non-genomic biological responses. The AP forms complexes with an array of 1,25(OH)2D3 molecule conformations, while the GP is more restricted with its binding affinity.15
Evidence for a bi-functional VDR comes from a study showing 1,25(OH)2D3 induced rapid chloride fluxes in wild type mouse osteoblasts, while mouse osteoblasts lacking a functional VDR showed no response.14 The VDR-KO mice used in the aforementioned study were previously reported to exhibit phenotypic defects similar those seen in human vitamin D-resistant rickets type II, such as alopecia, growth retardation, impaired bone formation and rickets, among many others.23 Importantly, VDR-KO mice are more susceptible to photocarcinogenesis as well as chemical skin carcinogenesis.24-26
Skin Carcinogenesis
UVR is classified as a class one carcinogen by the World Health Organisation.27 Carcinogenesis is a multistep process which requires initiation, promotion and progression28 and UVR is known to be both an initiator and a promoter. UVR is known to be responsible for more than 90% of all skin cancers in humans including non-melanoma skin cancers and malignant melanoma, through well characterized mechanisms including inflammation,29 DNA damage,30-32 mutagenesis33 and immune suppression,34 all of which are believed to be involved in combination or sequentially leading to photocarcinogenesis.
DNA damage
UV-induced DNA lesions, such as cyclobutane pyrimidine dimers (CPD) and 6–4 photoproducts35 are thought to be the initiating events to photocarcinogenesis. UV radiation also causes other types of DNA damage through production of reactive oxygen species (ROS) and nitric oxide (NO), products which are known to be not only mutagenic but also carcinogenic on their own.36-38 UV-induced DNA damage is known to be repaired at various rates through nucleotide excision repair or base excision repair. CPDs are known to take many hours for nucleotide excision repair, with different rates of repair in different species.39,40 Xeroderma pigmentosum is an autosomal recessive disorder with dysfunctional nucleotide excision repair and sufferers are known to have a very high incidence of skin cancer.41
p53
UVR induces the upregulation and accumulation of the tumor suppressor protein p53 in the nucleus, a process first described by Maltzman.42-45 Lane proposed p53 to be a DNA guardian since it has been shown to cause growth arrest, which could allow enough time for adequate DNA repair to take place.46 p53 is also believed to cause apoptosis to remove cells with severe and unrepairable DNA damage. This was evidenced by a markedly reduced number of sunburn cells after UVR in an inactivated p53 transgenic mouse model.47 Inactivating mutations in p53 as a result of UVR therefore would prevent G1 arrest and the initiation of apoptosis, and therefore would lead to continued division of mutated cells and subsequent carcinogenesis.
Immunosuppression
Lewis Thomas proposed a new concept of immune surveillance in 1959 for the first time.48 Since then, many studies have built on this proposal and confirmed that UV radiation causes immune suppression in skin.49,50 Immunosuppression was suspected to contribute to carcinogenesis when observations were made showing increased cancer development, including skin cancers, in immunocompromised patients.51 UV- induced immunosuppression is thought to be mediated through suppressor T cells and antigen presenting cells (APCs) or Langerhans cells.52 UV is known to reduce contact and delayed type hypersensitivity, which would mean decreased skin immune surveillance.53,54 Applegate and Kripke et al.55 also suggested that UV-induced immunosuppression may be triggered or worsened by DNA damage proposing that UV-induced DNA damage and photoimmunosuppression play significant roles together in photocarcinogenesis.
Oxidative stress
Finally, UV-induced oxidative stress is known to contribute to photocarcinogenesis. Oxidative stress is believed to be caused mainly by UVA rather than UVB,54 and when combined with the damaging effects of UVB, may have a very significant role in photocarcinogenesis.56,57 The most frequent product from oxidative stress to the DNA guanine base is 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG).37 8-oxodG production was shown to be proportional to the degree of UV exposure58 and hence believed to be caused by UVR. 8-oxodG is repaired through base excision repair (BER) and OGG1 has been identified as the important enzyme for the repair process.54 Kunisada et al. showed OGG1 knockout mice had significantly increased incidence of basal cell carcinoma,36 which supports the importance of ROS in photocarcinogenesis.
Notch signaling pathway
Notch signaling pathways are responsible for cell proliferation, migration, differentiation and apoptosis59 and maintain homeostasis of epidermis. Aberrant notch signaling is implicated in carcinogenesis.60 Although the exact mechanism of this is not fully understood, it is believed to have two contrasting sides where it may act either as a tumor promoter or a tumor suppressor depending on the cell type and tissue context, level of expression and potential contemporary cross talk with other signaling systems.61 It is believed upregulation in Notch signaling is associated with melanoma while downregulation was observed in non-melanoma skin cancers,60 except for basal cell carcinoma (BCC) where nodular or superficial subtypes showed overexpression of Notch-1 protein.60
Analogs of 1,25(OH)2D3 and a Vitamin D-Like Compound
Previous studies have identified the photoprotective properties of 1,25(OH)2D3, the active derivative of vitamin D. 1,25(OH)2D3 has been shown to enhance survival of skin cells following exposure to UV radiation, by reducing the level of damage to DNA and thus UV-induced apoptosis.62 Further, 1,25(OH)2D3 has been shown to reduce post-irradiation edema and inflammation in mouse skin, as well as photocarcinogenesis.62 These findings suggest that 1,25(OH)2D3 might be beneficial as an addition to sunscreens or even as an after-sun topical therapy, to boost skin protection against the harmful effects of UV radiation. However, 1,25(OH)2D3 is chemically unstable in the presence of light. Further, it is an extremely expensive compound to synthesize and potentially causes hypercalcemia when large areas of skin are covered.63 Analogs of 1,25(OH)2D3 are currently being studied for their potential commercial use in after-sun lotions; however, they are also expensive to produce.
1α,25-dihydroxylumisterol3 (JN)
1α,25(OH)2-lumisterol3 (JN) is a low-calcemic 6 sec-cis-locked analog (Fig. 1), which has been shown to be a full agonist of the non-genomic pathway that can only weakly bind to the VDR.64 This compound is potentially a metabolite of the over-irradiation product, lumisterol.65,66 JN was shown to generate non-genomic effects in pancreatic β cells and endothelial cells.67,68
1α-hydroxymethyl-16-ene-24,24-difluoro-25-hydroxy-26,27-bis-homovitaminD3 (QW; QW-1624F2–2)
1α-hydroxymethyl-16-ene-24,24-difluoro-25-hydroxy-26,27-bis-homovitaminD3 (QW; QW-1624F2–2) has some transcriptional activity and is approximately 80–100 times less calciuric than 1,25(OH)2D3.69,70 This hybrid analog does not cause cachexia in animals71 and has been shown to be non-genotoxic71 (Fig. 1). QW has been demonstrated to have anti-proliferative and pro-differentiating activity and proved effective in the inhibition of skin tumor formation and latency in a model of chemical-induced skin tumorigenesis.72
Bufalin
Toad venom isolated from various Bufo species has been used in the preparation of Ch’an Su and Senso, traditional Chinese medicines, for centuries.73 Extraction and characterization of this medicinal remedy identified bufalin from the family Bufadienolide as the most active component. Bufalin exhibits cardiotonic and local anesthetic properties via its action on the α subunit of Na+, K+-ATPase, inhibiting the movement of Na+ and K+ across the cell membrane.73 Subsequent intracellular accumulation of Na+ modulates the Na+/ Ca2+ exchanger. This causes a build-up of Ca2+ ions within the cell, leading to enhanced cardiomyocyte contraction, preventing heart failure.74
Previous studies of bufalin have revealed it to have some effects in common with 1,25(OH)2D3 in cancer cells.75 The ability of 1,25(OH)2D3 to differentiate human myeloid leukemia cell lines toward monocyte/macrophage-like cells was significantly enhanced in combination with low doses of bufalin.76 In these studies, bufalin did not appear to bind directly to the vitamin D receptor (VDR) but improved ligand-dependent activation of the VDR and upregulated VDR-mediated expression of endogenous target genes, such as CYP24.75,77 In the presence of 1,25(OH)2D3, bufalin maintained expression of the VDR in the nucleus of human leukemia cells, most likely through a reduction in nuclear VDR degradation or export.77 Bufalin was also shown to modulate the relationship and enhance the interaction of the VDR with its recruited coactivators, such as SCR-1.75 Bufalin treatment enhanced the association between the VDR and the corepressor N-CoR and abolished their dissociation in the presence of 1,25(OH)2D3. Bufalin-induced modification of cofactor complexes enhanced 1,25(OH)2D3-stimulated VDR transactivational activity.75 Since the only known receptor for bufalin is the Na+, K+-ATPase and the plasma membrane is impermeable to bufalin, it has been proposed that bufalin must functionally modify the VDR through a Na+, K+-ATPase dependent mechanism.78 Collectively, the findings suggest a new role for cardiotonic steroids, in particular bufalin, in the modulation of VDR function. More recently, it has been proposed that bufalin’s structural similarity to 6 sec-cis conformation of 1,25(OH)2D3 might allow it to bind to the alternative binding pocket of the VDR and activate the non-genomic pathway and thus confer protection to skin cells following UV-exposure (Fig. 1).
Effects of Vitamin D, Its Metabolites and Its Photoproducts on Skin Cells
Vitamin D and UV-induced DNA damage in skin cells
UV induces various types of DNA damage either photochemically or by UV activation of endogenous photoreceptors that create genotoxic free radicals that modify the DNA molecular structure. The most frequently occurring photolesion in sun-exposed human skin is the cyclobutane pyrimidine dimer (CPD),79,80 particularly thymine dimers, which are induced primarily by UVB, and also by UVA to a lesser extent.80-82 CPDs are produced by the dislocation of double bonds in two adjacent pyrimidines by UV absorption, resulting in a cyclobutane ring conformation linking the two nucleobases as a dimer.83,84
Our group and others have shown that 1,25(OH)2D3 reduces thymine dimers in irradiated skin cells in vitro85-90 and also in vivo in mouse62,87,89,91 and human skin.92,93 Thymine dimers are also reduced in irradiated skin cells in the presence of the low calcaemic rapid acting cis-locked non-genomic analogs, 1α,25(OH)2-lumisterol3 (JN) and 1α,25(OH)2-7-dehydrocholesterol (JM) in vitro85,87,94,95 and in mouse skin,62 and also by the transcriptionally active hybrid QW.85,95
Evidence that the vitamin D photoprotective effect on reductions in thymine dimer DNA damage is via the rapid non-genomic pathway is demonstrated with various vitamin D-like compounds. As noted above, studies by our group have shown that that the transcriptionally non-active 1α,25(OH)2-lumisterol3 protects against UV-induced thymine dimers.85,87,88 Of relevance to the mechanism of action of vitamin D compounds in photoprotection, the co-incubation of skin cells with 1,25(OH)2D3 and 25-dehydro-1α-hydroxyvitamin D3-26,23S-lactone (TEI-9647) (Fig. 1), an antagonist of the genomic action of 1,25(OH)2D3,96,97 did not alter the protective effects of 1,25(OH)2D3 on thymine dimers.87 In contrast, co-incubation with 1β,25-dihydroxyvitamin D3 (HL), an antagonist of the non-genomic pathway, abolished the photoprotective effect of 1,25(OH)2D3.87,88
As shown in Figure 2, bufalin caused a significant and dose-dependent reduction in thymine dimers in irradiated keratinocytes at nanomolar concentrations with 16 ± 1% to 18 ± 2% of nuclei carrying thymine dimers compared with 35 ± 3% of nuclei in vehicle-treated wells. This study shows for the first time that bufalin reduced UV-induced DNA damage. In Skh:hr1 mice given a single three minimal erythemal dose exposure of solar-simulated irradiation (as described in ref. 62) bufalin, at a concentration of 23 pmol/cm2 applied topically immediately after irradiation, reduced at 24 h, the proportion of keratinocytes exhibiting thymine dimers from 4.8 ± 2.5% in mice treated with vehicle to 2.3 ± 1.6%. This figure was not significantly different to the thymine dimers measured at 24 h in skin of mice treated topically with a similar concentration of 1,25(OH)2D3.
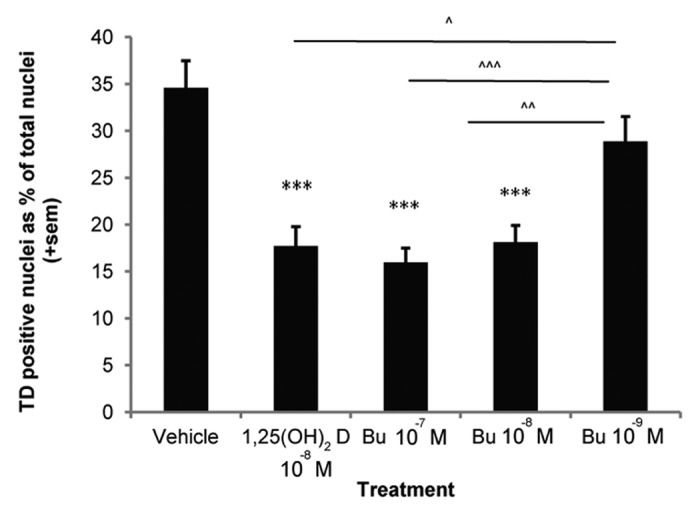
Figure 2. Photoprotection against thymine dimers by 1,25(OH)2D3 and Bufalin in human keratinocytes. DNA damage was assessed using a monoclonal antibody to thymine dimers,182 immunohistochemistry and image analysis as previously described89,94 at 3 h after UV irradiation, in human keratinocytes treated with vehicle, 1,25(OH)2D3 or three concentrations of bufalin and expressed as positively stained nuclei as a percent of total nuclei. The graph illustrates pooled data from a minimum of four independent experiments. *** denotes a significant difference compared with vehicle (***p < 0.001), ^, ^^, ^^^ denotes a significant difference compared with 10−9M bufalin (^p < 0.05, ^^p < 0.01, ^^^p < 0.001).
All the above studies were assessed by immunohistochemistry and image analysis. A reduction in thymine dimers (TD) by 1,25(OH)2D3 has also been demonstrated by an entirely different method in irradiated keratinocytes by digestion with the site specific DNA repair enzyme T4 endonuclease IV for CPDs in the comet assay.94,98
Two other major UV-induced photolesions found in human skin are 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG)84,99-101 and 8-nitroguanine.102-105 These are induced indirectly by UV through oxidation or nitrosylation of guanine by increased levels of reactive oxygen (ROS) and nitrogen (RNS) species respectively. Cellular levels of ROS are increased by UV activation of endogenous photoreceptors in the cell.106 Excess levels of nitric oxide (NO) accumulate by UV upregulation of nitric oxide synthases107-109 and UVA decomposition of NO stores.110-112 NO can act as a free radical on its own as well as react with ROS to form more powerful oxidating and nitrating intermediates, such as peroxynitrite.
Recently, we reported that 1,25(OH)2D3 reduces 8-oxodG in irradiated keratinocytes in culture, in mouse skin98 and in irradiated ex vivo human skin explants.93 This was demonstrated by reduced nuclear staining with a monoclonal antibody to 8-oxodG, immunohistochemistry and image analysis, and also by Comet assay incorporating digestion with the site specific DNA repair enzyme, human 8-oxoguanine DNA glycosylase (hOGG1) in cultured keratinocytes.93,98 Reduction of hOGG-sensitive sites by 1,25(OH)2D3 occurred within 30 min of UV irradiation.98 The 8-nitroguanosine lesion, considered a marker for inflammation and carcinogenesis,113 was also reduced by 1,25(OH)2D3. This was demonstrated by reduced nuclear staining with a monoclonal antibody to 8-nitroguanine in irradiated human ex vivo skin explants.93 The 8-nitroguanosine lesion is rapidly removed by depurination to leave an abasic site.114 We have also demonstrated that 1,25(OH)2D3 reduced abasic sites in irradiated keratinocytes by Comet assay with endonuclease IV digestion, which cleaves DNA at abasic sites.98
Vitamin D compounds reduce UV-induced cell death in skin cells
Cell death is suppressed in UV-irradiated skin cells treated with vitamin D compounds despite a further increase in expression of p53.62,89 This has been demonstrated in human skin cell cultures treated with 1,25(OH)2D385,89,91,115-119 and the vitamin D analogs calcipotriol,120 JN,85 JM85 and QW.88 Evidence suggests that this is facilitated by a non-genomic pathway as the non-genomic antagonist HL suppressed improved cell survival with 1,25(OH)2D3.85 Apoptotic cells (sunburn cells) in the epidermis of UV irradiated skin were also reduced by 1,25(OH)2D3 when applied systemically in mice,121 and topically in mice62,88,89 and human subjects.92 When applied to an in vivo model bufalin reduced the number of sunburn (apoptotic) cells as well as the aforementioned thymine dimers in the epidermis of Skh:hr1 hairless mice. At a concentration of 115 pmol/cm2, topical bufalin significantly (p < 0.01) reduced apoptotic keratinocytes (sunburn cells) in skin of Skh:hr1 mice irradiated with a single exposure of three minimal erythemal doses of solar-simulated UV radiation as described in ref. 62 compared with vehicle treatment and to a similar extent as 23 pmol/cm2 of 1,25(OH)2D3.
The mechanism for this improved cell survival in the presence of vitamin D compounds is almost certainly due to reduced DNA damage, and may be mediated by a reduced catalytic action of caspase 3 on poly(ADP-ribose) polymerase118 and through effects on sphingosine-1 phosphate, a breakdown product of membrane sphingolipid and second messenger in the apoptotic signaling pathway.116
Vitamin D compounds reduce nitric oxide derivatives
Nitric oxide (NO) levels are increased in irradiated skin112,122-126 as noted in the above section. High levels of NO cause nitrosylation of DNA repair enzymes,127 inhibit DNA repair128 and combine with excess levels of ROS to produce more toxic RNS intermediates that cause oxidative and nitrosative damage to DNA and proteins and peroxidation of lipids.102 The vitamin D hormone, 1,25(OH)2D3, has been shown to reduce nitrite89 and 3-nitrotyrosine,62 two stable end products of the nitric oxide pathway. Furthermore, similar to 1,25(OH)2D3, the inhibitors of nitric oxide synthase, aminoguanidine and L-N-monomethylarginine, reduced UV-induced nitrite and thymine dimers in skin cells,85,89 while a selective inhibitor of the inducible isoform (1400W) reduced both CPDs and 8-oxodG.98 It is likely that a reduction in post-UVR NO products is a mechanism whereby 1,25(OH)2D3 protects skin cells from oxidative and nitrative DNA damage, as well as enhancing DNA repair and improving cell survival. Whether a reduction in NO products contributes to a reduction in thymine dimers as well, is unclear, since in one study in human keratinocytes, the reduction in NO products with 1,25(OH)2D3 was dissociated from the reduction in thymine dimers.94
Mutagenesis
Most DNA damage is promutagenic, including that induced by RNS.100,129-131 If the lesions are not perfectly repaired before DNA replication, the damaged nucleobases are replaced with an adenine during transcription. This may alter the coding sequence.35 Mutations with sequence changes C to T transitions are associated with unrepaired CPDs, which are the predominant mutation due to the relatively high frequency and slow repair of CPD. These are now termed “solar signature mutations” rather than “UVB signature mutations,” as the contribution of UVA to mutations in unrepaired bipyrimidines is currently being debated (refer to refs. 80, 132–135). The hallmark of mutations from UV- induced 8-oxodG are G to T transversions, found predominantly in the basal layer of the epidermis where keratinocyte stem cells and melanocytes are situated. These mutations have implications for skin cancer development.100,136 The presence of mutations in the p53 tumor suppressor gene in many skin cancers, including premalignant precursors of SCC, correlate with both UVA- and UVB-induced lesions, providing evidence of their involvement in photocarcinogenesis.137-140 Although there is no direct data that 1,25(OH)2D3 reduces mutagenicity in UV-irradiated skin, the observation that photocarcinogenesis is substantially reduced by topical application of this compound suggests that this is indeed the case.62
D compounds and UV-induced immune suppression
The UVR component of sunlight causes immune suppression.141 These immunosuppressive effects suppress cell-mediated immune reactions that normally destroy developing skin tumors.142 The VDR is present in several immune cells, including monocytes, macrophages and activated T and B cells.143 In fact, it has been suggested that the UVB-induced immune suppression from sun exposure is mediated through vitamin D. Interestingly, 1,25(OH)2D3 was reported to increase mRNA expression of interleukin-10 (IL-10), an immune suppressive cytokine, and induce IL-10 secretion by mouse mast cells, thereby adding to the mast cell’s ability to suppress inflammation and skin pathology at sites of UV irradiation.144
The effects of vitamin D compounds on the immune system are complex and depend on many factors including concentration. Interestingly, high concentrations of vitamin D have been shown to be immunosuppressive,145 while vitamin D deficiency also causes immunosuppression.146 Studies by our group have shown that vitamin D compounds including 1,25(OH)2D3, JN and QW inhibit UV-induced immune suppression (contact hypersensitivity reaction to oxazolone) in hairless mice.62,87,88 Conversely, studies in human subjects showed that 1,25(OH)2D3 provided no protection against UV-induced suppression of a recall delayed-type hypersensitivity (DTH) (Mantoux) reaction and that topical 1,25(OH)2D3 (doses of 1 μg or higher) was immunosuppressive in a recall DTH model.92 This is supported by findings in another human study in which the vitamin D analog calcipotriene suppressed contact hypersensitivity responses to dinitrochlorobenzene by 64%, a level similar to that caused by solar simulated UV.147
Photoaging
Photoaging, also known as dermatoheliosis,148 is a premature aging process caused by excessive exposure to solar UV radiation determined by the degree of sun exposure and the level of skin pigmentation.149 This extrinsic aging process is distinguished from the natural chronological or intrinsic aging, in that it produces several complex and sequential cellular and molecular phenomena from photochemical reactions that are not seen in chronological aging including inflammation, oxidative stress, DNA lesions, mutation and immunosuppression caused by UV.
The acute effects of UVB on skin include the upregulation of TNFα by both keratinocytes and dermal fibroblasts within a couple of hours after UV exposure,150 which is an important step for development of the inflammatory cascade in skin. This is augmented by release of pro-inflammatory cytokines such as IL-1, and IL-6151 due to UV-induced DNA lesions such as CPDs and 6–4 photoproducts.152-154 These DNA lesions are also known to enhance expression of inducible nitric oxide synthetase (iNOS) that increases nitric oxide products.155 UVA is known to induce IL-10 in dermal macrophages and neutrophils which are, at least in part, responsible for UV-induced immunosuppression. Reeve and Tyrrell also reported the induction of IL-12 by UVA, which they proposed would counteract the UVB- induced immunosuppression through the hemoxygenase 1 pathway.156 UV exposure is also known to deplete cellular antioxidants such as glutathione,157 which increases reactive oxygen species (ROS) and reactive nitrogen species (RNS), which adds to the inflammatory process through peroxidation of membrane lipids and production of prostaglandins E2.158
The signs of photoaging include wrinkles, reduced elasticity, dyspigmentation, telangiectasia and the development of benign or malignant tumors.149 Wrinkles and reduced elasticity are produced, in part, through activation protein 1 (AP-1) transcription factor, which is known to be a critical mediator of photoaging and also involved in the overexpression of matrix metalloproteinases (MMPs) that in turn cause both the reduction of dermal collage and inhibition of collagen synthesis.159 Interestingly, MMPs are also known to be responsible for collagen breakdown during invasion of various malignancies.160
The mechanism of UV-induced melanogenesis is unclear. There is a constant basal melanogenesis determined by genetic makeup, and this is increased with exposure to UV.161 Since UV is responsible both for sunburn and tanning, it is believed the mechanism may be similar. Certainly, pigmentary disorders such as solar lentigo are commonly seen in photoaged skin, and it was postulated they were caused by UV-induced DNA mutations of keratinocytes and melanocytes with subsequently increased melanogenesis and transfer.162 The evidence of this is seen in patients suffering from xeroderma pigmentosum with excessive development of severe dyspigmentation and skin tumors due to impaired nucleotide excision repair, which is important for repair of UV-induced cyclobutane pyrimidine dimers and other photoproducts.41 Melanogenesis was once thought to be the negative feedback mechanism to control vitamin D synthesis to prevent hypercalcemia, but this is now unlikely and it has been proposed that the “over-irradiation” derivatives of the vitamin D pathway from excessive UVR reduce vitamin D synthesis in skin.163
To prevent photoaging, the most important and obvious strategy is to minimize UV exposure through lifestyle changes and using effective UV filters to prevent penetration of UVR into skin. Further to this, cellular and molecular protection to minimize some of the molecular events described may be attempted. In particular, prevention of UV-induced ROS has been suggested to be an important aspect in photoaging prevention, although these claims may be difficult to substantiate since there still are many challenges in accurately assessing the antioxidant activities required to provide cellular protection.164 Endogenous, naturally occurring cellular protective anti-oxidants such as superoxide dismutase, catalase, glutathione peroxidase and glutathione reductase are known to be depleted after UV exposure in animals165 as well as in photoaged human skin.166 There is some evidence that topical and systemic administration of antioxidants such as vitamin E, vitamin C, polyphenols and carotenoids provide some photoprotection in human skin.167-171 Several plant based polyphenolic compounds such as green tea, grape seed and pomegranate showed some protection in vitro, and it has been suggested that they may reduce photoaging and photocarcinogenesis.172 Topical all-trans-retinoic acids showed more convincing evidence of photo-aging protection through inhibition of UV-induced inflammation mediated by AP-1 and NFkB transcription factors.173,174 It does this through reduction in UV-induced c-jun both by reducing the accumulation and also stimulating the breakdown of c-jun through ubiquitin-proteasome degradation.173,174
There is some evidence that suggests vitamin D compounds may also contribute to reductions in photoaging. As noted earlier, vitamin D metabolites and other photoproducts reduce several types of UV-induced DNA damage including thymine dimers, 8-oxodG and 8-nitroguanosine.62,85,87,89,90,92,93,175 They also reduce NO products (8-nitrotyrosine and nitrite) and augment p53 expression,89 thereby probably reducing inflammation and improving DNA repair. Both 1,25(OH)2D3, and an analog, 1,24(OH)D2, reduced TNFα in human macrophages176 and also caused upregulation of IkappaBalpha levels by increased mRNA stability thereby reducing nuclear translocation of NFkB and downgrading its activity. We have also reported that 1,25(OH)2D3 reduced expression of the inflammatory marker IL-6 in mouse skin (Fig. 3),177 similar to the reduction in IL-6 production by irradiated keratinocytes treated with this compound in vitro.118 Treatment of mice with topical 1,25(OH)2D3 also reduced post-UV irradiation skin edema (Fig. 4).62
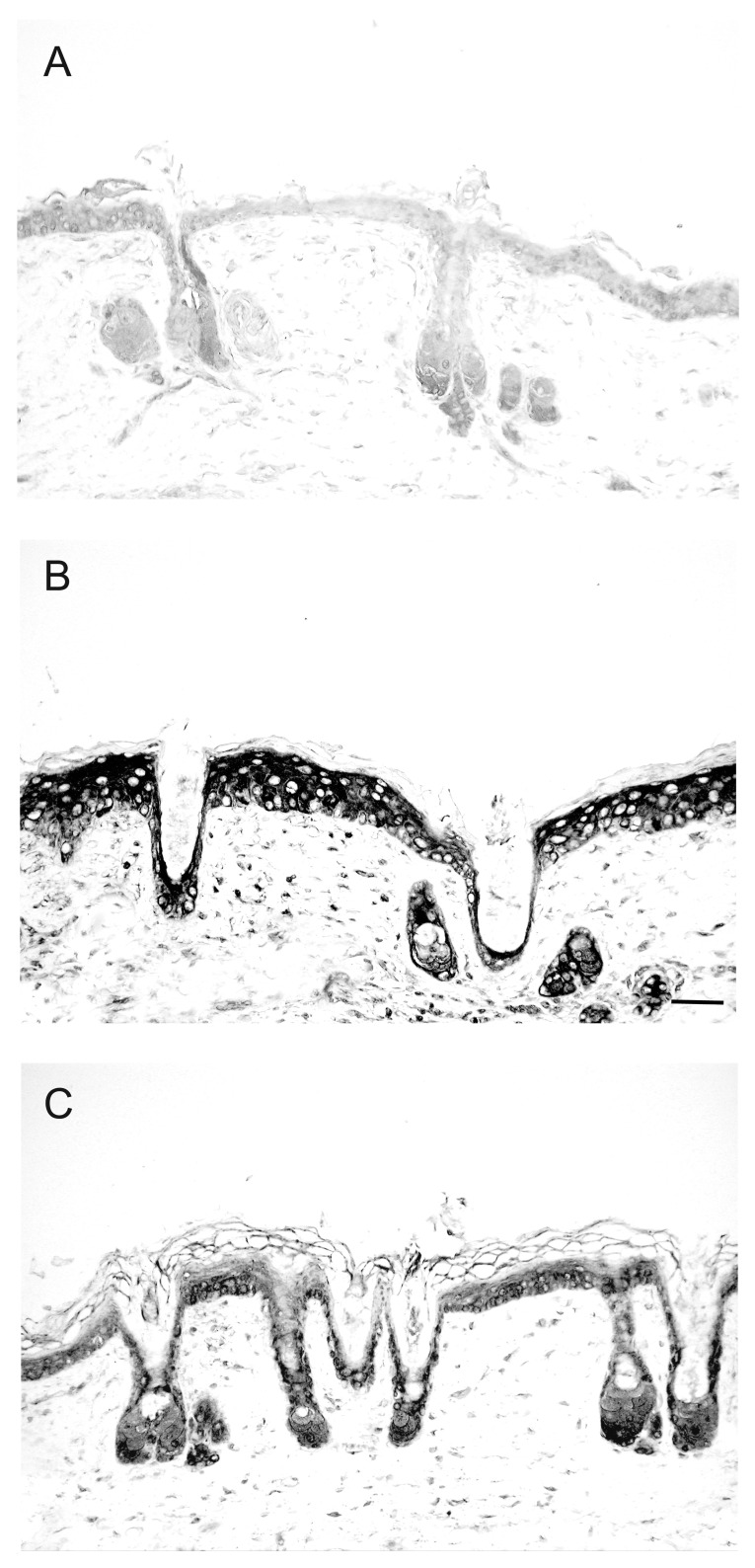
Figure 3. Reduction of UV-induced IL-6 expression by topical application of 1,25(OH)2D3 in mouse skin. Immunohistochemical detection of IL-6 in Skh:hr1 hairless mice skin was with a monoclonal antibody to IL-6 and a biotinylated secondary rabbit anti-goat IgG. Figures are representative dorsal skin sections (A) non-irradiated skin or (B) after solar simulated radiation followed by 48-h treatment with vehicle or (C) after solar simulated radiation followed by 48-h treatment with 1,25D (22.8 pmol/cm2). Reprinted from The Journal of Steroid Biochemistry and Molecular Biology. R.S. Mason, V.B. Sequeira, K.M. Dixon, C. Gordon-Thomson, K. Pobre, A. Dilley, M.T. Mizwicki, A.W. Norman, D. Feldman, G.M. Halliday, V.E. Reeve (2010).
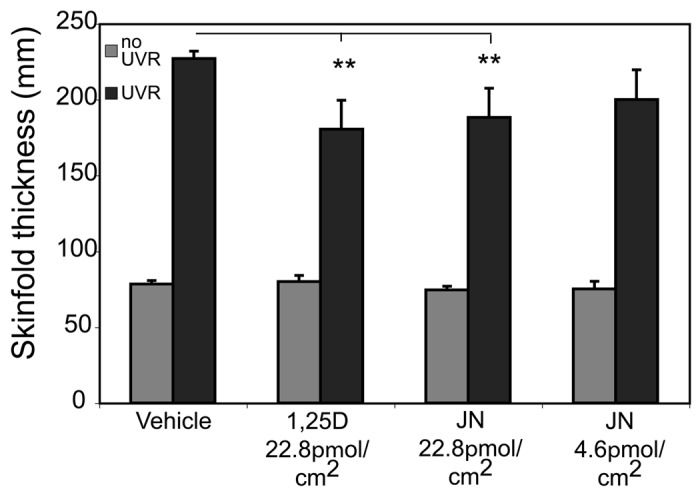
Figure 4. 1,25(OH)2D3 and JN protect against UV-induced edema. 1,25(OH)2D3 and JN reduce UV-induced edema in Skh:hr1 mouse skin. Mice were exposed to 1 × 3 MEDs (minimal erythemal doses) of solar-simulated UVR (3.98 kJ/m2 UVB and 63.8 kJ/m2 UVA) and were treated topically on the UV-irradiated dorsal surface immediately after UVR exposure with 100 mL of vehicle only, 1,25(OH)2D3, or JN. Edema was measured as dorsal skin-fold thickness in the mice 48 h after UVR exposure. Significantly different from vehicle-treated UV-irradiated mice. **p < 0.01; n = 5. Reproduced from Cancer Prevention Research. K.M. Dixon, A.W. Norman, V.B. Sequeira, R. Mohan, M.S. Rybchyn, V.E. Reeve, G.M. Halliday, R.S. Mason (2011).
1,25(OH)2D3 was found to have regulatory effects on AP-1, and MMPs via the VDR178 and a recent study suggested that 1,25(OH)2D3 attenuated TNFα induced MMP3, hence probably also reducing UV-induced collagen degradation in skin.179
In summary, vitamin D and photoproducts are likely to provide protection against photoaging by protecting keratinocytes and fibroblasts from UV-induced DNA damage, oxidative stress and excessive nitric oxide products and also protecting against dermal collagen breakdown through MMP regulation.
Vitamin D system and photocarcinogenesis
Vitamin D and its photoproducts provide photoprotection in several types of UV-induced DNA damage, as described earlier, including CPD,62,85,87,89,90,92,93,177 8-oxodG93,98 and 8-nitroguanosine.93,98 The further increase in p53 with 1,25(OH)2D3, which is known to promote DNA repair and thus reduce mutagenesis, is also thought to be an important mechanism of photoprotection by 1,25(OH)2D3.89 Skin cancers induced by UV or chemically were significantly increased in VDR knockout mice.24-26 Interestingly, the absence of a vitamin D receptor also blocked the ability of 1,25(OH)2D3 to reduce UV-induced thymine dimers in human skin fibroblasts.90 VDR is also implicated in the DNA repair process where VDR−/− mice showed significantly higher CPD levels when subjected to UVB, which failed to reduce over time compared with the wild-type mice.24 We also reported the key role of the alternate receptor ERp57/MARRS/PDIA3 in 1,25(OH)2D3-mediated photoprotection. Blockade of this protein by a neutralizing antibody or downregulation with siRNA, abolished the reduction in post-UV thymine dimers in skin cells treated with 1,25(OH)2D3.90 In this model the VDR and ERp57 co-immunoprecipitated in non-nuclear cell extracts.90
It has now been demonstrated that treatment with 1,25(OH)2D3 or the derivative of the over-irradiation product lumisterol, JN, reduced photocarcinogenesis in mice62 (Fig. 5). The low calcemic analog, QW, also protected against UV-induced cell death, DNA damage and immunosuppression,85,87 but at a comparable dose to 1,25(OH)2D3, did not confer protection in a model of chronic UV-induced skin carcinogenesis in hairless mice.180 It is possible that this analog might prove effective at higher doses. The non-steroid compound bufalin, also protected against UV-induced thymine dimers in vitro and in vivo, but has not yet been tested in a photocarcinogenesis model. Bufalin is more likely to be stable in the presence of light than vitamin D compounds, as it does not feature the broken B-ring seen in the molecular structure of 1,25(OH)2D3. Provided that bufalin can mimic the photoprotective properties of other low-calcemic analogs being trialled, it could provide a cheap and efficient way to increase the level of protection against UV-induced skin damage.
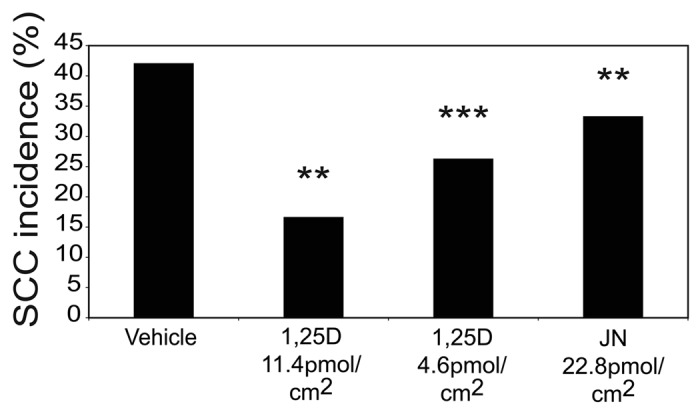
Figure 5. 1,25(OH)2D3 and JN protect against chronic UV-induced squamous cell carcinomas (SCC). Groups of 20 mice were exposed to a suberythemal dose of UVR on 5 d/wk for 10 wk. The solar-simulated UVR source was identical to that used in previous studies,88 as described earlier for acute exposures, but the daily dose was lowered to mimic chronic human sunlight exposure without burning. The daily dose provided 0.658 kJ/m2 UVB and 20.30 kJ/m2 UVA, which is approximately one MED (minimal erythemal dose). Incidence of SCC was reduced by 1,25(OH)2D3 and JN at 40-wk time point. SCC incidence was calculated at each weekly time point as the percentage of mice in each group bearing at least 1 SCC. The Mantel-Haenszel log-rank test was performed to determine the difference in risk of developing an SCC between treatment groups and vehicle control. There was a significantly reduced risk of SCC development in both the 1,25(OH)2D3 and JN treatment groups compared with vehicle. The asterisks refer to the incidence risk assessed by the Mantel–Haenszel log-rank test over the whole time course. Significantly different from vehicle. ***p < 0.001; **p < 0.01; n = 20. Reproduced from Cancer Prevention Research. K.M. Dixon, A.W. Norman, V.B. Sequeira, R. Mohan, M.S. Rybchyn, V.E. Reeve, G.M. Halliday, R.S. Mason (2011).
Summary and Perspectives
It has been noted with some curiosity that while vitamin D receptor knockout mice are more susceptible to photocarcinogenesis, mice with a knockout of the 1α-hydroxylase enzyme and so unable to make 1,25(OH)2D3 are not.181 Given that a range of vitamin D compounds, and even the non-steroidal compound bufalin, appear to have some protective properties against UV-damage, it is likely that further metabolites of vitamin D, or even other over-irradiation products, will be found to contribute to endogenous photoprotection by the vitamin D system. The production of many vitamin D compounds in skin takes several hours, so it is likely that this system mostly protects against the next exposure to UV, in a similar manner to increased pigmentation and increased epidermal thickness, both well-known adaptive responses to UV. By applying vitamin D or vitamin D like compounds during or even immediately after UV exposure, it seems possible to enhance endogenous protection of skin from the various changes that lead to photocarcinogenesis. The search is now on to find relatively cheap and stable vitamin D-like compounds to add to sunscreens and possibly even after sun lotions to reduce the risk of UV damage.
Acknowledgments
Funding to support this work was granted by the National Health and Medical Research Council (NHMRC) and the Australian Research Council (ARC).
Disclosure of Potential Conflicts of Interest
This work was funded in part by an Australian Research Council-Linkage Grant with commercial partner Ultraceuticals Pty Ltd.
Footnotes
Previously published online: www.landesbioscience.com/journals/dermatoendocrinology/article/23939
References
- 1.Nowson CA, McGrath JJ, Ebeling PR, Haikerwal A, Daly RM, Sanders KM, et al. Working Group of Australian and New Zealand Bone and Mineral Society, Endocrine Society of Australia and Osteoporosis Australia Vitamin D and health in adults in Australia and New Zealand: a position statement. Med J Aust. 2012;196:686–7. doi: 10.5694/mja11.10301. [DOI] [PubMed] [Google Scholar]
- 2.Holick MF. Vitamin D: evolutionary, physiological and health perspectives. Curr Drug Targets. 2011;12:4–18. doi: 10.2174/138945011793591635. [DOI] [PubMed] [Google Scholar]
- 3.AIHW. Non-melanoma skin cancer: general practice consultations, hospitalisation and mortality. Australian Institute for Health and Welfare 2008: http://australia.gov.au/directories/australia/aihw.
- 4.Mason RS, Dixon KM, Sequeira VB, Gordon-Thomson C. Sunlight protection by vitamin D compounds. In: Feldman D, Pike JW, Adams JS, eds. Vitamin D. 3rd edition. San Diego: Elsevier, 2011: 1943-53. [Google Scholar]
- 5.Mason RS, Reichrath J. Sunlight vitamin d and skin cancer. Anticancer Agents Med Chem. 2013;13:83–97. doi: 10.2174/187152013804487272. [DOI] [PubMed] [Google Scholar]
- 6.Malloy PJ, Pike JW, Feldman D. The vitamin D receptor and the syndrome of hereditary 1,25-dihydroxyvitamin D-resistant rickets. Endocr Rev. 1999;20:156–88. doi: 10.1210/er.20.2.156. [DOI] [PubMed] [Google Scholar]
- 7.Norman AW, Song XD, Zanello L, Bula C, Okamura WH. Rapid and genomic biological responses are mediated by different shapes of the agonist steroid hormone, 1alpha,25(OH)2vitamin D3. Steroids. 1999;64:120–8. doi: 10.1016/S0039-128X(98)00091-9. [DOI] [PubMed] [Google Scholar]
- 8.Norman AW, Henry HL, Bishop JE, Song XD, Bula C, Okamura WH. Different shapes of the steroid hormone 1alpha,25(OH)(2)-vitamin D(3) act as agonists for two different receptors in the vitamin D endocrine system to mediate genomic and rapid responses. Steroids. 2001;66:147–58. doi: 10.1016/S0039-128X(00)00165-3. [DOI] [PubMed] [Google Scholar]
- 9.Holick MF, MacLaughlin JA, Clark MB, Holick SA, Potts JT, Jr., Anderson RR, et al. Photosynthesis of previtamin D3 in human skin and the physiologic consequences. Science. 1980;210:203–5. doi: 10.1126/science.6251551. [DOI] [PubMed] [Google Scholar]
- 10.Bikle DD, Nemanic MK, Whitney JO, Elias PW. Neonatal human foreskin keratinocytes produce 1,25-dihydroxyvitamin D3. Biochemistry. 1986;25:1545–8. doi: 10.1021/bi00355a013. [DOI] [PubMed] [Google Scholar]
- 11.Lehmann B, Genehr T, Knuschke P, Pietzsch J, Meurer M. UVB-induced conversion of 7-dehydrocholesterol to 1alpha,25-dihydroxyvitamin D3 in an in vitro human skin equivalent model. J Invest Dermatol. 2001;117:1179–85. doi: 10.1046/j.0022-202x.2001.01538.x. [DOI] [PubMed] [Google Scholar]
- 12.Sequeira VB, Rybchyn MS, Tongkao-On W, Gordon-Thomson C, Malloy PJ, Nemere I, et al. The role of the vitamin D receptor and ERp57 in photoprotection by 1α,25-dihydroxyvitamin D3. Mol Endocrinol. 2012;26:574–82. doi: 10.1210/me.2011-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nemere I, Dormanen MC, Hammond MW, Okamura WH, Norman AW. Identification of a specific binding protein for 1 alpha,25-dihydroxyvitamin D3 in basal-lateral membranes of chick intestinal epithelium and relationship to transcaltachia. J Biol Chem. 1994;269:23750–6. [PubMed] [Google Scholar]
- 14.Zanello LP, Norman AW. Electrical responses to 1alpha,25(OH)2-Vitamin D3 and their physiological significance in osteoblasts. Steroids. 2004;69:561–5. doi: 10.1016/j.steroids.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Mizwicki MT, Norman AW. The vitamin D sterol-vitamin D receptor ensemble model offers unique insights into both genomic and rapid-response signaling. Sci Signal. 2009;2:re4. doi: 10.1126/scisignal.275re4. [DOI] [PubMed] [Google Scholar]
- 16.Norman AW. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr. 2008;88:491S–9S. doi: 10.1093/ajcn/88.2.491S. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Zhu J, DeLuca HF. Where is the vitamin D receptor? Arch Biochem Biophys. 2012;523:123–33. doi: 10.1016/j.abb.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 18.Haussler MR, Jurutka PW, Mizwicki M, Norman AW. Vitamin D receptor (VDR)-mediated actions of 1α,25(OH)₂vitamin D₃: genomic and non-genomic mechanisms. Best Pract Res Clin Endocrinol Metab. 2011;25:543–59. doi: 10.1016/j.beem.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 19.Zella LA, Meyer MB, Nerenz RD, Lee SM, Martowicz ML, Pike JW. Multifunctional enhancers regulate mouse and human vitamin D receptor gene transcription. Mol Endocrinol. 2010;24:128–47. doi: 10.1210/me.2009-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huhtakangas JA, Olivera CJ, Bishop JE, Zanello LP, Norman AW. The vitamin D receptor is present in caveolae-enriched plasma membranes and binds 1 alpha,25(OH)2-vitamin D3 in vivo and in vitro. Mol Endocrinol. 2004;18:2660–71. doi: 10.1210/me.2004-0116. [DOI] [PubMed] [Google Scholar]
- 21.Nemere I, Ray R, McManus W. Immunochemical studies on the putative plasmalemmal receptor for 1, 25(OH)(2)D(3). I. Chick intestine. Am J Physiol Endocrinol Metab. 2000;278:E1104–14. doi: 10.1152/ajpendo.2000.278.6.E1104. [DOI] [PubMed] [Google Scholar]
- 22.Mizwicki MT, Keidel D, Bula CM, Bishop JE, Zanello LP, Wurtz JM, et al. Identification of an alternative ligand-binding pocket in the nuclear vitamin D receptor and its functional importance in 1alpha,25(OH)2-vitamin D3 signaling. Proc Natl Acad Sci U S A. 2004;101:12876–81. doi: 10.1073/pnas.0403606101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshizawa T, Handa Y, Uematsu Y, Takeda S, Sekine K, Yoshihara Y, et al. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat Genet. 1997;16:391–6. doi: 10.1038/ng0897-391. [DOI] [PubMed] [Google Scholar]
- 24.Teichert AE, Elalieh H, Elias PM, Welsh J, Bikle DD. Overexpression of hedgehog signaling is associated with epidermal tumor formation in vitamin D receptor-null mice. J Invest Dermatol. 2011;131:2289–97. doi: 10.1038/jid.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ellison TI, Smith MK, Gilliam AC, MacDonald PN. Inactivation of the vitamin D receptor enhances susceptibility of murine skin to UV-induced tumorigenesis. J Invest Dermatol. 2008;128:2508–17. doi: 10.1038/jid.2008.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quigley DA, To MD, Pérez-Losada J, Pelorosso FG, Mao JH, Nagase H, et al. Genetic architecture of mouse skin inflammation and tumour susceptibility. Nature. 2009;458:505–8. doi: 10.1038/nature07683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Twombly R. New carcinogen list includes estrogen, UV radiation. J Natl Cancer Inst. 2003;95:185–6. doi: 10.1093/jnci/95.3.185. [DOI] [PubMed] [Google Scholar]
- 28.Weinstein IB. Disorders in cell circuitry during multistage carcinogenesis: the role of homeostasis. Carcinogenesis. 2000;21:857–64. doi: 10.1093/carcin/21.5.857. [DOI] [PubMed] [Google Scholar]
- 29.Hruza LL, Pentland AP. Mechanisms of UV-induced inflammation. J Invest Dermatol. 1993;100:35S–41S. doi: 10.1038/jid.1993.21. [DOI] [PubMed] [Google Scholar]
- 30.Douki T, Court M, Sauvaigo S, Odin F, Cadet J. Formation of the main UV-induced thymine dimeric lesions within isolated and cellular DNA as measured by high performance liquid chromatography-tandem mass spectrometry. J Biol Chem. 2000;275:11678–85. doi: 10.1074/jbc.275.16.11678. [DOI] [PubMed] [Google Scholar]
- 31.Cooke MS, Podmore ID, Mistry N, Evans MD, Herbert KE, Griffiths HR, et al. Immunochemical detection of UV-induced DNA damage and repair. J Immunol Methods. 2003;280:125–33. doi: 10.1016/S0022-1759(03)00269-2. [DOI] [PubMed] [Google Scholar]
- 32.Mouret S, Charveron M, Favier A, Cadet J, Douki T. Differential repair of UVB-induced cyclobutane pyrimidine dimers in cultured human skin cells and whole human skin. DNA Repair (Amst) 2008;7:704–12. doi: 10.1016/j.dnarep.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Ikehata H, Ono T. Significance of CpG methylation for solar UV-induced mutagenesis and carcinogenesis in skin. Photochem Photobiol. 2007;83:196–204. doi: 10.1562/2006-02-28-IR-822. [DOI] [PubMed] [Google Scholar]
- 34.Halliday GM. Inflammation, gene mutation and photoimmunosuppression in response to UVR-induced oxidative damage contributes to photocarcinogenesis. Mutat Res. 2005;571:107–20. doi: 10.1016/j.mrfmmm.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 35.Matsumura Y, Ananthaswamy HN. Toxic effects of ultraviolet radiation on the skin. Toxicol Appl Pharmacol. 2004;195:298–308. doi: 10.1016/j.taap.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 36.Kunisada M, Sakumi K, Tominaga Y, Budiyanto A, Ueda M, Ichihashi M, et al. 8-Oxoguanine formation induced by chronic UVB exposure makes Ogg1 knockout mice susceptible to skin carcinogenesis. Cancer Res. 2005;65:6006–10. doi: 10.1158/0008-5472.CAN-05-0724. [DOI] [PubMed] [Google Scholar]
- 37.Huang XX, Scolyer RA, Abubakar A, Halliday GM. Human 8-oxoguanine-DNA glycosylase-1 is downregulated in human basal cell carcinoma. Mol Genet Metab. 2012;106:127–30. doi: 10.1016/j.ymgme.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 38.Niles JC, Wishnok JS, Tannenbaum SR. Peroxynitrite-induced oxidation and nitration products of guanine and 8-oxoguanine: structures and mechanisms of product formation. Nitric Oxide. 2006;14:109–21. doi: 10.1016/j.niox.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Sutherland BM, Harber LC, Kochevar IE. Pyrimidine dimer formation and repair in human skin. Cancer Res. 1980;40:3181–5. [PubMed] [Google Scholar]
- 40.Young AR, Chadwick CA, Harrison GI, Hawk JL, Nikaido O, Potten CS. The in situ repair kinetics of epidermal thymine dimers and 6-4 photoproducts in human skin types I and II. J Invest Dermatol. 1996;106:1307–13. doi: 10.1111/1523-1747.ep12349031. [DOI] [PubMed] [Google Scholar]
- 41.Ichihashi M, Fujiwara Y. Clinical and photobiological characteristics of Japanese xeroderma pigmentosum variant. Br J Dermatol. 1981;105:1–12. doi: 10.1111/j.1365-2133.1981.tb00876.x. [DOI] [PubMed] [Google Scholar]
- 42.Healy E, Reynolds NJ, Smith MD, Campbell C, Farr PM, Rees JL. Dissociation of erythema and p53 protein expression in human skin following UVB irradiation, and induction of p53 protein and mRNA following application of skin irritants. J Invest Dermatol. 1994;103:493–9. doi: 10.1111/1523-1747.ep12395637. [DOI] [PubMed] [Google Scholar]
- 43.Hall PA, McKee PH, Menage HD, Dover R, Lane DP. High levels of p53 protein in UV-irradiated normal human skin. Oncogene. 1993;8:203–7. [PubMed] [Google Scholar]
- 44.Campbell C, Quinn AG, Angus B, Farr PM, Rees JL. Wavelength specific patterns of p53 induction in human skin following exposure to UV radiation. Cancer Res. 1993;53:2697–9. [PubMed] [Google Scholar]
- 45.Maltzman W, Czyzyk L. UV irradiation stimulates levels of p53 cellular tumor antigen in nontransformed mouse cells. Mol Cell Biol. 1984;4:1689–94. doi: 10.1128/mcb.4.9.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lane DP. Cancer. p53, guardian of the genome. Nature. 1992;358:15–6. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- 47.Ziegler A, Jonason AS, Leffell DJ, Simon JA, Sharma HW, Kimmelman J, et al. Sunburn and p53 in the onset of skin cancer. Nature. 1994;372:773–6. doi: 10.1038/372773a0. [DOI] [PubMed] [Google Scholar]
- 48.Thomas L. Discussion. In: Lawrence HS, ed. Cellular and humoral aspects of the hypersensitive states. New York: Hoeber-Harper, 1959:529-33. [Google Scholar]
- 49.Kripke ML, Cox PA, Alas LG, Yarosh DB. Pyrimidine dimers in DNA initiate systemic immunosuppression in UV-irradiated mice. Proc Natl Acad Sci U S A. 1992;89:7516–20. doi: 10.1073/pnas.89.16.7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Daynes RA, Bernhard EJ, Gurish MF, Lynch DH. Experimental photoimmunology: immunologic ramifications of UV-induced carcinogenesis. J Invest Dermatol. 1981;77:77–85. doi: 10.1111/1523-1747.ep12479260. [DOI] [PubMed] [Google Scholar]
- 51.Kinlen LJ, Sheil AG, Peto J, Doll R. Collaborative United Kingdom-Australasian study of cancer in patients treated with immunosuppressive drugs. Br Med J. 1979;2:1461–6. doi: 10.1136/bmj.2.6203.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schwarz T. 25 years of UV-induced immunosuppression mediated by T cells-from disregarded T suppressor cells to highly respected regulatory T cells. Photochem Photobiol. 2008;84:10–8. doi: 10.1111/j.1751-1097.2007.00223.x. [DOI] [PubMed] [Google Scholar]
- 53.De Fabo EC, Noonan FP. Mechanism of immune suppression by ultraviolet irradiation in vivo. I. Evidence for the existence of a unique photoreceptor in skin and its role in photoimmunology. J Exp Med. 1983;158:84–98. doi: 10.1084/jem.158.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Halliday GM, Byrne SN, Damian DL. Ultraviolet A radiation: its role in immunosuppression and carcinogenesis. Semin Cutan Med Surg. 2011;30:214–21. doi: 10.1016/j.sder.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 55.Applegate LA, Ley RD, Alcalay J, Kripke ML. Identification of the molecular target for the suppression of contact hypersensitivity by ultraviolet radiation. J Exp Med. 1989;170:1117–31. doi: 10.1084/jem.170.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vile GF, Tyrrell RM. UVA radiation-induced oxidative damage to lipids and proteins in vitro and in human skin fibroblasts is dependent on iron and singlet oxygen. Free Radic Biol Med. 1995;18:721–30. doi: 10.1016/0891-5849(94)00192-M. [DOI] [PubMed] [Google Scholar]
- 57.Black H. The defensive role of antioxidants in skin carcinogenesis. In : Fuchs J, Parker L, eds. Oxidative Stress in Demartology. New York: Marcel Dekker, 1993: 243-69. [Google Scholar]
- 58.Floyd RA, West MS, Eneff KL, Hogsett WE, Tingey DT. Hydroxyl free radical mediated formation of 8-hydroxyguanine in isolated DNA. Arch Biochem Biophys. 1988;262:266–72. doi: 10.1016/0003-9861(88)90188-9. [DOI] [PubMed] [Google Scholar]
- 59.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–6. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 60.Panelos J, Massi D. Emerging role of Notch signaling in epidermal differentiation and skin cancer. Cancer Biol Ther. 2009;8:1986–93. doi: 10.4161/cbt.8.21.9921. [DOI] [PubMed] [Google Scholar]
- 61.Miele L, Golde T, Osborne B. Notch signaling in cancer. Curr Mol Med. 2006;6:905–18. doi: 10.2174/156652406779010830. [DOI] [PubMed] [Google Scholar]
- 62.Dixon KM, Norman AW, Sequeira VB, Mohan R, Rybchyn MS, Reeve VE, et al. 1α,25(OH)₂-vitamin D and a nongenomic vitamin D analogue inhibit ultraviolet radiation-induced skin carcinogenesis. Cancer Prev Res (Phila) 2011;4:1485–94. doi: 10.1158/1940-6207.CAPR-11-0165. [DOI] [PubMed] [Google Scholar]
- 63.Langner A, Verjans H, Stapór V, Mol M, Fraczykowska M. Topical calcitriol in the treatment of chronic plaque psoriasis: a double-blind study. Br J Dermatol. 1993;128:566–71. doi: 10.1111/j.1365-2133.1993.tb00237.x. [DOI] [PubMed] [Google Scholar]
- 64.Norman AW, Okamura WH, Hammond MW, Bishop JE, Dormanen MC, Bouillon R, et al. Comparison of 6-s-cis- and 6-s-trans-locked analogs of 1alpha,25-dihydroxyvitamin D3 indicates that the 6-s-cis conformation is preferred for rapid nongenomic biological responses and that neither 6-s-cis- nor 6-s-trans-locked analogs are preferred for genomic biological responses. Mol Endocrinol. 1997;11:1518–31. doi: 10.1210/me.11.10.1518. [DOI] [PubMed] [Google Scholar]
- 65.Janjetovic Z, Zmijewski MA, Tuckey RC, DeLeon DA, Nguyen MN, Pfeffer LM, et al. 20-Hydroxycholecalciferol, product of vitamin D3 hydroxylation by P450scc, decreases NF-kappaB activity by increasing IkappaB alpha levels in human keratinocytes. PLoS One. 2009;4:e5988. doi: 10.1371/journal.pone.0005988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Slominski AT, Zmijewski MA, Semak I, Sweatman T, Janjetovic Z, Li W, et al. Sequential metabolism of 7-dehydrocholesterol to steroidal 5,7-dienes in adrenal glands and its biological implication in the skin. PLoS One. 2009;4:e4309. doi: 10.1371/journal.pone.0004309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kajikawa M, Ishida H, Fujimoto S, Mukai E, Nishimura M, Fujita J, et al. An insulinotropic effect of vitamin D analog with increasing intracellular Ca2+ concentration in pancreatic beta-cells through nongenomic signal transduction. Endocrinology. 1999;140:4706–12. doi: 10.1210/en.140.10.4706. [DOI] [PubMed] [Google Scholar]
- 68.Rebsamen MC, Sun JX, Norman AW, Liao JK. 1alpha,25-dihydroxyvitamin D3 induces vascular smooth muscle cell migration via activation of phosphatidylinositol 3-kinase. Circ Res. 2002;91:17–24. doi: 10.1161/01.RES.0000025269.60668.0F. [DOI] [PubMed] [Google Scholar]
- 69.Posner GH, Jeon HB, Sarjeant A, Riccio ES, Doppalapudi RS, Kapetanovic IM, et al. Low-calcemic, efficacious, 1alpha,25-dihydroxyvitamin D3 analog QW-1624F2-2: calcemic dose-response determination, preclinical genotoxicity testing, and revision of A-ring stereochemistry. Steroids. 2004;69:757–62. doi: 10.1016/j.steroids.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 70.Reddy CD, Patti R, Guttapalli A, Maris JM, Yanamandra N, Rachamallu A, et al. Anticancer effects of the novel 1alpha, 25-dihydroxyvitamin D3 hybrid analog QW1624F2-2 in human neuroblastoma. J Cell Biochem. 2006;97:198–206. doi: 10.1002/jcb.20629. [DOI] [PubMed] [Google Scholar]
- 71.Posner GH, Lee JK, Wang Q, Peleg S, Burke M, Brem H, et al. Noncalcemic, antiproliferative, transcriptionally active, 24-fluorinated hybrid analogues of the hormone 1alpha, 25-dihydroxyvitamin D3. Synthesis and preliminary biological evaluation. J Med Chem. 1998;41:3008–14. doi: 10.1021/jm980031t. [DOI] [PubMed] [Google Scholar]
- 72.Kensler TW, Dolan PM, Gange SJ, Lee JK, Wang Q, Posner GH. Conceptually new deltanoids (vitamin D analogs) inhibit multistage skin tumorigenesis. Carcinogenesis. 2000;21:1341–5. doi: 10.1093/carcin/21.7.1341. [DOI] [PubMed] [Google Scholar]
- 73.Krenn L, Kopp B. Bufadienolides from animal and plant sources. Phytochemistry. 1998;48:1–29. doi: 10.1016/S0031-9422(97)00426-3. [DOI] [PubMed] [Google Scholar]
- 74.Schwinger RHG, Bundgaard H, Müller-Ehmsen J, Kjeldsen K. The Na, K-ATPase in the failing human heart. Cardiovasc Res. 2003;57:913–20. doi: 10.1016/S0008-6363(02)00767-8. [DOI] [PubMed] [Google Scholar]
- 75.Nakano H, Matsunawa M, Yasui A, Adachi R, Kawana K, Shimomura I, et al. Enhancement of ligand-dependent vitamin D receptor transactivation by the cardiotonic steroid bufalin. Biochem Pharmacol. 2005;70:1479–86. doi: 10.1016/j.bcp.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 76.Zhang LS, Nakaya K, Yoshida T, Kuroiwa Y. Induction by bufalin of differentiation of human leukemia cells HL60, U937, and ML1 toward macrophage/monocyte-like cells and its potent synergistic effect on the differentiation of human leukemia cells in combination with other inducers. Cancer Res. 1992;52:4634–41. [PubMed] [Google Scholar]
- 77.Amano Y, Cho Y, Matsunawa M, Komiyama K, Makishima M. Increased nuclear expression and transactivation of vitamin D receptor by the cardiotonic steroid bufalin in human myeloid leukemia cells. J Steroid Biochem Mol Biol. 2009;114:144–51. doi: 10.1016/j.jsbmb.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 78.Numazawa S, Shinoki MA, Ito H, Yoshida T, Kuroiwa Y. Involvement of Na+,K(+)-ATPase inhibition in K562 cell differentiation induced by bufalin. J Cell Physiol. 1994;160:113–20. doi: 10.1002/jcp.1041600114. [DOI] [PubMed] [Google Scholar]
- 79.Cooke MS, Podmore ID, Mistry N, Evans MD, Herbert KE, Griffiths HR, et al. Immunochemical detection of UV-induced DNA damage and repair. J Immunol Methods. 2003;280:125–33. doi: 10.1016/S0022-1759(03)00269-2. [DOI] [PubMed] [Google Scholar]
- 80.Mouret S, Baudouin C, Charveron M, Favier A, Cadet J, Douki T. Cyclobutane pyrimidine dimers are predominant DNA lesions in whole human skin exposed to UVA radiation. Proc Natl Acad Sci U S A. 2006;103:13765–70. doi: 10.1073/pnas.0604213103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mouret S, Philippe C, Gracia-Chantegrel J, Douki T. Long UVA directly generate cyclobutane pyrimidine dimers in isolated and cellular DNA. Journal of Investigative Dermatology. 2009;129:S34. [Google Scholar]
- 82.Tewari A, Sarkany RP, Young AR. UVA1 induces cyclobutane pyrimidine dimers but not 6-4 photoproducts in human skin in vivo. J Invest Dermatol. 2012;132:394–400. doi: 10.1038/jid.2011.283. [DOI] [PubMed] [Google Scholar]
- 83.Ravanat JL, Douki T, Cadet J. Direct and indirect effects of UV radiation on DNA and its components. J Photochem Photobiol B. 2001;63:88–102. doi: 10.1016/S1011-1344(01)00206-8. [DOI] [PubMed] [Google Scholar]
- 84.Pattison DI, Davies MJ. Actions of ultraviolet light on cellular structures. EXS. 2006;(96):131–57. doi: 10.1007/3-7643-7378-4_6. [DOI] [PubMed] [Google Scholar]
- 85.Wong G, Gupta R, Dixon KM, Deo SS, Choong SM, Halliday GM, et al. 1,25-Dihydroxyvitamin D and three low-calcemic analogs decrease UV-induced DNA damage via the rapid response pathway. J Steroid Biochem Mol Biol. 2004;89-90:567–70. doi: 10.1016/j.jsbmb.2004.03.072. [DOI] [PubMed] [Google Scholar]
- 86.De Haes P, Garmyn M, Verstuyf A, De Clercq P, Vandewalle M, Degreef H, et al. 1,25-Dihydroxyvitamin D3 and analogues protect primary human keratinocytes against UVB-induced DNA damage. J Photochem Photobiol B. 2005;78:141–8. doi: 10.1016/j.jphotobiol.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 87.Dixon KM, Deo SS, Wong G, Slater M, Norman AW, Bishop JE, et al. Skin cancer prevention: a possible role of 1,25dihydroxyvitamin D3 and its analogs. J Steroid Biochem Mol Biol. 2005;97:137–43. doi: 10.1016/j.jsbmb.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 88.Dixon KM, Deo SS, Norman AW, Bishop JE, Halliday GM, Reeve VE, et al. In vivo relevance for photoprotection by the vitamin D rapid response pathway. J Steroid Biochem Mol Biol. 2007;103:451–6. doi: 10.1016/j.jsbmb.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 89.Gupta R, Dixon KM, Deo SS, Holliday CJ, Slater M, Halliday GM, et al. Photoprotection by 1,25 dihydroxyvitamin D3 is associated with an increase in p53 and a decrease in nitric oxide products. J Invest Dermatol. 2007;127:707–15. doi: 10.1038/sj.jid.5700597. [DOI] [PubMed] [Google Scholar]
- 90.Sequeira VB, Rybchyn MS, Tongkao-On W, Gordon-Thomson C, Malloy PJ, Nemere I, et al. The role of the vitamin D receptor and ERp57 in photoprotection by 1α,25-dihydroxyvitamin D3. Mol Endocrinol. 2012;26:574–82. doi: 10.1210/me.2011-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee J, Youn JI. The photoprotective effect of 1,25-dihydroxyvitamin D3 on ultraviolet light B-induced damage in keratinocyte and its mechanism of action. J Dermatol Sci. 1998;18:11–8. doi: 10.1016/S0923-1811(98)00015-2. [DOI] [PubMed] [Google Scholar]
- 92.Damian DL, Kim YJ, Dixon KM, Halliday GM, Javeri A, Mason RS. Topical calcitriol protects from UV-induced genetic damage but suppresses cutaneous immunity in humans. Exp Dermatol. 2010;19:e23–30. doi: 10.1111/j.1600-0625.2009.00955.x. [DOI] [PubMed] [Google Scholar]
- 93.Song EJ, Gordon-Thomson C, Cole L, Stern H, Halliday GM, Damian DL, et al. 1α,25-Dihydroxyvitamin D(3) reduces several types of UV-induced DNA damage and contributes to photoprotection. J Steroid Biochem Mol Biol. 2012 doi: 10.1016/j.jsbmb.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 94.Sequeira VB, Rybchyn MS, Gordon-Thomson C, Tongkao-On W, Mizwicki MT, Norman AW, et al. Opening of chloride channels by 1α,25-dihydroxyvitamin D(3) contributes to photoprotection against UVR-induced thymine dimers in keratinocytes. J Invest Dermatol. 2012;133:776–82. doi: 10.1038/jid.2012.343. [DOI] [PubMed] [Google Scholar]
- 95.Dixon KM, Sequeira VB, Deo SS, Mohan R, Posner GH, Mason RS. Differential photoprotective effects of 1,25-dihydroxyvitamin D3 and a low calcaemic deltanoid. Photochem Photobiol Sci. 2012;11:1825–30. doi: 10.1039/c2pp25208b. [DOI] [PubMed] [Google Scholar]
- 96.Bula CM, Bishop JE, Ishizuka S, Norman AW. 25-Dehydro-1alpha-hydroxyvitamin D3-26,23S-lactone antagonizes the nuclear vitamin D receptor by mediating a unique noncovalent conformational change. Mol Endocrinol. 2000;14:1788–96. doi: 10.1210/me.14.11.1788. [DOI] [PubMed] [Google Scholar]
- 97.Ishizuka S, Miura D, Ozono K, Saito M, Eguchi H, Chokki M, et al. (23S)- and (23R)-25-dehydro-1alpha-hydroxyvitamin D(3)-26,23-lactone function as antagonists of vitamin D receptor-mediated genomic actions of 1alpha,25-dihydroxyvitamin D(3) Steroids. 2001;66:227–37. doi: 10.1016/S0039-128X(00)00146-X. [DOI] [PubMed] [Google Scholar]
- 98.Gordon-Thomson C, Gupta R, Tongkao-on W, Ryan A, Halliday GM, Mason RS. 1α,25 dihydroxyvitamin D3 enhances cellular defences against UV-induced oxidative and other forms of DNA damage in skin. Photochem Photobiol Sci. 2012;11:1837–47. doi: 10.1039/c2pp25202c. [DOI] [PubMed] [Google Scholar]
- 99.Kvam E, Tyrrell RM. Induction of oxidative DNA base damage in human skin cells by UV and near visible radiation. Carcinogenesis. 1997;18:2379–84. doi: 10.1093/carcin/18.12.2379. [DOI] [PubMed] [Google Scholar]
- 100.Agar NS, Halliday GM, Barnetson RS, Ananthaswamy HN, Wheeler M, Jones AM. The basal layer in human squamous tumors harbors more UVA than UVB fingerprint mutations: a role for UVA in human skin carcinogenesis. Proc Natl Acad Sci U S A. 2004;101:4954–9. doi: 10.1073/pnas.0401141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Halliday GM. Inflammation, gene mutation and photoimmunosuppression in response to UVR-induced oxidative damage contributes to photocarcinogenesis. Mutat Res. 2005;571:107–20. doi: 10.1016/j.mrfmmm.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 102.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Burney S, Caulfield JL, Niles JC, Wishnok JS, Tannenbaum SR. The chemistry of DNA damage from nitric oxide and peroxynitrite. Mutat Res. 1999;424:37–49. doi: 10.1016/S0027-5107(99)00006-8. [DOI] [PubMed] [Google Scholar]
- 104.Niles JC, Wishnok JS, Tannenbaum SR. Peroxynitrite-induced oxidation and nitration products of guanine and 8-oxoguanine: structures and mechanisms of product formation. Nitric Oxide. 2006;14:109–21. doi: 10.1016/j.niox.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 105.Murata M, Thanan R, Ma N, Kawanishi S. Role of nitrative and oxidative DNA damage in inflammation-related carcinogenesis. J Biomed Biotechnol. 2012;2012:623019. doi: 10.1155/2012/623019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Epe B. DNA damage spectra induced by photosensitization. Photochem Photobiol Sci. 2012;11:98–106. doi: 10.1039/c1pp05190c. [DOI] [PubMed] [Google Scholar]
- 107.Deliconstantinos G, Villiotou V, Stavrides JC. Increase of particulate nitric oxide synthase activity and peroxynitrite synthesis in UVB-irradiated keratinocyte membranes. Biochem J. 1996;320:997–1003. doi: 10.1042/bj3200997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bruch-Gerharz D, Suschek C, Krischel V, Ruzicka T, Kolb-Bachofen V. Induction of nitric oxide synthase in endothelial cells: Different molecular mechanisms for UVA and UVB irradiation. J Invest Dermatol. 1998;110:649. [Google Scholar]
- 109.Cals-Grierson MM, Ormerod AD. Nitric oxide function in the skin. Nitric Oxide. 2004;10:179–93. doi: 10.1016/j.niox.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 110.Paunel AN, Dejam A, Thelen S, Kirsch M, Horstjann M, Gharini P, et al. Enzyme-independent nitric oxide formation during UVA challenge of human skin: characterization, molecular sources, and mechanisms. Free Radic Biol Med. 2005;38:606–15. doi: 10.1016/j.freeradbiomed.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 111.Mowbray M, McLintock S, Weerakoon R, Lomatschinsky N, Jones S, Rossi AG, et al. Enzyme-independent NO stores in human skin: quantification and influence of UV radiation. J Invest Dermatol. 2009;129:834–42. doi: 10.1038/jid.2008.296. [DOI] [PubMed] [Google Scholar]
- 112.Hess DT, Matsumoto A, Nudelman R, Stamler JS. S-nitrosylation: spectrum and specificity. Nat Cell Biol. 2001;3:E46–9. doi: 10.1038/35055152. [DOI] [PubMed] [Google Scholar]
- 113.Hiraku Y, Kawanishi S. Immunohistochemical analysis of 8-nitroguanine, a nitrative DNA lesion, in relation to inflammation-associated carcinogenesis. In: Kozlov S, ed. Inflammation and cancer: methods and Protocols. Humana Press, 2009:3-14. [DOI] [PubMed] [Google Scholar]
- 114.Ohshima H, Sawa T, Akaike T. 8-nitroguanine, a product of nitrative DNA damage caused by reactive nitrogen species: formation, occurrence, and implications in inflammation and carcinogenesis. Antioxid Redox Signal. 2006;8:1033–45. doi: 10.1089/ars.2006.8.1033. [DOI] [PubMed] [Google Scholar]
- 115.Mason R. Effect of vitamin D on melanocytes and its Role in melanogenesis. In: Kragballe K, ed. Vitamin D in cermatology. New York: Marcel Dekker, 2000:123-32. [Google Scholar]
- 116.Manggau M, Kim DS, Ruwisch L, Vogler R, Korting HC, Schäfer-Korting M, et al. 1Alpha,25-dihydroxyvitamin D3 protects human keratinocytes from apoptosis by the formation of sphingosine-1-phosphate. J Invest Dermatol. 2001;117:1241–9. doi: 10.1046/j.0022-202x.2001.01496.x. [DOI] [PubMed] [Google Scholar]
- 117.Mason RS, Holliday CJ, Gupta R. 1,25 dihydroxyvitamin D and photoprotection in skin cells. In: Tsambos D, Merk H, eds. Modern trends in skin pharmacology. Athens, Greece: Parissianos Medical Publications S.A. Athens, 2002:59-66. [Google Scholar]
- 118.De Haes P, Garmyn M, Degreef H, Vantieghem K, Bouillon R, Segaert S. 1,25-Dihydroxyvitamin D3 inhibits ultraviolet B-induced apoptosis, Jun kinase activation, and interleukin-6 production in primary human keratinocytes. J Cell Biochem. 2003;89:663–73. doi: 10.1002/jcb.10540. [DOI] [PubMed] [Google Scholar]
- 119.De Haes P, Garmyn M, Verstuyf A, De Clercq P, Vandewalle M, Vantieghem K, et al. Two 14-epi analogues of 1,25-dihydroxyvitamin D3 protect human keratinocytes against the effects of UVB. Arch Dermatol Res. 2004;295:527–34. doi: 10.1007/s00403-004-0451-x. [DOI] [PubMed] [Google Scholar]
- 120.Youn JI, Park BS, Chung JH, Lee JH. Photoprotective effect of calcipotriol upon skin photoreaction to UVA and UVB. Photodermatol Photoimmunol Photomed. 1997;13:109–14. doi: 10.1111/j.1600-0781.1997.tb00126.x. [DOI] [PubMed] [Google Scholar]
- 121.Hanada K, Sawamura D, Nakano H, Hashimoto I. Possible role of 1,25-dihydroxyvitamin D3-induced metallothionein in photoprotection against UVB injury in mouse skin and cultured rat keratinocytes. J Dermatol Sci. 1995;9:203–8. doi: 10.1016/0923-1811(94)00378-R. [DOI] [PubMed] [Google Scholar]
- 122.Deliconstantinos G, Villiotou V, Stravrides JC. Release by ultraviolet B (u.v.B) radiation of nitric oxide (NO) from human keratinocytes: a potential role for nitric oxide in erythema production. Br J Pharmacol. 1995;114:1257–65. doi: 10.1111/j.1476-5381.1995.tb13341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Paunel-Gorgulu A, Dejam A, Thelen S, Kirsch M, Horstjann M, Gharini P, et al. UVA induces immediate and enzyme-independent nitric oxide formation in healthy human skin leading to no-specific signalling. Eur J Cell Biol. 2005;84:37–8. [Google Scholar]
- 124.Mowbray M, McLintock S, Weerakoon R, Lomatschinsky N, Jones S, Rossi AG, et al. Enzyme-independent NO stores in human skin: quantification and influence of UV radiation. J Invest Dermatol. 2009;129:834–42. doi: 10.1038/jid.2008.296. [DOI] [PubMed] [Google Scholar]
- 125.Bruch-Gerharz D, Ruzicka T, Kolb-Bachofen V. Nitric oxide in human skin: current status and future prospects. J Invest Dermatol. 1998;110:1–7. doi: 10.1046/j.1523-1747.1998.00084.x. [DOI] [PubMed] [Google Scholar]
- 126.Cals-Grierson MM, Ormerod AD. Nitric oxide function in the skin. Nitric Oxide. 2004;10:179–93. doi: 10.1016/j.niox.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 127.Jaiswal M, LaRusso NF, Burgart LJ, Gores GJ. Inflammatory cytokines induce DNA damage and inhibit DNA repair in cholangiocarcinoma cells by a nitric oxide-dependent mechanism. Cancer Res. 2000;60:184–90. [PubMed] [Google Scholar]
- 128.Bau DT, Gurr JR, Jan KY. Nitric oxide is involved in arsenite inhibition of pyrimidine dimer excision. Carcinogenesis. 2001;22:709–16. doi: 10.1093/carcin/22.5.709. [DOI] [PubMed] [Google Scholar]
- 129.Ferguson LR. Chronic inflammation and mutagenesis. Mutat Res. 2010;690:3–11. doi: 10.1016/j.mrfmmm.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 130.Yang YC, Chou HY, Shen TL, Chang WJ, Tai PH, Li TK. Topoisomerase II-mediated DNA cleavage and mutagenesis activated by nitric oxide underlie the inflammation-associated tumorigenesis. Antioxid Redox Signal. 2013;18:1129–40. doi: 10.1089/ars.2012.4620. [DOI] [PubMed] [Google Scholar]
- 131.Tang CH, Wei W, Liu LM. Regulation of DNA repair by S-nitrosylation. Biochim Biophys Acta. 2012;1820:730–5. doi: 10.1016/j.bbagen.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.You YH, Pfeifer GP. Similarities in sunlight-induced mutational spectra of CpG-methylated transgenes and the p53 gene in skin cancer point to an important role of 5-methylcytosine residues in solar UV mutagenesis. J Mol Biol. 2001;305:389–99. doi: 10.1006/jmbi.2000.4322. [DOI] [PubMed] [Google Scholar]
- 133.Chen PZ, Hu PT, Xie D, Qin Y, Wang FD, Wang H. Meta-analysis of vitamin D, calcium and the prevention of breast cancer. Breast Cancer Res Treat. 2010;121:469–77. doi: 10.1007/s10549-009-0593-9. [DOI] [PubMed] [Google Scholar]
- 134.Pfeifer GP, Besaratinia A. UV wavelength-dependent DNA damage and human non-melanoma and melanoma skin cancer. Photochem Photobiol Sci. 2012;11:90–7. doi: 10.1039/c1pp05144j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tewari A, Grage MML, Harrison GI, Sarkany R, Young AR. UVA1 is skin deep: molecular and clinical implications. Photochem Photobiol Sci. 2013;12:95–103. doi: 10.1039/c2pp25323b. [DOI] [PubMed] [Google Scholar]
- 136.Yogianti F, Kunisada M, Ono R, Sakumi K, Nakabeppu Y, Nishigori C. Skin tumours induced by narrowband UVB have higher frequency of p53 mutations than tumours induced by broadband UVB independent of Ogg1 genotype. Mutagenesis. 2012;27:637–43. doi: 10.1093/mutage/ges029. [DOI] [PubMed] [Google Scholar]
- 137.Matsumura Y, Ananthaswamy HN. Molecular mechanisms of photocarcinogenesis. Front Biosci. 2002;7:d765–83. doi: 10.2741/matsumur. [DOI] [PubMed] [Google Scholar]
- 138.Nataraj AJ, Trent JC, 2nd, Ananthaswamy HN. p53 gene mutations and photocarcinogenesis. Photochem Photobiol. 1995;62:218–30. doi: 10.1111/j.1751-1097.1995.tb05262.x. [DOI] [PubMed] [Google Scholar]
- 139.Besaratinia A, Kim SI, Pfeifer GP. Rapid repair of UVA-induced oxidized purines and persistence of UVB-induced dipyrimidine lesions determine the mutagenicity of sunlight in mouse cells. FASEB J. 2008;22:2379–92. doi: 10.1096/fj.07-105437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Weihrauch M, Bader M, Lehnert G, Wittekind C, Tannapfel A, Wrbitzky R. Carcinogen-specific mutation pattern in the p53 tumour suppressor gene in UV radiation-induced basal cell carcinoma. Int Arch Occup Environ Health. 2002;75:272–6. doi: 10.1007/s00420-001-0307-z. [DOI] [PubMed] [Google Scholar]
- 141.Kripke ML, Fisher MS. Immunologic parameters of ultraviolet carcinogenesis. J Natl Cancer Inst. 1976;57:211–5. doi: 10.1093/jnci/57.1.211. [DOI] [PubMed] [Google Scholar]
- 142.Nghiem DX, Kazimi N, Mitchell DL, Vink AA, Ananthaswamy HN, Kripke ML, et al. Mechanisms underlying the suppression of established immune responses by ultraviolet radiation. J Invest Dermatol. 2002;119:600–8. doi: 10.1046/j.1523-1747.2002.01845.x. [DOI] [PubMed] [Google Scholar]
- 143.Matheu V, Bäck O, Mondoc E, Issazadeh-Navikas S, et al. Dual effects of vitamin D-induced alteration of TH1/TH2 cytokine expression: enhancing IgE production and decreasing airway eosinophilia in murine allergic airway disease. J Allergy Clin Immunol. 2003;112:585–92. doi: 10.1016/S0091-6749(03)01855-4. [see comment] [DOI] [PubMed] [Google Scholar]
- 144.Biggs L, Yu C, Fedoric B, Lopez AF, Galli SJ, Grimbaldeston MA. Evidence that vitamin D(3) promotes mast cell-dependent reduction of chronic UVB-induced skin pathology in mice. J Exp Med. 2010;207:455–63. doi: 10.1084/jem.20091725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yang S, Smith C, DeLuca HF. 1 alpha, 25-Dihydroxyvitamin D3 and 19-nor-1 alpha, 25-dihydroxyvitamin D2 suppress immunoglobulin production and thymic lymphocyte proliferation in vivo. Biochim Biophys Acta. 1993;1158:279–86. doi: 10.1016/0304-4165(93)90026-5. [DOI] [PubMed] [Google Scholar]
- 146.Yang S, Smith C, Prahl JM, Luo X, DeLuca HF. Vitamin D deficiency suppresses cell-mediated immunity in vivo. Arch Biochem Biophys. 1993;303:98–106. doi: 10.1006/abbi.1993.1260. [DOI] [PubMed] [Google Scholar]
- 147.Hanneman KK, Scull HM, Cooper KD, Baron ED. Effect of topical vitamin D analogue on in vivo contact sensitization. Arch Dermatol. 2006;142:1332–4. doi: 10.1001/archderm.142.10.1332. [DOI] [PubMed] [Google Scholar]
- 148.Gordon JR, Brieva JC. Images in clinical medicine. Unilateral dermatoheliosis. N Engl J Med. 2012;366:e25. doi: 10.1056/NEJMicm1104059. [DOI] [PubMed] [Google Scholar]
- 149.Fisher GJ, Kang S, Varani J, Bata-Csorgo Z, Wan Y, Datta S, et al. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 2002;138:1462–70. doi: 10.1001/archderm.138.11.1462. [DOI] [PubMed] [Google Scholar]
- 150.Bashir MM, Sharma MR, Werth VP. TNF-alpha production in the skin. Arch Dermatol Res. 2009;301:87–91. doi: 10.1007/s00403-008-0893-7. [DOI] [PubMed] [Google Scholar]
- 151.Kondo S, Sauder DN, Kono T, Galley KA, McKenzie RC. Differential modulation of interleukin-1 alpha (IL-1 alpha) and interleukin-1 beta (IL-1 beta) in human epidermal keratinocytes by UVB. Exp Dermatol. 1994;3:29–39. doi: 10.1111/j.1600-0625.1994.tb00263.x. [DOI] [PubMed] [Google Scholar]
- 152.Setlow RB, Carrier WL. Pyrimidine dimers in ultraviolet-irradiated DNA’s. J Mol Biol. 1966;17:237–54. doi: 10.1016/S0022-2836(66)80105-5. [DOI] [PubMed] [Google Scholar]
- 153.Mitchell DL. The relative cytotoxicity of (6-4) photoproducts and cyclobutane dimers in mammalian cells. Photochem Photobiol. 1988;48:51–7. doi: 10.1111/j.1751-1097.1988.tb02785.x. [DOI] [PubMed] [Google Scholar]
- 154.Mitchell DL, Nairn RS. The biology of the (6-4) photoproduct. Photochem Photobiol. 1989;49:805–19. doi: 10.1111/j.1751-1097.1989.tb05578.x. [DOI] [PubMed] [Google Scholar]
- 155.Rhodes LE, Belgi G, Parslew R, McLoughlin L, Clough GF, Friedmann PS. Ultraviolet-B-induced erythema is mediated by nitric oxide and prostaglandin E2 in combination. J Invest Dermatol. 2001;117:880–5. doi: 10.1046/j.0022-202x.2001.01514.x. [DOI] [PubMed] [Google Scholar]
- 156.Reeve VE, Tyrrell RM. Heme oxygenase induction mediates the photoimmunoprotective activity of UVA radiation in the mouse. Proc Natl Acad Sci U S A. 1999;96:9317–21. doi: 10.1073/pnas.96.16.9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wright D, Zampagni M, Evangelisti E, Conti S, D’Adamio G, Goti A, et al. Protective properties of novel S-acyl-glutathione thioesters against ultraviolet-induced oxidative stress. Photochem Photobiol. 2012 doi: 10.1111/j.1751-1097.2012.01231.x. In press. [DOI] [PubMed] [Google Scholar]
- 158.Wilgus TA, Koki AT, Zweifel BS, Kusewitt DF, Rubal PA, Oberyszyn TM. Inhibition of cutaneous ultraviolet light B-mediated inflammation and tumor formation with topical celecoxib treatment. Mol Carcinog. 2003;38:49–58. doi: 10.1002/mc.10141. [DOI] [PubMed] [Google Scholar]
- 159.Fisher GJ. The pathophysiology of photoaging of the skin. Cutis. 2005;75(Suppl):5–8, discussion 8-9. [PubMed] [Google Scholar]
- 160.Offersen BV, Knap MM, Horsman MR, Verheijen J, Hanemaaijer R, Overgaard J. Matrix metalloproteinase-9 measured in urine from bladder cancer patients is an independent prognostic marker of poor survival. Acta Oncol. 2010;49:1283–7. doi: 10.3109/0284186X.2010.509109. [DOI] [PubMed] [Google Scholar]
- 161.Parrish JA, Jaenicke KF, Anderson RR. Erythema and melanogenesis action spectra of normal human skin. Photochem Photobiol. 1982;36:187–91. doi: 10.1111/j.1751-1097.1982.tb04362.x. [DOI] [PubMed] [Google Scholar]
- 162.Ichihashi M, Ando H, Yoshida M, Niki Y, Matsui M. Photoaging of the skin. Anti-Aging Med. 2009;6:46–59. doi: 10.3793/jaam.6.46. [DOI] [Google Scholar]
- 163.Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79:362–71. doi: 10.1093/ajcn/79.3.362. [DOI] [PubMed] [Google Scholar]
- 164.Chen L, Wang S. From the bottle to the skin: challenges in evaluating antioxidants. Photodermatol. Photoimmunol Photomed. 2012;28:228–34. doi: 10.1111/j.1600-0781.2012.00674.x. [DOI] [PubMed] [Google Scholar]
- 165.Hashimoto Y, Ohkuma N, Iizuka H. Reduced superoxide dismutase activity in UVB-induced hyperproliferative pig epidermis. Arch Dermatol Res. 1991;283:317–20. doi: 10.1007/BF00376620. [DOI] [PubMed] [Google Scholar]
- 166.Rhie G, Shin MH, Seo JY, Choi WW, Cho KH, Kim KH, et al. Aging- and photoaging-dependent changes of enzymic and nonenzymic antioxidants in the epidermis and dermis of human skin in vivo. J Invest Dermatol. 2001;117:1212–7. doi: 10.1046/j.0022-202x.2001.01469.x. [DOI] [PubMed] [Google Scholar]
- 167.Eberlein-König B, Placzek M, Przybilla B. Protective effect against sunburn of combined systemic ascorbic acid (vitamin C) and d-alpha-tocopherol (vitamin E) J Am Acad Dermatol. 1998;38:45–8. doi: 10.1016/S0190-9622(98)70537-7. [DOI] [PubMed] [Google Scholar]
- 168.Murad H, Tabibian MP. The effect of an oral supplement containing glucosamine, amino acids, minerals, and antioxidants on cutaneous aging: a preliminary study. J Dermatolog Treat. 2001;12:47–51. doi: 10.1080/095466301750163590. [DOI] [PubMed] [Google Scholar]
- 169.Nusgens BV, Humbert P, Rougier A, Colige AC, Haftek M, Lambert CA, et al. Topically applied vitamin C enhances the mRNA level of collagens I and III, their processing enzymes and tissue inhibitor of matrix metalloproteinase 1 in the human dermis. J Invest Dermatol. 2001;116:853–9. doi: 10.1046/j.0022-202x.2001.01362.x. [DOI] [PubMed] [Google Scholar]
- 170.Humbert PG, Haftek M, Creidi P, Lapière C, Nusgens B, Richard A, et al. Topical ascorbic acid on photoaged skin. Clinical, topographical and ultrastructural evaluation: double-blind study vs. placebo. Exp Dermatol. 2003;12:237–44. doi: 10.1034/j.1600-0625.2003.00008.x. [DOI] [PubMed] [Google Scholar]
- 171.Yoshida E, Watanabe T, Takata J, Yamazaki A, Karube Y, Kobayashi S. Topical application of a novel, hydrophilic gamma-tocopherol derivative reduces photo-inflammation in mice skin. J Invest Dermatol. 2006;126:1633–40. doi: 10.1038/sj.jid.5700236. [DOI] [PubMed] [Google Scholar]
- 172.Hsu S. Green tea and the skin. J Am Acad Dermatol. 2005;52:1049–59. doi: 10.1016/j.jaad.2004.12.044. [DOI] [PubMed] [Google Scholar]
- 173.Fisher GJ, Voorhees JJ. Molecular mechanisms of photoaging and its prevention by retinoic acid: ultraviolet irradiation induces MAP kinase signal transduction cascades that induce Ap-1-regulated matrix metalloproteinases that degrade human skin in vivo. J Investig Dermatol Symp Proc. 1998;3:61–8. [PubMed] [Google Scholar]
- 174.Fisher GJ, Datta S, Wang Z, Li XY, Quan T, Chung JH, et al. c-Jun-dependent inhibition of cutaneous procollagen transcription following ultraviolet irradiation is reversed by all-trans retinoic acid. J Clin Invest. 2000;106:663–70. doi: 10.1172/JCI9362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mason RS, Sequeira VB, Dixon KM, Gordon-Thomson C, Pobre K, Dilley A, et al. Photoprotection by 1alpha,25-dihydroxyvitamin D and analogs: further studies on mechanisms and implications for UV-damage. J Steroid Biochem Mol Biol. 2010;121:164–8. doi: 10.1016/j.jsbmb.2010.03.082. [DOI] [PubMed] [Google Scholar]
- 176.Cohen-Lahav M, Shany S, Tobvin D, Chaimovitz C, Douvdevani A. Vitamin D decreases NFkappaB activity by increasing IkappaBalpha levels. Nephrol Dial Transplant. 2006;21:889–97. doi: 10.1093/ndt/gfi254. [DOI] [PubMed] [Google Scholar]
- 177.Mason RS, Sequeira VB, Dixon KM, Gordon-Thomson C, Pobre K, Dilley A, et al. Photoprotection by 1α,25-dihydroxyvitamin D and analogs: further studies on mechanisms and implications for UV-damage. J Steroid Biochem Mol Biol. 2010;121:164–8. doi: 10.1016/j.jsbmb.2010.03.082. [DOI] [PubMed] [Google Scholar]
- 178.Tetlow LC, Woolley DE. Expression of vitamin D receptors and matrix metalloproteinases in osteoarthritic cartilage and human articular chondrocytes in vitro. Osteoarthritis Cartilage. 2001;9:423–31. doi: 10.1053/joca.2000.0408. [DOI] [PubMed] [Google Scholar]
- 179.Bahar-Shany K, Ravid A, Koren R. Upregulation of MMP-9 production by TNFalpha in keratinocytes and its attenuation by vitamin D. J Cell Physiol. 2010;222:729–37. doi: 10.1002/jcp.22004. [DOI] [PubMed] [Google Scholar]
- 180.Dixon KM, Sequeira VB, Camp AJ, Mason RS. Vitamin D and skin cancer. In: Preedy VR, ed. Handbook of Diet, Nutrition and the Skin. Wageningen: Wageningen Academic Publishers, 2012: 395-411. [Google Scholar]
- 181.Bikle DD. The vitamin D receptor: a tumor suppressor in skin. Discov Med. 2011;11:7–17. [PMC free article] [PubMed] [Google Scholar]
- 182.Roza L, van der Wulp KJ, MacFarlane SJ, Lohman PH, Baan RA. Detection of cyclobutane thymine dimers in DNA of human cells with monoclonal antibodies raised against a thymine dimer-containing tetranucleotide. Photochem Photobiol. 1988;48:627–33. doi: 10.1111/j.1751-1097.1988.tb02873.x. [DOI] [PubMed] [Google Scholar]