Abstract
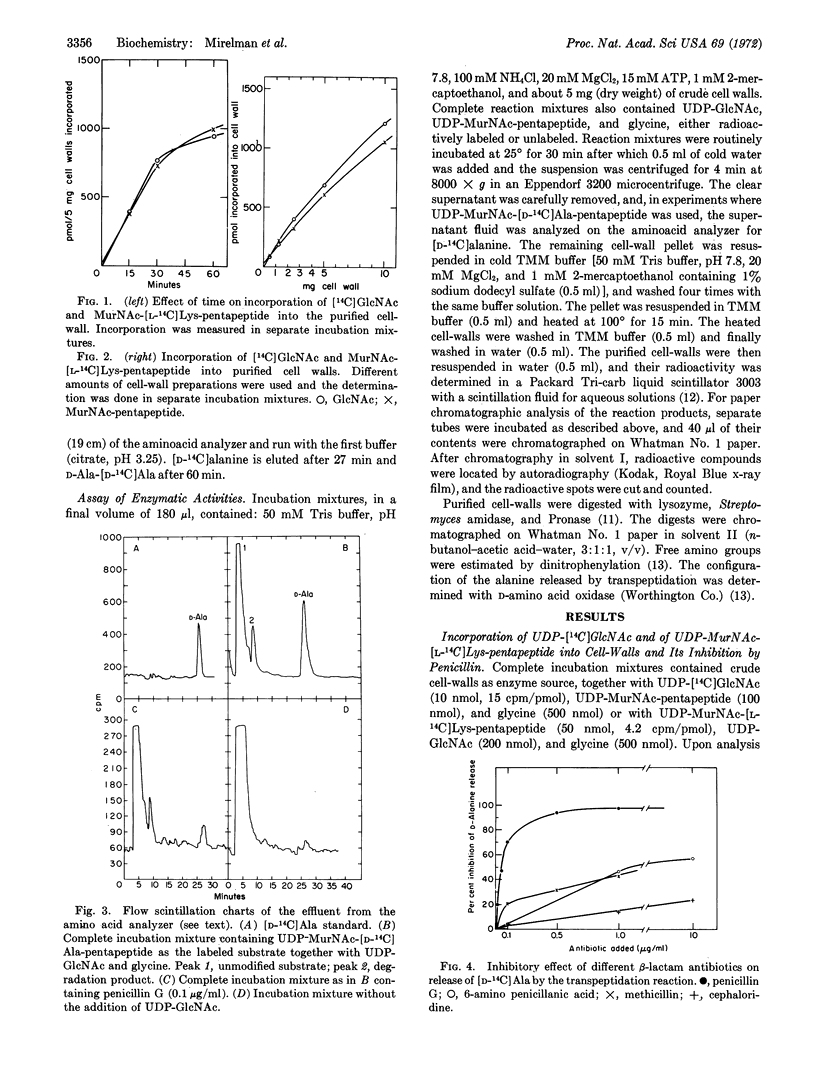
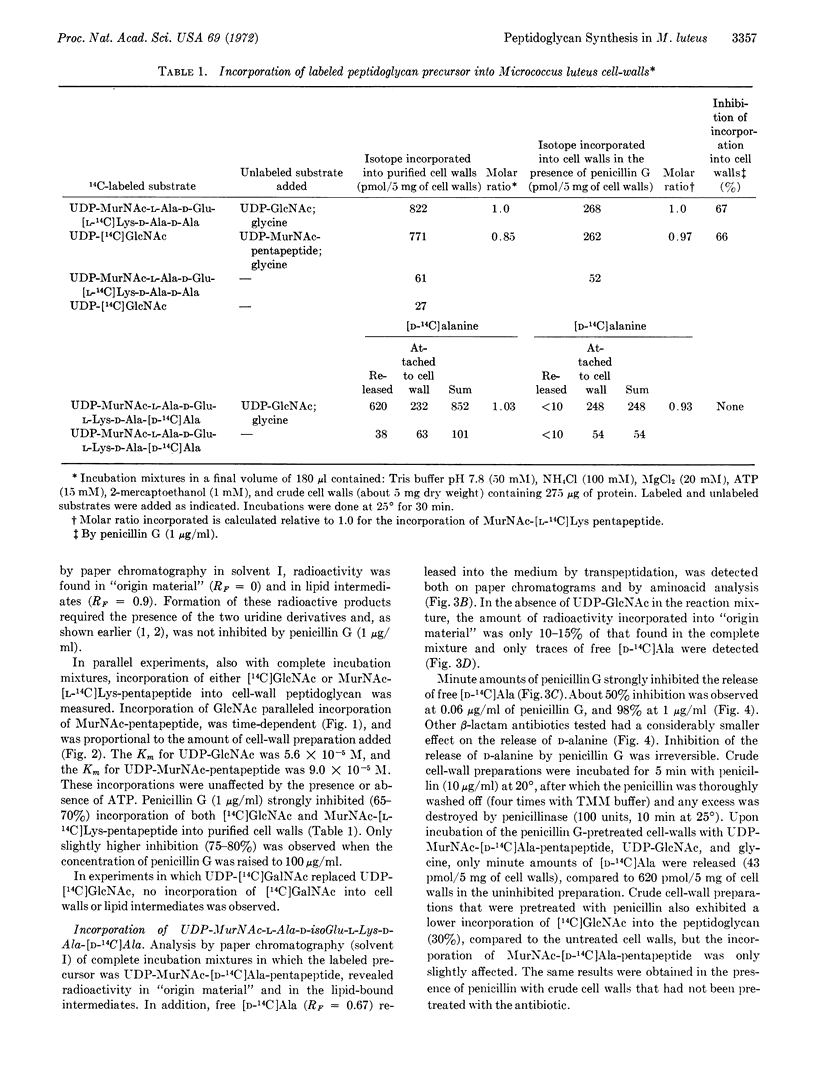
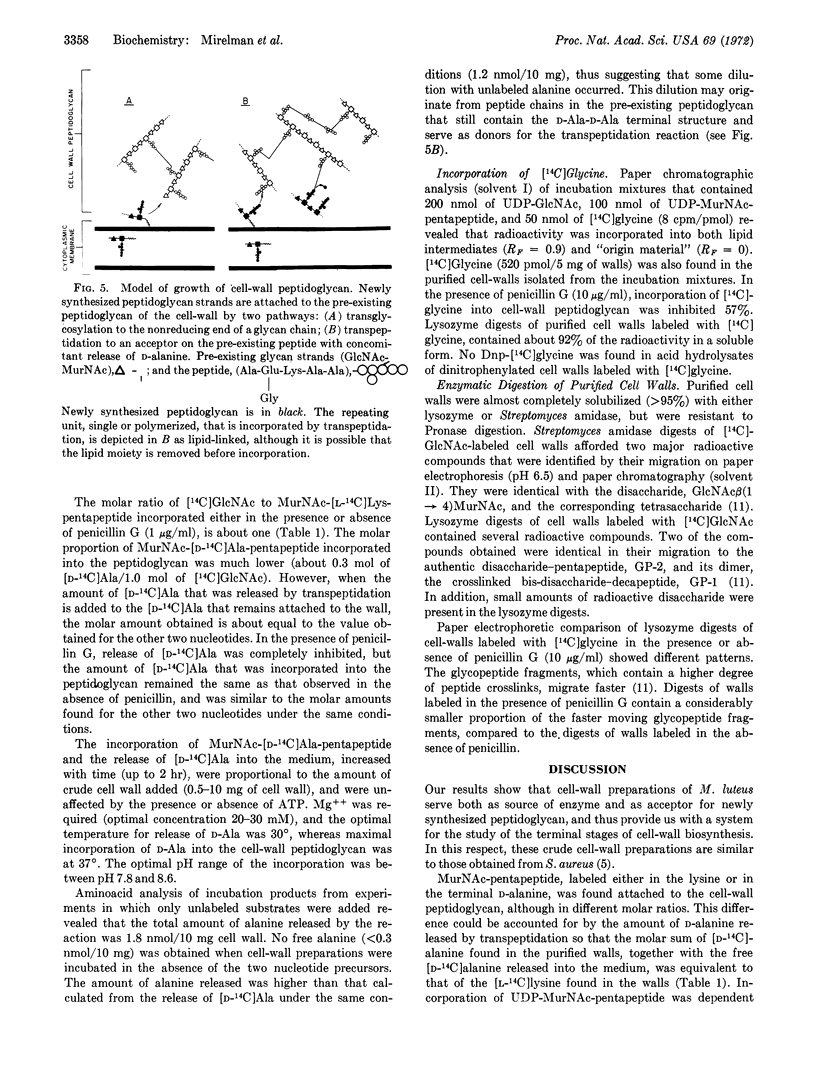
Cell-wall preparations of Micrococcus luteus (lysodeikticus) catalyze in vitro peptidoglycan synthesis from UDP N-acetyl-D-glucosamine, UDP N-acetylmuramic acid-pentapeptide, and glycine. Newly synthesized peptidoglycan is partially cross-linked by a transpeptidation reaction with concomitant release of C-terminal D-alanine. Penicillin not only strongly inhibits release of D-alanine (98% at 1 μg/ml), but also markedly inhibits incorporation of acetylglucosamine and N-acetylmuramic acid-pentapeptide into the preformed cell-wall peptidoglycan. The simplest explanation for the results is that incorporation of newly synthesized strands of peptidoglycan and their attachment to “older” cell-wall peptidoglycan proceeds mainly by transpeptidation and that transglycosylation is responsible only for part of the elongation of the pre-existing peptidoglycan. Another possibility is that incorporation occurs by transglycosylation, but it cannot continue without concurrent formation of peptide cross-bridges.
Keywords: bacterial cell-wall biosynthesis, transglycosylation, β-lactam antibiotics
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON J. S., MATSUHASHI M., HASKIN M. A., STROMINGER J. L. LIPID-PHOSPHOACETYLMURAMYL-PENTAPEPTIDE AND LIPID-PHOSPHODISACCHARIDE-PENTAPEPTIDE: PRESUMED MEMBRANE TRANSPORT INTERMEDIATES IN CELL WALL SYNTHESIS. Proc Natl Acad Sci U S A. 1965 Apr;53:881–889. doi: 10.1073/pnas.53.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. S., Meadow P. M., Haskin M. A., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. I. Utilization of uridine diphosphate acetylmuramyl pentapeptide and uridine diphosphate acetylglucosamine for peptidoglycan synthesis by particulate enzymes from Staphylococcus aureus and Micrococcus lysodeikticus. Arch Biochem Biophys. 1966 Sep 26;116(1):487–515. doi: 10.1016/0003-9861(66)90056-7. [DOI] [PubMed] [Google Scholar]
- CHATTERJEE A. N., PARK J. T. BIOSYNTHESIS OF CELL WALL MUCOPEPTIDE BY A PARTICULATE FRACTION FROM STAPHYLOCOCCUS AUREUS. Proc Natl Acad Sci U S A. 1964 Jan;51:9–16. doi: 10.1073/pnas.51.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghuysen J. M., Bricas E., Lache M., Leyh-Bouille M. Structure of the cell walls of Micrococcus lysodeikticus. 3. Isolation of a new peptide dimer, N-alpha-[L-alanyl-gamma-(alpha-D-glutamylglycine)]-L-lysyl-D-alanyl-N-alpha-[L-alanyl-gamma-(alpha-D-glutamylglycine)]-L-lysyl-D-alanine. Biochemistry. 1968 Apr;7(4):1450–1460. doi: 10.1021/bi00844a030. [DOI] [PubMed] [Google Scholar]
- Ghuysen J. M., Leyh-Bouille M., Bonaly R., Nieto M., Perkins H. R., Schleifer K. H., Kandler O. Isolation of DD carboxypeptidase from Streptomyces albus G culture filtrates. Biochemistry. 1970 Jul 21;9(15):2955–2961. doi: 10.1021/bi00817a004. [DOI] [PubMed] [Google Scholar]
- Katz W., Matsuhashi M., Dietrich C. P., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. IV. Incorporation of glycine in Micrococcus lysodeikticus. J Biol Chem. 1967 Jul 10;242(13):3207–3217. [PubMed] [Google Scholar]
- MANDELSTAM J., ROGERS H. J. The incorporation of amino acids into the cell-wall mucopeptide of staphylococci and the effect of antibiotics on the process. Biochem J. 1959 Aug;72:654–662. doi: 10.1042/bj0720654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirelman D., Sharon N. Biosynthesis of peptidoglycan by a cell wall preparation of Staphylococcus aureus and its inhibition by penicillin. Biochem Biophys Res Commun. 1972 Mar 10;46(5):1909–1917. doi: 10.1016/0006-291x(72)90069-1. [DOI] [PubMed] [Google Scholar]
- Mirelman D., Shaw D. R., Park J. T. Nature and origins of phosphorus compounds in isolated cell walls of Staphylococcus aureus. J Bacteriol. 1971 Jul;107(1):239–244. doi: 10.1128/jb.107.1.239-244.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEUHAUS F. C., STRUVE W. G. ENZYMATIC SYNTHESIS OF ANALOGS OF THE CELL-WALL PRECURSOR. I. KINETICS AND SPECIFICITY OF URIDINE DIPHOSPHO-N-ACETYLMURAMYL-L-ALANYL-D-GLUTAMYL-L-LYSINE:D-ALANYL-D-ALANINE LIGASE (ADENOSINE DIPHOSPHATE) FROM STREPTOCOCCUS FAECALIS R. Biochemistry. 1965 Jan;4:120–131. doi: 10.1021/bi00877a020. [DOI] [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Micrococcus lysodeikticus: a new type of cross-linkage of the murein. Biochem Biophys Res Commun. 1967 Sep 27;28(6):965–972. doi: 10.1016/0006-291x(67)90074-5. [DOI] [PubMed] [Google Scholar]
- Strominger J. L., Ghuysen J. M. Mechanisms of enzymatic bacteriaolysis. Cell walls of bacteri are solubilized by action of either specific carbohydrases or specific peptidases. Science. 1967 Apr 14;156(3772):213–221. doi: 10.1126/science.156.3772.213. [DOI] [PubMed] [Google Scholar]
- Strominger J. L., Izaki K., Matsuhashi M., Tipper D. J. Peptidoglycan transpeptidase and D-alanine carboxypeptidase: penicillin-sensitive enzymatic reactions. Fed Proc. 1967 Jan-Feb;26(1):9–22. [PubMed] [Google Scholar]
- Strominger J. L. Penicillin-sensitive enzymatic reactions in bacterial cell wall synthesis. Harvey Lect. 1968 1969;64:179–213. [PubMed] [Google Scholar]
- Wise E. M., Jr, Park J. T. Penicillin: its basic site of action as an inhibitor of a peptide cross-linking reaction in cell wall mucopeptide synthesis. Proc Natl Acad Sci U S A. 1965 Jul;54(1):75–81. doi: 10.1073/pnas.54.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]


