Abstract
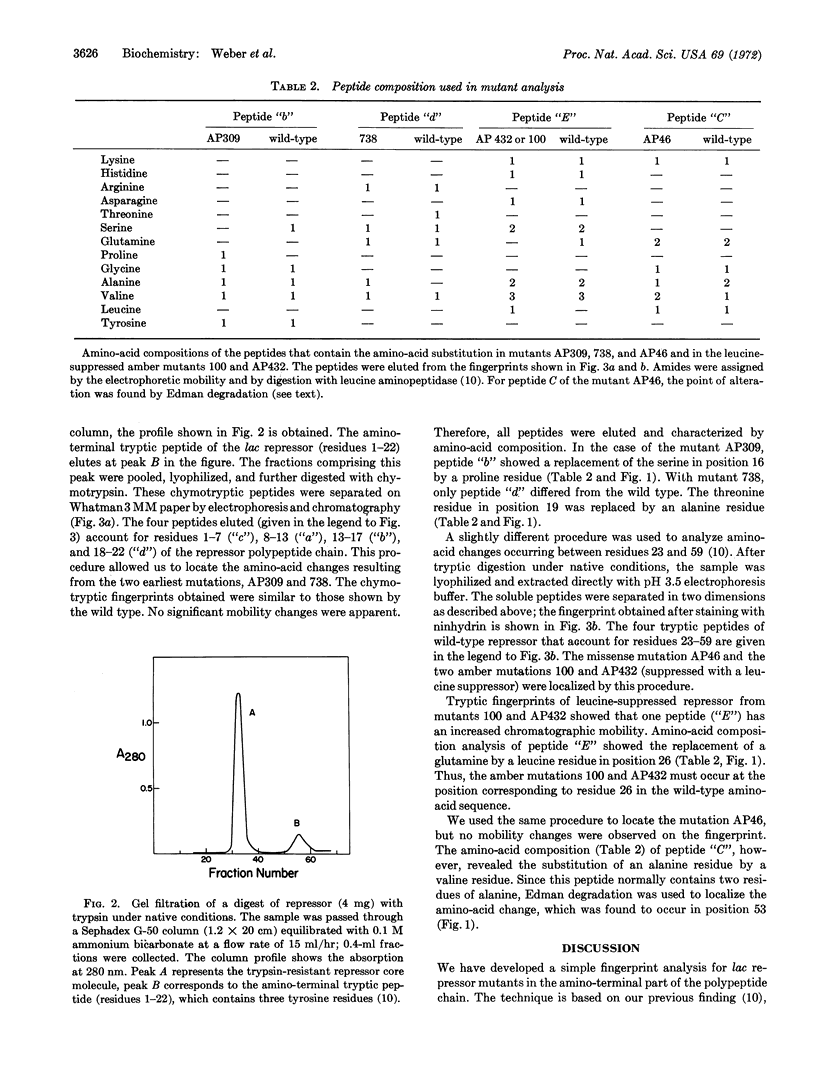
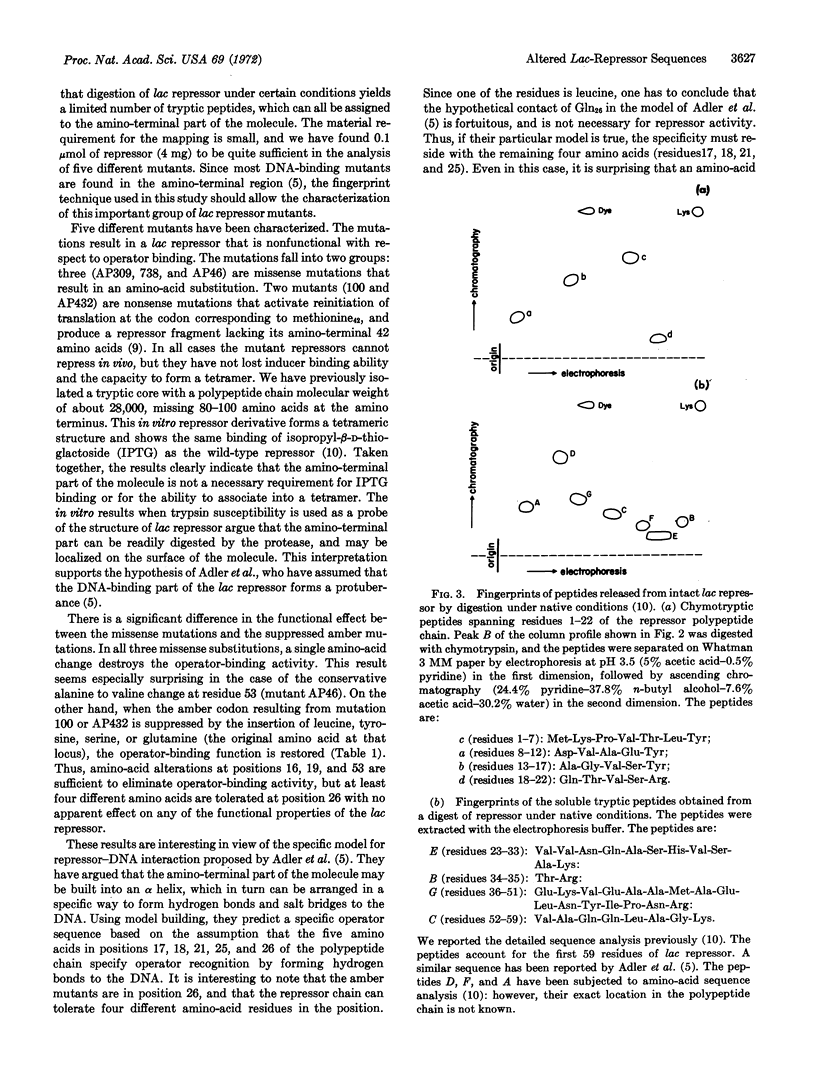
A technique is described for mapping point mutations in the first 59 amino-acid residues of the lac repressor from Escherichia coli, using less than 0.1 μmol (4 mg) of the purified protein. This technique was used to localize five mutations affecting the ability of the i-gene product to repress in vivo. These alterations are located at four different sites in the amino-terminal region of the repressor molecule. Three of these are missense mutations and result in changes from serine to proline (residue 16), threonine to alanine (residue 19), and alanine to valine (residue 53). Each amino-acid substitution alone is sufficient to eliminate repression in vivo, presumably by altering the operator binding activity. The remaining two independently-isolated mutations are identical, and result in a change from a glutamine codon at position 26 to an amber (UAG) codon. Since suppression of this nonsense mutation with amber suppressors that insert leucine, tyrosine, serine, or glutamine restores repressor activity to the molecule, glutamine26 cannot be crucial for the operator-binding function. A comparison of the position of each altered residue with the genetic map enabled us to estimate the physical distance between several deletion-group endpoints.
Keywords: amino-acid substitutions, amino-terminal, proteolysis, mutant, E. coli, suppressor
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler K., Beyreuther K., Fanning E., Geisler N., Gronenborn B., Klemm A., Müller-Hill B., Pfahl M., Schmitz A. How lac repressor binds to DNA. Nature. 1972 Jun 9;237(5354):322–327. doi: 10.1038/237322a0. [DOI] [PubMed] [Google Scholar]
- Gilbert W., Müller-Hill B. The lac operator is DNA. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2415–2421. doi: 10.1073/pnas.58.6.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- Kumar S., Szybalski W. Orientation of transcription of the lac operon and its repressor gene in Escherichia coli. J Mol Biol. 1969 Feb 28;40(1):145–151. doi: 10.1016/0022-2836(69)90303-9. [DOI] [PubMed] [Google Scholar]
- Miller J. H., Beckwith J., Muller-Hill B. Direction of transcription of a regulatory gene in E. coli. Nature. 1968 Dec 28;220(5174):1287–1290. doi: 10.1038/2201287a0. [DOI] [PubMed] [Google Scholar]
- Müller-Hill B., Crapo L., Gilbert W. Mutants that make more lac repressor. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1259–1264. doi: 10.1073/pnas.59.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt T., Weber K., Ganem D., Miller J. H. Translational restarts: AUG reinitiation of a lac repressor fragment. Proc Natl Acad Sci U S A. 1972 Apr;69(4):897–901. doi: 10.1073/pnas.69.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs A. D., Bourgeois S., Newby R. F., Cohn M. DNA binding of the lac repressor. J Mol Biol. 1968 Jul 14;34(2):365–368. doi: 10.1016/0022-2836(68)90261-1. [DOI] [PubMed] [Google Scholar]
- Smith T. F., Sadler J. R. The nature of lactose operator constitive mutations. J Mol Biol. 1971 Jul 28;59(2):273–305. doi: 10.1016/0022-2836(71)90051-9. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yanofsky C., Ito J., Horn V. Amino acid replacements and the genetic code. Cold Spring Harb Symp Quant Biol. 1966;31:151–162. doi: 10.1101/sqb.1966.031.01.023. [DOI] [PubMed] [Google Scholar]