Abstract
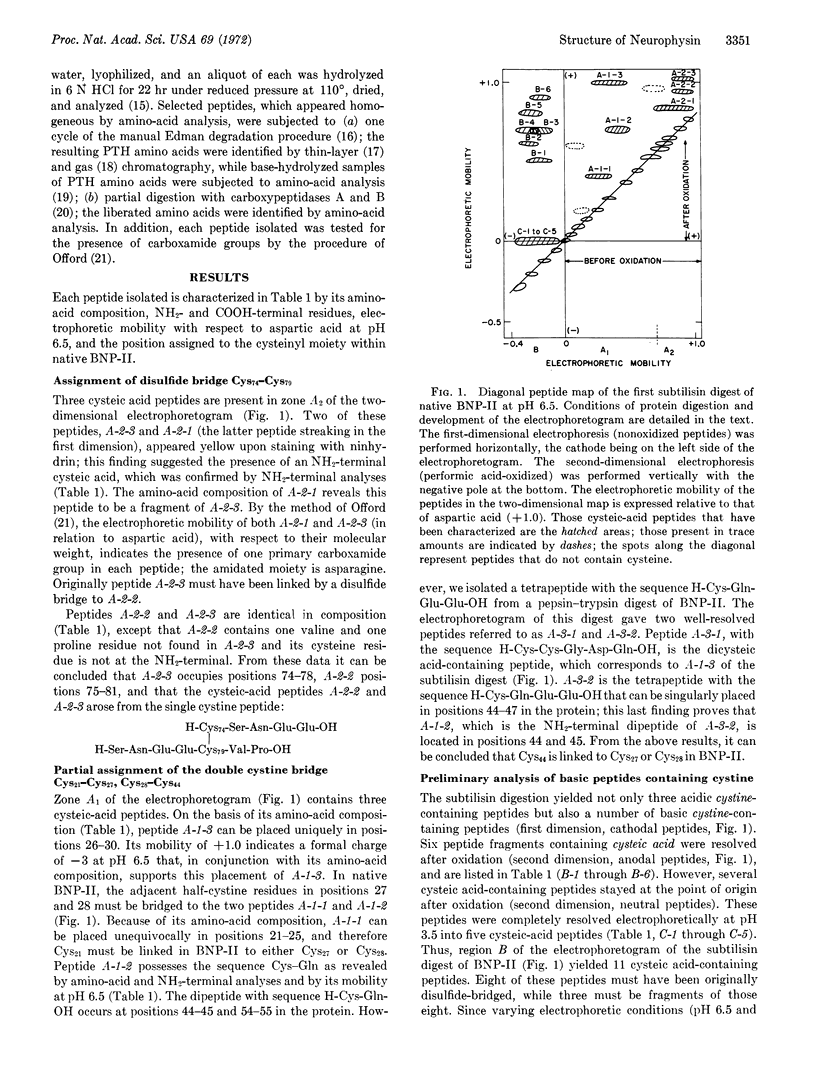
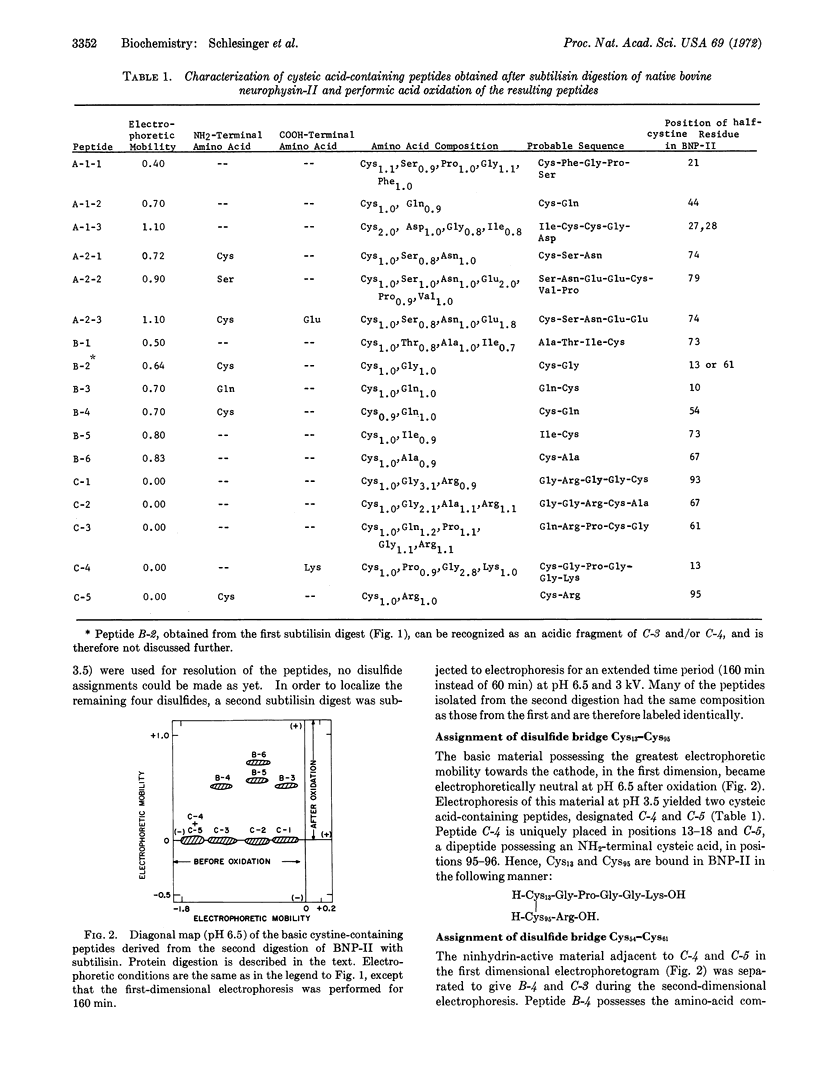
The completed amino-acid sequence of bovine neurophysin-II, a major neurohypophyseal hormone-binding protein in the hypothalamo-neurohypophyseal complex of cows, set the stage for the localization of the disulfide bonds of this sulfur-rich molecule. Neurophysin-II was digested with subtilisin or a pepsin-trypsin mixture. The resulting peptides were subjected to first-dimensional electrophoresis at pH 6.5, oxidized with performic acid, and subjected to second-dimensional electrophoresis under identical conditions as the first-dimensional separation, but in a perpendicular direction. Cysteic acid peptides were eluted (several after additional electrophoretic purification at pH 3.5) for amino-acid composition and NH2- and COOH-terminal analyses. Our assignment of the seven disulfide bridges present in neurophysin-II is as follows: Cys10-Cys93; Cys13-Cys95; Cys21-Cys27; Cys28-Cys44; Cys54-Cys61; Cys67-Cys73; Cys74-Cys79. The assignment of disulfide bridges associated with Cys27 and Cys28 is tentative as it is derived from evolutionary consideration. The high disulfide content reduces drastically the allowed number of biofunctional conformers of neurophysin-II. It is suggested that neurophysin-II possesses a globular topography with minimal α-helix structure.
Keywords: binding protein, conformation, molecular evolution, disulfides
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blombäck B., Blombäck M., Edman P., Hessel B. Human fibrinopeptides. Isolation, characterization and structure. Biochim Biophys Acta. 1966 Feb 28;115(2):371–396. doi: 10.1016/0304-4165(66)90437-5. [DOI] [PubMed] [Google Scholar]
- Breslow E., Aanning H. L., Abrash L., Schmir M. Physical and chemical properties of the bovine neurophysins. J Biol Chem. 1971 Sep 10;246(17):5179–5188. [PubMed] [Google Scholar]
- Breslow E., Abrash L. The binding of oxytocin and oxytocin analogues by purified bovine neurophysins. Proc Natl Acad Sci U S A. 1966 Aug;56(2):640–646. doi: 10.1073/pnas.56.2.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslow E., Walter R. Binding properties of bovine neurophysins I and II: an equilibrium dialysis study. Mol Pharmacol. 1972 Jan;8(1):75–81. [PubMed] [Google Scholar]
- Brown J. R., Hartley B. S. Location of disulphide bridges by diagonal paper electrophoresis. The disulphide bridges of bovine chymotrypsinogen A. Biochem J. 1966 Oct;101(1):214–228. doi: 10.1042/bj1010214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capra J. D., Kehoe J. M., Kotelchuck D., Walter R., Breslow E. Evolution of neurophysin proteins: the partial sequence of bovine neurophysin-I (vasopressin-oxytocin-carrier proteins-automated amino-acid-sequence analysis-homology-protein evolution). Proc Natl Acad Sci U S A. 1972 Feb;69(2):431–434. doi: 10.1073/pnas.69.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C. R., Hope D. B., Kazić T. Evidence for the storage of oxytocin with neurophysin-I and of vasopressin with neurophysin-II in separate neurosecretory granules. Br J Pharmacol. 1968 Sep;34(1):192P–193P. [PMC free article] [PubMed] [Google Scholar]
- Hollenberg M. D., Hope D. B. The isolation of the native hormone-binding proteins from bovine pituitary posterior lobes. Crystallization of neurophysin-I and-II as complexes with [8-arginine]-vasopressin. Biochem J. 1968 Jan;106(2):557–564. doi: 10.1042/bj1060557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOSTKA V., CARPENTER F. H. INHIBITION OF CHYMOTRYPSIN ACTIVITY IN CRYSTALLINE TRYPSIN PREPARATIONS. J Biol Chem. 1964 Jun;239:1799–1803. [PubMed] [Google Scholar]
- Kotelchuck D., Scheraga H. A. The influence of short-range interactions on protein onformation. II. A model for predicting the alpha-helical regions of proteins. Proc Natl Acad Sci U S A. 1969 Jan;62(1):14–21. doi: 10.1073/pnas.62.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LABELLA F. S., REIFFENSTEIN R. J., BEAULIEU G. Subcellular fractionation of bovine posterior pituitary glands by centrifugation. Arch Biochem Biophys. 1963 Mar;100:399–408. doi: 10.1016/0003-9861(63)90104-8. [DOI] [PubMed] [Google Scholar]
- LIU T. Y., STEIN W. H., MOORE S., ELLIOTT S. D. THE SEQUENCE OF AMINO ACID RESIDUES AROUND THE SULFHYDRYL GROUP AT THE ACTIVE SITE OF STREPTOCOCCAL PROTEINASE. J Biol Chem. 1965 Mar;240:1143–1149. [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- Pisano J. J., Bronzert T. J. Analysis of amino acid phenylthiohydantoins by gas chromatography. J Biol Chem. 1969 Oct 25;244(20):5597–5607. [PubMed] [Google Scholar]
- RYLE A. P., SANGER F., SMITH L. F., KITAI R. The disulphide bonds of insulin. Biochem J. 1955 Aug;60(4):541–556. doi: 10.1042/bj0600541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAWYER W. H. Neurophypophysial hormones. Pharmacol Rev. 1961 Jun;13:225–277. [PubMed] [Google Scholar]
- Van Orden H. O., Carpenter F. H. Hydrolysis of phenylthiohydantoins of amino acids. Biochem Biophys Res Commun. 1964;14:399–403. doi: 10.1016/0006-291x(64)90075-0. [DOI] [PubMed] [Google Scholar]
- Walter R., Schlesinger D. H., Schwartz I. L., Capra J. D. Complete amino acid sequence of bovine neurophysin II. Biochem Biophys Res Commun. 1971 Jul 16;44(2):293–298. doi: 10.1016/0006-291x(71)90598-5. [DOI] [PubMed] [Google Scholar]
- Yang C. C., Yang H. J., Chiu R. H. The position of disulfide bonds in cobrotoxin. Biochim Biophys Acta. 1970 Aug 21;214(2):355–363. doi: 10.1016/0005-2795(70)90013-9. [DOI] [PubMed] [Google Scholar]


