Abstract
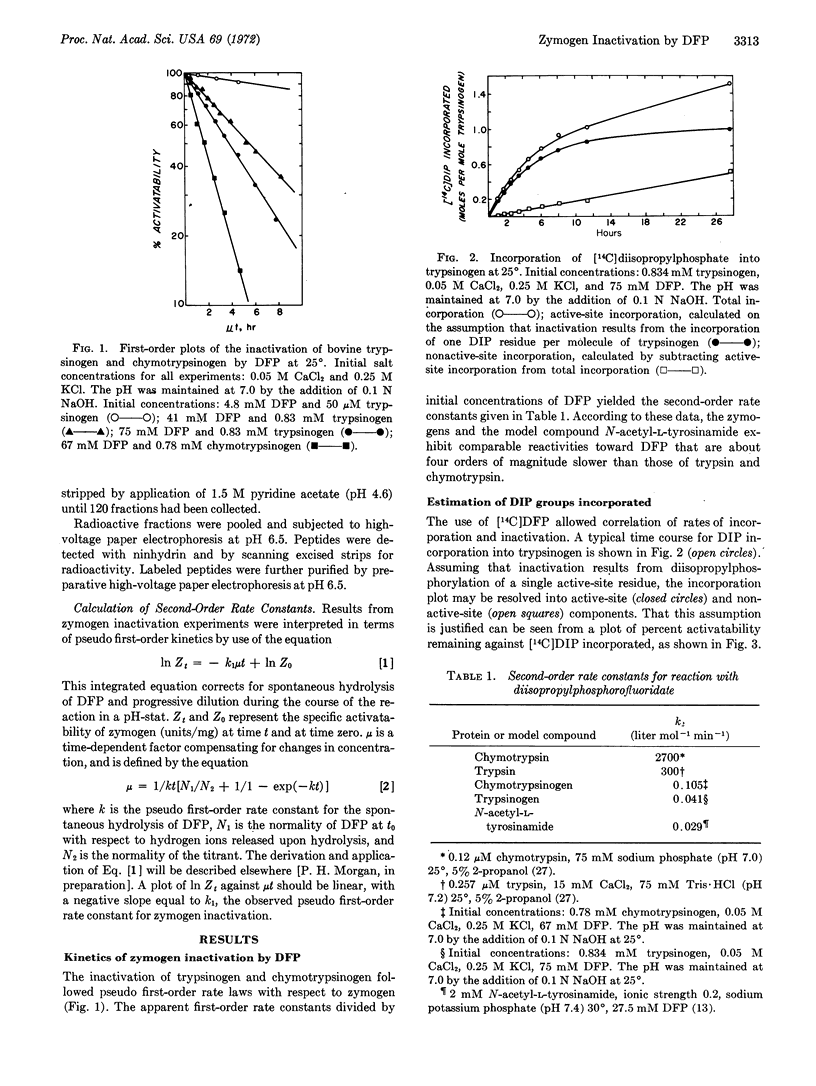
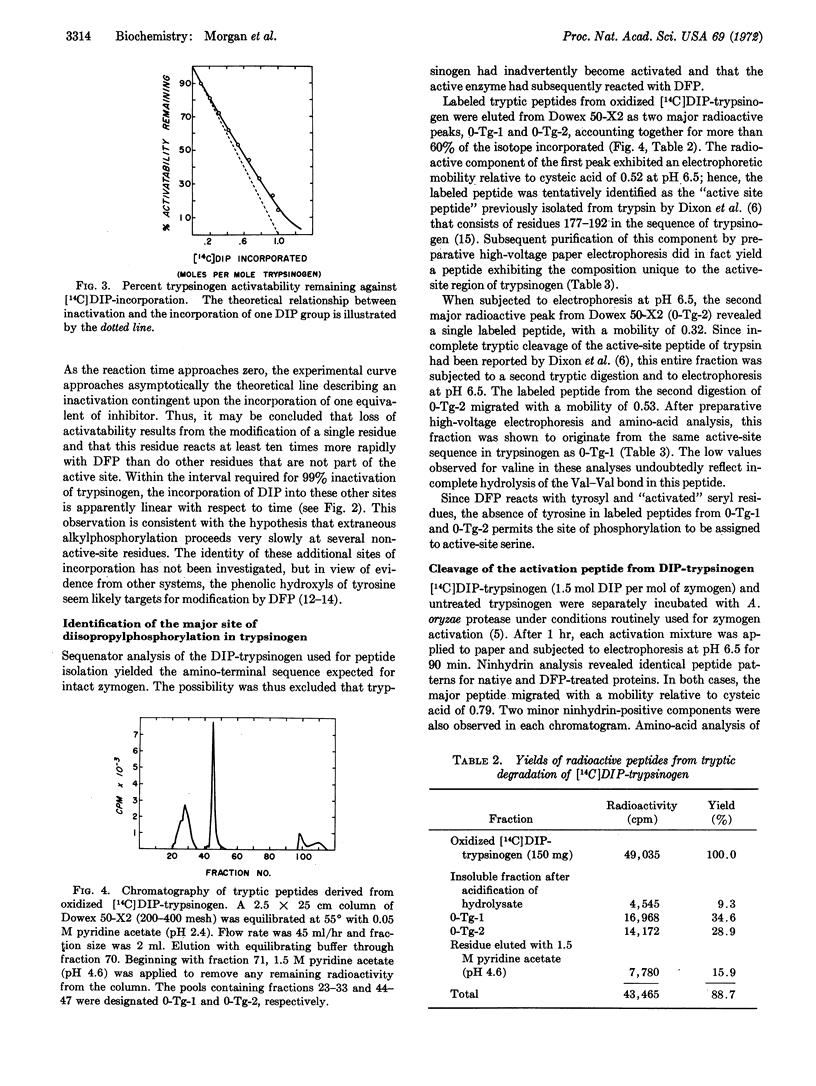
Diisopropylphosphorofluoridate reacts with trypsinogen and chymotrypsinogen and inhibits the potential activity of both zymogens. The reactions follow pseudo first-order kinetics and proceed approximately four orders of magnitude slower than diisopropylphosphorylation of the corresponding enzymes. Correlation of initial rates of inactivation with incorporation of the reagent indicates that zymogen inactivation results from incorporation of 1 mol of organic phosphate per mol of protein. Peptides isolated from the active-site region of trypsinogen account for more than 60% of the label originally present in the [14C]diisopropylphosphoryl zymogen. It is concluded that loss of activation of trypsinogen is due to alkylphosphorylation of Ser183. It is proposed that reduced reactivity of the zymogen, as compared to the enzyme, primarily reflects inefficient binding of substrates and inhibitors, and that Ser183 of the active site exists in trypsinogen in an activated state.
Keywords: zymogen activation, active site, serine proteases
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bustin M., Conway-Jacobs A. Intramolecular activation of porcine pepsinogen. J Biol Chem. 1971 Feb 10;246(3):615–620. [PubMed] [Google Scholar]
- DAVIE E. W., NEURATH H. Identification of a peptide released during autocatalytic activation of trypsinogen. J Biol Chem. 1955 Feb;212(2):515–529. [PubMed] [Google Scholar]
- DIXON G. H., KAUFFMAN D. L., NEURATH H. Amino acid sequence in the region of diisopropylphosphoryl binding in diisopropylphosphoryl-trypsin. J Biol Chem. 1958 Dec;233(6):1373–1381. [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Foltmann B. Prochymosin and chymosin (prorennin and rennin). Biochem J. 1969 Nov;115(3):3P–4P. doi: 10.1042/bj1150003p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freer S. T., Kraut J., Robertus J. D., Wright H. T., Xuong N. H. Chymotrypsinogen: 2.5-angstrom crystal structure, comparison with alpha-chymotrypsin, and implications for zymogen activation. Biochemistry. 1970 Apr 28;9(9):1997–2009. doi: 10.1021/bi00811a022. [DOI] [PubMed] [Google Scholar]
- Glazer A. N. Specific binding of thionine to the active site of trypsin. J Biol Chem. 1967 Jul 25;242(14):3326–3331. [PubMed] [Google Scholar]
- HARTLEY B. S. Proteolytic enzymes. Annu Rev Biochem. 1960;29:45–72. doi: 10.1146/annurev.bi.29.070160.000401. [DOI] [PubMed] [Google Scholar]
- Inagami T., Hatano H. Effect of alkylguanidines on the inactivation of trypsin by alkylation and phosphorylation. J Biol Chem. 1969 Mar 10;244(5):1176–1182. [PubMed] [Google Scholar]
- Inagami T., Murachi T. Reaction of diisopropylphosphorofluoridate with N-acetyl-L-tyrosinamide. J Biochem. 1970 Sep;68(3):419–421. doi: 10.1093/oxfordjournals.jbchem.a129371. [DOI] [PubMed] [Google Scholar]
- Kato K., Murachi T. Chemical modification of tyrosyl residues of hen egg-white lysozyme by diisopropylphosphorofluoridate. J Biochem. 1971 Apr;69(4):725–737. doi: 10.1093/oxfordjournals.jbchem.a129521. [DOI] [PubMed] [Google Scholar]
- Kay J., Kassell B. The autoactivation of trypsinogen. J Biol Chem. 1971 Nov;246(21):6661–6665. [PubMed] [Google Scholar]
- Lacko A. G., Neurath H. Studies on procarboxypeptidase A and carboxypeptidase A of the spiny pacific dogfish (Squalus acanthias). Biochemistry. 1970 Nov 24;9(24):4680–4690. doi: 10.1021/bi00826a010. [DOI] [PubMed] [Google Scholar]
- Murachi T., Inagami T., Yasui M. Evidence for alkylphosphorylation of tyrosyl residues of stem bromelain by diisopropylphosphorofluoridate. Biochemistry. 1965 Dec;4(12):2815–2825. doi: 10.1021/bi00888a036. [DOI] [PubMed] [Google Scholar]
- NEURATH H., DIXON G. H. Structure and activation of trypsinogen and chymotrypsinogen. Fed Proc. 1957 Sep;16(3):791–801. [PubMed] [Google Scholar]
- NEURATH H. MECHANISM OF ZYMOGEN ACTIVATION. Fed Proc. 1964 Jan-Feb;23:1–7. [PubMed] [Google Scholar]
- TIETZE F. Molecular-kinetic properties of crystalline trypsinogen. J Biol Chem. 1953 Sep;204(1):1–11. [PubMed] [Google Scholar]
- WALSH K. A., NEURATH H. TRYPSINOGEN AND CHYMOTRYPSINOGEN AS HOMOLOGOUS PROTEINS. Proc Natl Acad Sci U S A. 1964 Oct;52:884–889. doi: 10.1073/pnas.52.4.884. [DOI] [PMC free article] [PubMed] [Google Scholar]


