Abstract
Metastatic breast cancer cannot be treated successfully. Currently, the targeted therapies for metastatic disease are limited to human epidermal growth factor receptor 2 and hormone receptor antagonists. Understanding the mechanisms of breast cancer growth and metastasis is therefore crucial for the development of new intervention strategies. Here, we show that FER kinase (FER) controls migration and metastasis of invasive human breast cancer cell lines by regulating α6- and β1-integrin-dependent adhesion. Conversely, the overexpression of FER in non-metastatic breast cancer cells induces pro-invasive features. FER drives anoikis resistance, regulates tumour growth and is necessary for metastasis in a mouse model of human breast cancer. In human invasive breast cancer, high FER expression is an independent prognostic factor that correlates with high-grade basal/triple-negative tumours and worse overall survival, especially in lymph node-negative patients. These findings establish FER as a promising target for the prevention and inhibition of metastatic breast cancer.
Keywords: breast cancer, cell adhesion, cell migration, FER kinase, metastasis
Introduction
Breast cancer is the second most commonly occurring cancer worldwide with 1.38 million new cases diagnosed in 2008, and is the leading cause of cancer mortality in women.1 Advancements in diagnostics and treatment have increased breast cancer survival rates, but still approximately one-third of patients will develop distant metastases and eventually die of the disease.2 Targeted therapies have improved survival in patients with human epidermal growth factor receptor 2-positive metastatic disease.3 However, further insight into the molecular processes underlying metastasis is needed to identify new potential drug targets.
The feline sarcoma and feline sarcoma-related (FER) proteins are the only two members of a unique family of non-receptor tyrosine kinases. They are distinguished from other tyrosine kinases by an N-terminal feline sarcoma/FER/CIP4 homology/Bin1/Amphiphysin/RVS (F-BAR) domain, which is also found in Rho GTPase-activating proteins and adaptor proteins involved in the regulation of cytoskeletal rearrangements, cell polarity, vesicular trafficking and endocytosis.4
FER is ubiquitously expressed and its subcellular localization is cytoplasmic.5, 6 Upon activation by growth factors, FER can associate with and phosphorylate the adherens junction molecule p120-catenin and actin-binding protein cortactin.7, 8 FER indirectly associates with N-cadherin via p120-catenin and is necessary for maintaining adherens junction stability.9, 10 However, others have reported that FER overexpression may lead to adherens junction dissolution and loss of focal adhesions (FAs).11 FER can shuttle between N-cadherin-containing adherens junctions and β1-integrin FA complexes, thus coordinately regulating cell–cell and cell–extracellular matrix (ECM) adhesion.12 FER-mediated cortactin phosphorylation is associated with fibroblast migration.13 In addition, FER has been shown to regulate migration in several other cell types.14, 15, 16
Several lines of evidence support a role of FER in malignant progression. FER regulates cell cycle progression in prostate and breast cancer, as well as in acute myeloid leukaemia cells.17, 18 Further, FER is highly expressed in prostate cancer as compared with normal prostate epithelium and regulates prostate cancer cell proliferation by activating signal transducer and activator of transcription 3.19, 20 High FER expression is associated with hepatocellular carcinoma metastasis21 and poor prognosis in clear cell renal cell carcinoma.22, 23 Also, recent data indicate that FER can mediate resistance to the anticancer agent quinacrine via activation of nuclear factor-κB, which could have therapeutic implications.24
These observations prompted us to investigate the role of FER in breast cancer progression. Here, we show that FER regulates breast cancer cell adhesion, migration and anoikis resistance, and is necessary for tumour growth and metastasis formation in mice. Moreover, FER expression is high in poorly differentiated breast carcinomas and independently predicts decreased patient survival.
Results
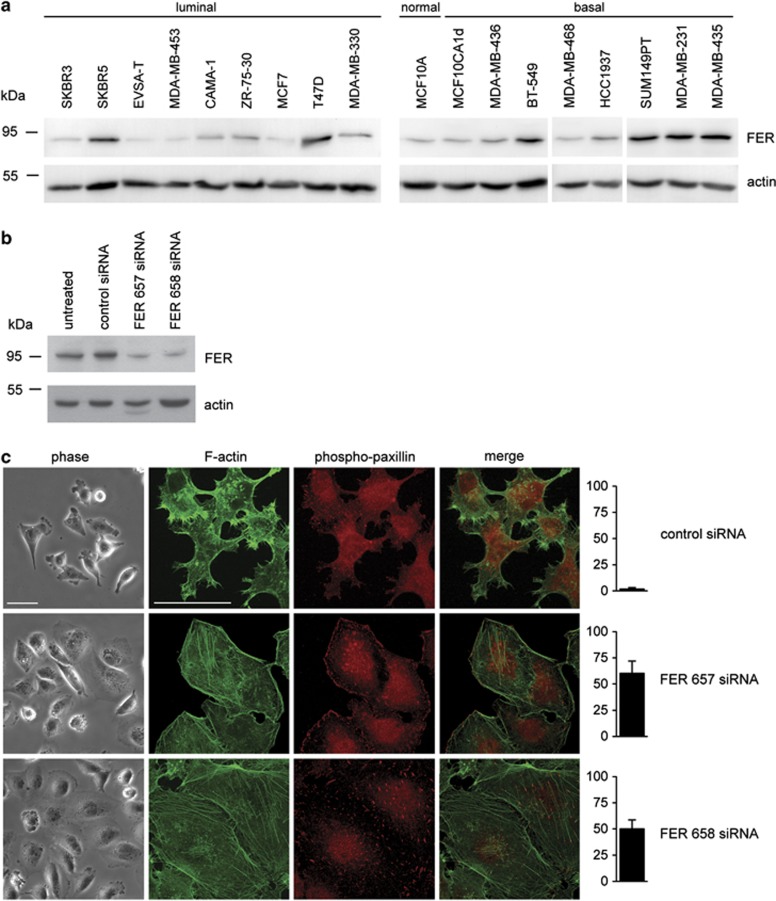
To establish a working model to study the role of FER in breast cancer, we examined FER expression in a panel of 18 breast carcinoma cell lines (Figure 1a). FER was almost undetectable in non-transformed MCF10A cells, and its level was low-to-moderate in non-invasive cells and cell lines with low metastatic potential. Notably, FER was highly expressed in MDA-MB-231, SUM149PT and MDA-MB-435, basal-like and metastatic breast cancer cell lines.
Figure 1.
Inhibition of FER induces cell spreading, actin stress fibre and FA formation. (a) Total cell lysates of the indicated breast cancer cell lines were prepared and resolved by SDS-polyacrylamide gel electrophoresis. FER kinase expression was analysed by immunoblot. (b) KD of FER in MDA-MB-231 cells using siRNA transfection. FER expression was analysed by immunoblot 72 h after transfection. Actin was used as a loading control. (c) Control and FER KD MDA-MB-231 cells were plated on collagen I-coated glass coverslips. The morphology of live cells was analysed by phase contrast microscopy (phase). F-actin (green) and phospho-paxillin (red) distribution was analysed in fixed cells by immunofluorescence (IF) microscopy. The percentage of cells showing stress fibers is shown in the right bar graphs. Scale bar=50 μm. The results shown are representative of three independent experiments.
FER regulates breast cancer cell morphology, actin cytoskeleton organization and ECM adhesion
As FER has been linked to actin cytoskeleton regulation,8 we analysed the effect of RNAi-mediated FER inhibition on cellular morphology and actin distribution in MDA-MB-231 and SUM149PT cells. Although MDA-MB-435 express high levels of FER, recent evidence suggest that these cells may originate from melanoma rather than breast cancer.25 Because of their unclear heritage we did not proceed to test the effect of FER downregulation in MDA-MB-435 cells. Transfection with two independent small interfering RNAs (siRNAs) significantly downregulated FER expression as compared with a scrambled, control siRNA (Figure 1b and Supplementary Figure 1A). FER knockdown (KD) induced cell spreading accompanied by the formation of prominent F-actin stress fibres. In addition, we observed FA reorganization (Figure 1c and Supplementary Figure 1B). These data suggest that FER controls actin dynamics, FA distribution and cell spreading, and that FER inhibition may lead to increased cell–ECM adhesion. To determine whether this is the case, we seeded control and FER KD cells on different ECM substrates and measured their ability to adhere. We found that FER KD induced a modest, but significant increase in MDA-MB-231 cell adhesion to collagen I and laminin (Figure 2a).
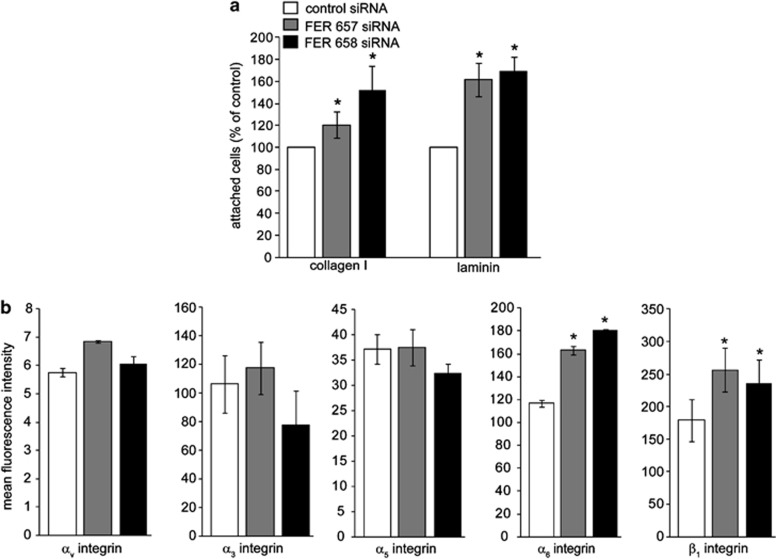
Figure 2.
FER regulates cell–matrix adhesion and integrin expression in breast cancer cells. (a) MDA-MB-231 FER KD cells were generated by transient transfection with the indicated siRNAs. FER KD cells were plated on 96-well plates coated with collagen I, fibronectin or laminin and the percentage of attached cells was determined. Values represent the relative proportion of attached cells ±s.e.m. * indicates significantly different proportions of adherent cells, relative to control (P<0.05, one-way analysis of variance (ANOVA)). (b) FER KD cells were trypsinized and surface expression of the indicated integrin subunits was analysed by fluorescence-activated cell sorting (FACS). Values represent mean fluorescence units ±s.e.m. * indicates significantly different integrin levels as compared with control (P<0.05, one-way ANOVA). The results shown are representative of three independent experiments.
Integrin receptors regulate cell–ECM adhesion, motility and anchorage-dependent survival. To investigate whether the increased cell adhesion we observed upon FER KD was due to changes in integrin levels, we measured cell surface expression of α3-, α5-, α6-, αv- and β1-integrin subunits in control and FER KD cells. Although we did not find significant changes in the expression of α3-, αv- and α5-integrins, cell surface levels of β1- and α6-integrins were significantly upregulated in FER KD as compared with control cells (Figure 2b). These results suggest that FER may regulate β1- and α6-integrin subcellular distribution.
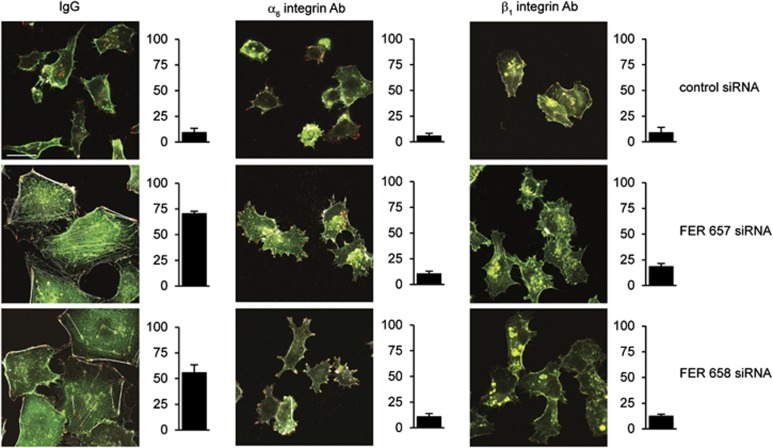
To investigate the effect of FER downregulation on β1- and α6-integrin, localization control and FER KD cells were seeded on different ECM substrata and analysed by immunofluorescence microscopy. Both α6- and β1-integrins colocalized with phosphorylated paxillin in FAs of FER KD cells (Figure 3 and Supplementary Figure 2). Further, we observed accumulation of α6-integrin on the cell membrane and in endosomal vesicles upon FER inhibition (Supplementary Figure 3). To determine whether cell spreading and stress fibre formation in FER KD cells are dependent on integrin activation, we treated cells with blocking antibodies before plating them on collagen I or laminin. Cell spreading and stress fibre formation were inhibited upon treatment with a β1-integrin-blocking antibody, indicating that FER KD induced β1-integrin-dependent adhesion to laminin and collagen I (Figure 3 and Supplementary Figure 2). Similar results were observed with an α6-integrin-blocking antibody in cells seeded on laminin (Figure 3 and Supplementary Figure 2C). These results are consistent with the concept that FER inhibition promotes cell adhesion via β1- and α6-integrin-dependent formation of FAs.
Figure 3.
FA and actin stress fibre formation in FER-depleted cells is β1- and α6-integrin dependent. MDA-MB-231 cells were transfected with the indicated siRNAs and cultured for 96 h. Cells were trypsinized and incubated with isotype control (IgG) or integrin-blocking antibodies and were re-plated on glass coverslips coated with laminin. Cells were processed for immunofluorescence (IF) microscopy using anti-β1 or -α6 integrin antibodies (green), anti-phospho-paxillin (red) and Alexa 633-labelled phalloidin (grey) to visualize FAs and F-actin, respectively. The percentage of cells showing stress fibers is shown in the right bar graphs. Scale bar=20 μm. The results shown are representative of three independent experiments.
FER controls breast cancer cell migration, invasion and anoikis resistance
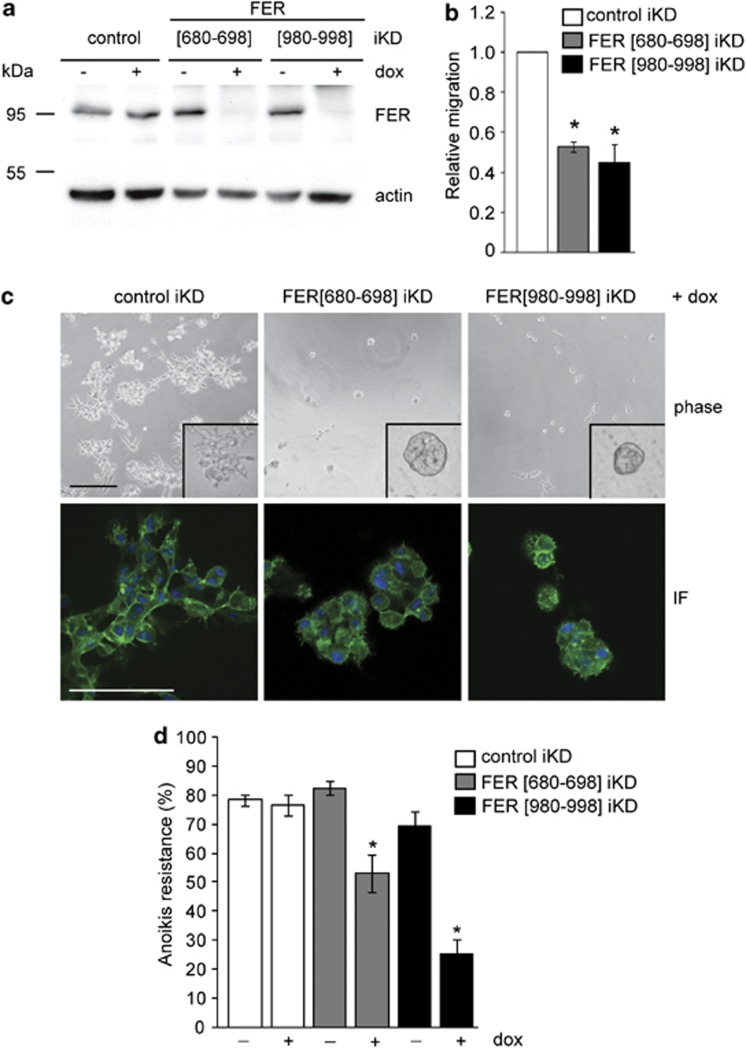
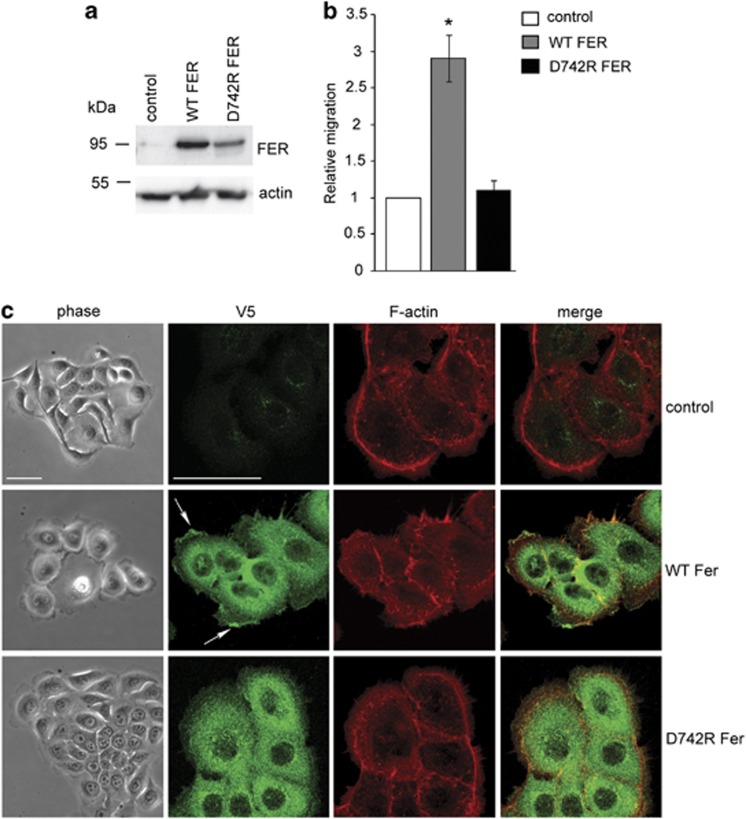
The ability of FER to regulate actin dynamics, lamellipodia formation and integrin-dependent adhesion suggested that it may control cell motility and invasion of basal breast cancer cells. To determine the effect of FER downregulation on MDA-MB-231 cell migration, we started by using a modified wound-healing assay. We employed a doxycycline-inducible and lentivirus-based small hairpin RNA (shRNA) approach to enable inducible KD (iKD) of FER in MDA-MB-231 cells (Figure 4a). We confirmed that the FER iKD cells exhibit the same phenotype of cell spreading, stress fibre formation and α6β1-integrin upregulation upon doxycycline treatment (Supplementary Figures 4A and B), as previously observed in cells transfected with FER siRNAs (Figures 1 and 2). FER iKD using two independent shRNA sequences significantly inhibited the ability of MDA-MB-231 cells to migrate on a collagen I-coated surface, as compared with control cells (Figure 4b). In a complementary experiment, we transduced MCF10A.CA1d, a basal-like breast cancer cell line with low endogenous FER expression, using lentiviruses encoding V5-tagged wild-type and kinase-deficient (D742R) mutant FER (Figure 5a). Overexpression of wild-type, but not D742R FER, significantly increased lamellipodia formation and migration of MCF10A.CA1d cells (Figures 5b and c). In addition, wild-type FER localized to the leading edges of lamellipodia, whereas D742R FER did not (Figure 5c, arrows). These results indicate that regulation of lamellipodia formation and cell migration by FER is dependent on its kinase activity.
Figure 4.
FER kinase regulates migration, invasion and anoikis resistance in breast cancer cells. (a) MDA-MB-231 iKD cells were cultured for 96 h in the absence (−) or presence (+) of doxycycline (dox; 2 μg/ml) to induce shRNA expression, and FER kinase was detected by immunoblot. Actin was used as a loading control. (b) MDA-MB-231 iKD cells treated as in a were seeded on collagen I-coated plates and the rate of migration was measured over 24 h using a modified wound-healing assay. Values represent the ratio of the migration rate of untreated versus dox-treated cells ±s.e.m. All values were normalized to control. * indicates significantly different migration rates, relative to control (P<0.05, one-way analysis of variance (ANOVA)). (c) MDA-MB-231 iKD cells treated as in a were plated on laminin-rich ECM substrate (Matrigel) and cultured for 96 h in the presence of dox. Cells were imaged by phase contrast (phase) or immunofluorescence (IF) microscopy using Alexa 488-labelled phalloidin and 4',6-diamidino-2-phenylindole to visualize F-actin (green) and DNA (blue), respectively. Images represent a single Z-position in the centre of three-dimensional cell structures. Scale bar=100 μm. (d) Adherent cultures of MDA-MB-231 iKD cells were treated as in a. Cells were trypsinized and cultured in suspension conditions for 72 h in the absence or presence of dox (2 μg/ml), followed by labelling with Cy5-conjugated Annexin V (AnnV) and propidium iodide (PI) and fluorescence-activated cell sorting (FACS) analysis. Values represent the percentage of anoikis-resistant cells (PINEG/AnnVNEG) ±s.e.m. * indicates a significant difference compared with untreated cells (-dox); (P<0.05, one-way ANOVA). The results shown are representative of three independent experiments.
Figure 5.
FER regulates lamellipodia formation and migration. (a) The expression of V5-tagged wild-type (WT) and kinase-deficient (D742R) FER in stably transduced MCF10.CA1d cells was determined by immunoblot using an anti-V5 antibody. Actin was used as a loading control. (b) Control, WT and D742R FER-expressing MCF10.CA1d cells were seeded on collagen I-coated plates. The rate of migration was measured over 24 h using a modified wound-healing assay. Values represent the migration rate normalized to control ±s.e.m. * indicates significantly different migration rates relative to control (P<0.05, one-way analysis of variance (ANOVA)). (c) Control and WT or D742R FER-expressing MCF10A.CA1d cells were plated on collagen I-coated glass coverslips. The morphology of live cells was analysed by phase contrast microscopy (phase) after 24 h. Cells were then fixed and processed for immunofluorescence (IF) microscopy using an anti-V5 antibody to detect exogenously expressed FER-V5 (green) and Alexa 555-conjugated phalloidin to visualize F-actin (red). The arrow indicates accumulation of WT FER at the leading edges of lamellipodia. Scale bar=50 μm.
The ability of FER to regulate cell motility and lamellipodia formation suggested that it may be involved in breast cancer cell invasion. The invasive in vivo behaviour of MDA-MB-231 cells can be recapitulated ex vivo by culturing the cells on a laminin-rich ECM substrate (lrECM; Matrigel, BD Biosciences, San Jose, CA, USA) in three dimension.26 Control iKD cells formed highly branched, invasive and disorganized colonies when cultured on lrECM (Figure 4c). In contrast, FER iKD resulted in non-invasive cell colonies (Figure 4c), suggesting that FER is necessary for breast cancer cell invasion and migration in lrECM. Thus, our results indicate that downregulation of FER increases integrin-mediated cell adhesion, while inhibiting migration and invasion.
Normal epithelial cells require ECM attachment for survival. Detachment from the ECM or inappropriate engagement of integrin receptors results in programmed cell death in a process termed anoikis.27 Anoikis resistance has been strongly implicated in the formation of distant metastases.28 Having observed increased integrin-dependent adhesion upon FER KD in breast cancer cells, we reasoned that this could lead to decreased anoikis resistance. To test this hypothesis, we cultured control and FER iKD MDA-MB-231 and SUM149PT cells in suspension and measured anoikis resistance. Interestingly, we found a significant decrease in anoikis resistance in both cell lines upon FER iKD using two independent shRNA sequences (Figure 4d and Supplementary Figure 5B). These results suggest that FER may regulate an anchorage-independent survival in breast cancer cells.
FER promotes breast tumour growth and metastasis formation
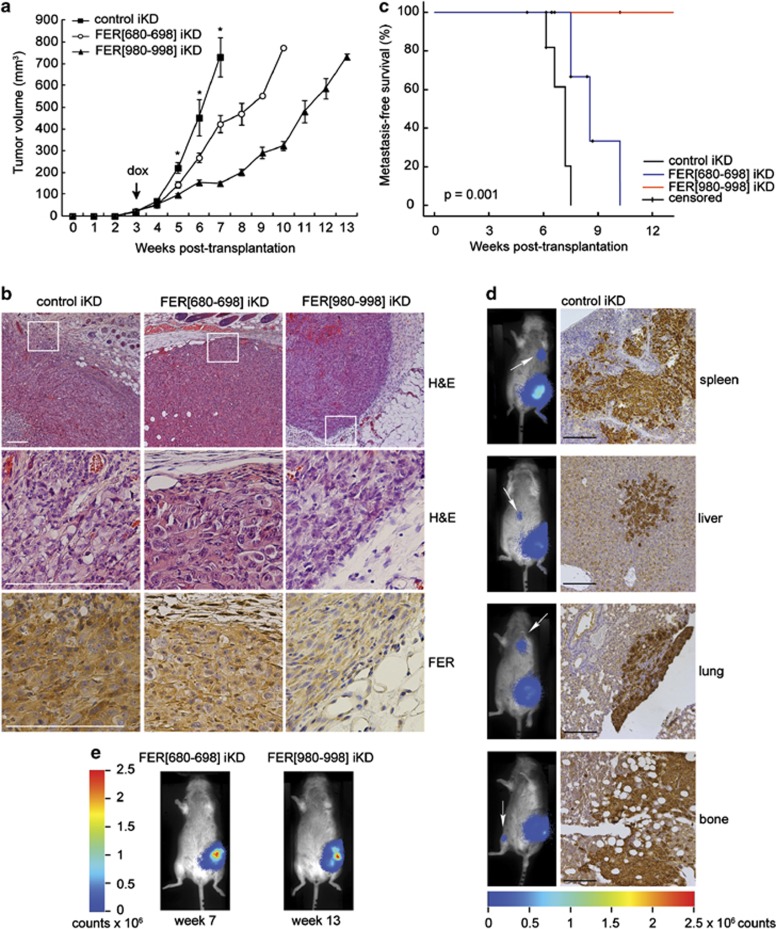
To study the role of FER in breast tumour growth and metastasis, we orthotopically transplanted luciferase-expressing MDA-MB-231 FER iKD cells in RAG2−/−;IL-2Rγc−/− immunodeficient mice29 and measured tumour growth over time. As FER KD can inhibit cell cycle progression in several cancer cell lines, including MDA-MB-231, Pasder et al.17 and (Supplementary Figure 6) we transplanted untreated cells, monitored animals until palpable tumours of ∼50 mm3 formed in all three groups and then started doxycycline administration to induce FER shRNA expression (Figure 6a, arrow). Expression of both FER shRNAs significantly inhibited tumour growth as compared with the control shRNA (Figure 6a). Furthermore, control tumours were highly invasive, whereas FER iKD resulted in non-invasive tumours with clearly defined borders that were confined to the mammary fat pad (Figure 6b). Next, we used immunohistochemistry (IHC) to detect FER protein in tumour samples. We could confirm that FER expression was downregulated in tumours expressing both FER shRNAs as compared with controls (Figure 6b). In addition, we detected higher FER levels in FER (680–698) iKD than FER (980–998) iKD tumours (Figure 6b), which is consistent with the different rates of tumour growth between the two groups (Figure 6a). Moreover, we could show using bioluminescence imaging that FER iKD inhibited the development of distant metastases (Figure 6c). All of the mice in the control group developed metastases. In total, we observed five spleen, five liver, two lung and one bone (femur) metastases (Figure 6d). In the FER(680–698) iKD group, 6/12 animals developed metastases (three lung, two spleen and one liver). Consistent with the low level FER expression in the primary tumours, mice in the FER (980–998) iKD group did not develop metastases by 14 weeks of follow-up (Figures 6c and e). As the tumour volumes in these mice at 14 weeks were comparable to those in the control group at 7 weeks (Figure 6a), the inhibition of metastasis formation was not due to reduced primary tumour size. These results indicate that FER promotes breast tumour growth and metastasis formation.
Figure 6.
FER regulates breast tumour growth and metastasis. (a) Luciferase-expressing MDA-MB-231 iKD cells were orthotopically transplanted into recipient mice. Upon development of palpable tumours, mice were switched to a doxycycline-containing diet (dox; arrow) to induce shRNA expression. Values represent the mean tumour volume±s.e.m. *P<0.05, n=12 (control and FER (980–998) iKD), n=13 (FER (680–698) iKD). (b) Haematoxylin and eosin (H&E) and IHC staining for FER in primary tumours from mice transplanted with the indicated cell lines. Scale bar=200 μm. (c) Kaplan–Meier metastasis-free survival plot. Animals were monitored by bioluminescence and killed when distant metastases developed. (d) Representative images of mice from the control group at end point. Metastatic spread was assessed using bioluminescence imaging (left; arrow). IHC staining for vimentin was performed to confirm distant metastases of control tumours. Scale bar=100 μm. (e) Representative images of mice from the FER (680–698) and FER (980–998) iKD groups at end point.
FER expression is associated with aggressive breast cancer and correlates with decreased patient survival
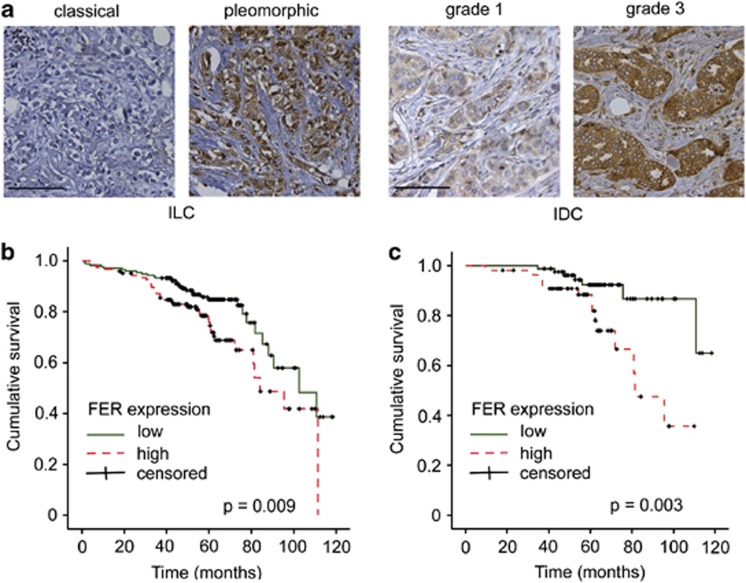
To extend our experimental findings that implicate FER in breast cancer progression, we examined its expression in a tissue microarray or 485 cases of invasive breast carcinoma (Supplementary Table 1) by IHC. Although high FER expression did not correlate with histological breast cancer subtypes, we observed that basal-type tumours showed higher FER levels than luminal-type carcinomas (P=0.003; Table 1). Within the invasive lobular carcinoma group, FER levels were significantly higher in pleomorphic (P<0.001) as compared with classical tumours (Figure 7a, Table 1). Overall, high FER expression was significantly associated with tumour size (P=0.003), tumour grade (P<0.001) and mitotic activity (P<0.001; Table 1). Consistently, high FER expression was correlated with epidermal growth factor receptor positivity (P=0.002) and was inversely correlated with oestrogen receptor-α expression (P=0.001). These results indicate that FER is predominantly expressed in breast tumours with an aggressive phenotype. We also evaluated FER expression in primary tumours of 51 breast cancer patients with confirmed distant metastases (Supplementary Table 1). Notably, FER was highly expressed in 75% of these cases (Supplementary Table 2), suggesting a link between FER and metastatic disease.
Table 1. Correlation of FER kinase expression with clinicopathological and molecular features of invasive breast cancer.
| Feature | N |
FER expression |
||
|---|---|---|---|---|
| Low (0-1+) N (%) | High (2-3+) N (%) | P-value | ||
| Histological type | ||||
| Invasive ductal cancer | 320 | 171 (53.4) | 149 (46.6) | |
| Invasive lobular cancer | 126 | 78 (61.9) | 48 (38.1) | |
| Other | 39 | 23 (59.0) | 16 (41.0) | 0.250 |
| Histological grade | ||||
| 1 | 88 | 63 (71.6) | 25 (28.4) | |
| 2 | 171 | 110 (64.3) | 61 (35.7) | |
| 3 | 219 | 94 (42.9) | 125 (57.1) | <0.001 |
| MAI (per 2 mm2) | ||||
| ⩽12 | 239 | 164 (68.6) | 75 (31.4) | |
| ⩾13 | 246 | 108 (43.9) | 138 (56.1) | <0.001 |
| Tumour size (cm) | ||||
| ⩽2 | 208 | 134 (64.4) | 74 (35.6) | |
| >2 and ⩽5 | 220 | 106 (48.2) | 114 (51.8) | |
| >5 | 49 | 29 (59.2) | 20 (40.8) | 0.003 |
| Lymph node status | ||||
| Negative | 227 | 133 (58.6) | 94 (41.4) | |
| Positive | 231 | 127 (55.0) | 104 (45.0) | 0.435 |
| Invasive lobular carcinomas | ||||
| Classical | 34 | 33 (97.1) | 1 (2.9) | |
| Pleomorphic | 56 | 17 (30.4) | 39 (69.6) | <0.001 |
| BRCA1 | ||||
| Sporadic | 459 | 266 (58.0) | 193 (42.0) | |
| Mutation carrier | 24 | 6 (25.0) | 18 (75.0) | 0.002 |
| Perou/Sorlie classification | ||||
| Luminal | 391 | 234 (59.8) | 157 (40.2) | |
| HER2 driven | 22 | 10 (45.5) | 12 (54.5) | |
| Basal/TN | 72 | 28 (38.9) | 44 (61.1) | 0.003 |
| ER | ||||
| Negative | 104 | 44 (42.3) | 60 (57.7) | |
| Positive | 381 | 228 (59.8) | 153 (40.2) | 0.001 |
| PR | ||||
| Negative | 200 | 106 (53.0) | 94 (47.0) | |
| Positive | 284 | 165 (58.1) | 119 (41.9) | 0.266 |
| HER2 | ||||
| Negative | 435 | 246 (56.6) | 189 (43.4) | |
| Positive | 49 | 25 (51.0) | 24 (49.0) | 0.460 |
| EGFR | ||||
| Negative | 393 | 233 (59.3) | 160 (40.7) | |
| Positive | 83 | 34 (41.0) | 49 (59.0) | 0.002 |
Abbreviations: EFGR, epidermal growth factor receptor; ER, oestrogen receptor; HER2, human epidermal growth factor receptor 2; MAI, mitotic activity index; PR, progesterone receptor; TN, triple negative.
Statistical significance estimated by χ2-test.
Statistically significant P-values are indicated in bold.
Figure 7.
FER kinase expression correlates with decreased patient survival. (a) Clinical specimens of invasive lobular carcinoma (ILC) and invasive ductal carcinoma (IDC) were analysed for FER kinase expression by immunohistochemistry. Note the differential FER expression in classical versus pleomorphic ILC, and grade 1 versus grade 3 IDC. Scale bar=100 μm. (b) Kaplan–Meier survival curves according to FER expression status (n=293). (c) Kaplan–Meier survival curves of patients with lymph node-negative breast cancer according to FER expression status (n=141).
In agreement with the association between FER expression and unfavourable clinicopathological variables, we found that patients with high FER-expressing tumours had a significantly worse prognosis based on overall survival (P=0.009, Figure 7b). The correlation between high FER expression and poor clinical outcome was even more pronounced in the lymph node-negative group of patients (P=0.003, Figure 7c). Multivariate analysis revealed that high FER expression was a significant independent predictor of decreased overall survival (hazard ratio: 1.785, 95% confidence interval: 1.068–2.982, P=0.027), as were lymph node status (hazard ratio: 2.868, 95% confidence interval: 1.675–4.910, P<0.001), age (hazard ratio: 1.050, 95% confidence interval: 1.030–1.069, P<0.001) and tumour grade (hazard ratio: 1.578, 95% confidence interval: 1.064–2.340, P=0.023). Other clinicopathological features were not independent prognostic factors. These clinical findings support the pro-metastatic function of FER, which we have identified in vitro and in a mouse model of breast cancer. These data indicate that the ability of FER to potentiate breast cancer cell motility and invasiveness may lead to clinically more aggressive disease and decreased patient survival.
Discussion
We established that FER is highly expressed in aggressive breast carcinomas and has a negative impact on prognosis. To our knowledge, this is the first report that indicates a role of FER in breast cancer. We found a strong correlation between high FER expression and most unfavourable clinicopathological variables, except for lymph node status. However, high FER expression correlated with a poor prognosis in the lymph node-negative group of patients. Approximately 5–10% of patients have metastatic disease at the time of surgery in the absence of lymph node involvement30 and up to 20% of lymph node-negative patients experience recurrence with distant metastases within 10 years after surgery.31 Haematogenous tumour cell dissemination in lymph node-negative breast cancer patients is associated with decreased distant disease-free survival.32 Our results indicate that FER promotes breast cancer cell migration and inhibits anchorage dependence, resulting in increased formation of distant metastases. Others have shown that haematogenous, rather than lymphatic, tumour cell dissemination leads to formation of distant metastases in a breast cancer mouse model.33 Thus, the correlation between high FER expression and decreased survival in lymph node-negative patients suggests that FER may facilitate haematogenous metastasis.
Metastatic MDA-MB-231 and SUM149PT breast cancer cells showed higher FER protein expression, as compared with other breast cancer cell lines tested. MDA-MB-231 and SUM149PT cells are hormone receptor-negative, overexpress epidermal growth factor receptor and are classified as basal-type breast cancer based on their gene expression profile.34, 35 Inhibition of FER in MDA-MB-231 and SUM149PT cells induces changes in cell morphology, including formation of actin stress fibres and FAs, which is consistent with RhoA activation. Indeed, actin stress fibre and FA formation in MDA-MB-231 cells are Rho-associated kinase-dependent events that can lead to decreased migration and increased anoikis sensitivity.36, 37 In agreement with our data that loss of FER increases α6- and β1-integrin expression and adhesion to collagen I and laminin in breast cancer cells, others have shown that FER can regulate cell adhesion in other cell types. Inhibition of FER activity increased bone marrow-derived mast cell adhesion to fibronectin,14 whereas FER overexpression in Rat-2 fibroblasts led to cell detachment and anoikis.11 Further, accumulation of FER in FA kinase/β1-integrin complexes was associated with decreased cell adhesion in neural retinal cells.12 Recent evidence suggests that FER can promote breast cancer cell migration by downregulating the synthesis of laminin-binding glycans and thus decreasing cell adhesion to laminin.16 It is therefore likely that along with increased α6β1-integrin cell surface expression, upregulation of laminin-binding glycans upon FER KD may contribute to increased adhesion to laminin. However, we also observed increased cell spreading, FA redistribution and decreased migration in FER KD cells plated on collagen I. Together with our functional integrin-blocking studies, these data suggest that the phenotypes we observe are primarily integrin dependent.
Although the exact mechanism whereby FER regulates FA formation is still unclear, these studies suggest that FER activity destabilizes FAs, thus inhibiting cell–ECM attachment. Conversely, we have shown that FER inhibition leads to FA formation, increased ECM attachment and reduced cell migration. Apart from decreased p130cas phosphorylation upon FER overexpression, the phosphorylation status of other FA molecules was unaltered.11 It is possible that FER does not directly phosphorylate FA components, but rather regulates the localization of integrin receptors. FER contains an N-terminal F-BAR domain, which is also found in proteins involved in vesicular transport and endocytosis.38 Although it has been suggested that feline sarcoma may be involved in vesicular transport,6 it has not been established whether FER can regulate integrin trafficking. Our data indicated that FER KD induces accumulation of α6-integrin at the cell membrane, as well as in endosomal vesicles, which suggests that FER may control recycling of α6-integrin in metastatic breast cancer cells.
Cortactin can associate with endocytic vesicles and regulate the internalization of several receptors.39 However, while re-secretion of internalized ECM was inhibited in cortactin KD cells, surface levels of β1-integrin were unaffected. These results suggest that cortactin regulates the trafficking of ECM components, but not β1-integrins.40 Although, cortactin was established as a FER substrate in other cell types, we have not consistently observed decreased Y421 and Y466 cortactin phosphorylation in our FER KD cell lines (data not shown). Thus, although we cannot exclude a role for cortactin in the FER-dependent phenotypes in our system, it seems unlikely that they are reliant on FER-mediated tyrosine phosphorylation. The association and coordinate trafficking of the fibronectin-binding α5β1-integrin and epidermal growth factor receptor by Rab-coupling protein promotes tumour cell migration and invasion.41 However, this mechanism may be tumour type-specific as breast cancer cells express primarily collagen- and laminin-binding integrin heterodimers.42
FER activation downstream of the integrin-reactive oxygen species pathway is necessary for cortactin phosphorylation and efficient migration in mouse embryonic fibroblasts.13 FER is also activated by phospholipase D signalling, leading to Rac1-dependent and cortactin-mediated lamellipodia formation and increased cell migration.15 The role of FER in Rho signalling is largely unexplored, but there is some evidence that it can regulate Rac1 by phosphorylating Rho guanine dissociation inhibitor-α, thereby preventing the formation of Rac1/Rho guanine dissociation inhibitor-α complexes.43 Also, FER overexpression leads to increased phosphorylation of Vav2, a direct Rac1 activator.15 Our data show that the inhibition of lamellipodia formation and migration upon FER KD is accompanied by stress fibre formation, a hallmark of active RhoA. Conversely, we observed that FER overexpression in MCF10.CA1d cells leads to increased lamellipodia formation and migration, which is consistent with Rac1 activation. As it is assumed that active Rac counterbalances the activity of Rho,44 these results imply that FER KD may lead to indirect activation of Rho through the inhibition of Rac. This in turn would inhibit migration and invasion, while promoting cell–ECM adhesion.
In this study, we have demonstrated that FER regulates breast tumour growth and metastasis. FER promotes breast cancer growth and metastasis in a cell autonomous manner through inhibition of adhesion and anchorage dependence. Significantly, we have also shown that FER expression is correlated to prognosis in breast cancer patients. Thus, the development of therapeutic strategies based on inhibition of FER signalling may be beneficial in the treatment of metastatic breast cancer.
Materials and methods
Cell culture and transfections
MCF10A, MCF10CA1a, MCF10CA1a.cl1 and MCF10CA1d.cl1 cells45 were obtained from the Barbara Ann Karmanos Cancer Institute (Detroit, MI, USA). All other cell lines, except MDA-MB-453, ZR-75–30, CAMA-1, SKBR3, MDA-MB-330 (ATCC, Manassas, VA, USA), were from Cell Lines Service (Eppelheim, Germany). SUM44PE and SUM149PT were obtained from Asterand, Inc. (Detroit, MI, USA), and MDA-MB-231/Luc+ have been previously described.46 Cell lines from non-commercial sources were authenticated by short tandem repeat profiling. Cell culture conditions are described in the Supplementary Methods.
Silencer Negative Control #1 (AM4611) and Silencer Validated siRNAs targeting FER kinase (AM51323; siRNA ID# 657 and 658) were purchased from Ambion (Breda, The Netherlands). MDA-MB-231 cells were transfected with 80 nM siRNA using Lipofectamine 2000 transfection reagent (11668500; Invitrogen, Breda, The Netherlands).
Constructs, virus generation and cell transduction
The cloning strategy used to generate lentiviral vectors encoding wild-type and kinase dead (D742R)47 FER (D742D FER) is described in the Supplementary Methods. Inducible shRNA-expressing lentivirus construct generation, lentivirus production and transduction have been described previously.48 Oligonucleotide sequences are shown in the Supplementary Methods.
Immunoblotting
Cell lysates were prepared and analysed as described.49
Immunofluorescence analysis
Specimens for immunofluorescence microscopy were prepared as described.50 Antibody sources and dilutions are described in the Supplementary Methods. Confocal analysis was conducted with a Plan-Apochromat 63 × /1.40 Oil DIC M27 objective mounted on an inverted Carl Zeiss LSM 700 Laser Scanning Microscope. Images were acquired and analysed with ZEN 2010 software (Carl Zeiss International, Sliedrecht, The Netherlands).
Adhesion assay
Adhesion assays were performed as described,51 with some modifications. Wells of 96-well tissue culture plates were coated with collagen I (8 μg/cm2), fibronectin (3 μg/cm2) or laminin (10 μg/cm2) overnight at 4 °C.
Integrin expression measurement
Cells were prepared for flow cytometry as described.51 Antibody sources and dilutions are described in the Supplementary Methods. Integrin expression was measured with a FACSCalibur flow cytometer (BD Biosciences) using CellQuest Pro software.
Migration assay
The 96-well Oris Cell Migration Kit (CMAU101; Platypus Technologies, Fitchburg, WI, USA) was used for cell migration assays. Wells were coated with 8 μg/cm2 collagen I (C3867; Sigma, Gillingham, UK) for 2 h at room temperature. Following two washes in phosphate-buffered saline, silicone stoppers were inserted in the wells to create ‘exclusion zones'. Cells were plated in triplicate wells (15 000 cells per well) and incubated at 37 °C overnight. The medium was replaced with serum-free Dulbecco's modified Eagle's medium/2.5% bovine serum albumin and cells were incubated for 8 h at 37 °C before removing the silicone stoppers to start the assay by allowing cells to migrate into the exclusion zone. Cells were incubated for 24 h at 37 °C, fixed with 3.7% formaldehyde and stained with 0.05% crystal violet. Migration was quantified by taking low magnification images of each well, measuring the remaining area of the exclusion zone using Adobe Photoshop CS3 Extended and comparing it with the exclusion zone area in reference wells (no migration).
Matrigel outgrowth assay
BD Matrigel Basement Membrane Matrix (356234; BD Biosciences) was diluted 1:1 with normal growth medium, and 40 μl per well were added to 96-well, flat bottom, optical plastic plates (3720; Corning, Tewksbury, MA, USA). Plates were incubated at 37 °C for 15 min to allow the Matrigel to solidify. MDA-MB-231 cells were seeded at a density of 3000 cells per well in 200 μl of normal growth medium and cultured for 4 days at 37 °C. Cultures were fixed with 3.7% formaldehyde/phosphate-buffered saline for 30–40 min, followed by permeabilization with 0.1% Triton-X/phosphate-buffered saline for 20 min at room temperature. Confocal analysis was conducted with a long working distance LD Plan-Neofluar 40 × /0.6 Korr M27 objective mounted on an inverted Carl Zeiss LSM 700 Laser Scanning Microscope. Images were acquired and analysed with ZEN 2010 software (Carl Zeiss International).
Anoikis assay
MDA-MB-231 cells were seeded in Ultra Low Attachment 24-well plates (3473; Corning) at 100 000 cells per well in duplicate and cultured in Dulbecco's modified Eagle's medium containing 1% fetal bovine serum at 37 °C for 72 h. The percentage of anoikis-resistant cells was determined as described.48
Mouse studies
Female RAG2−/−;IL-2Rγc−/− immunodeficient mice29 were a kind gift from The Netherlands Cancer Institute, Amsterdam, The Netherlands. Orthotopic transplantations and bioluminescence imaging were performed as described,48 with some modifications. Approximately 1 × 106 luciferase-expressing MDA-MB-231 cells were injected using a 50-μl Hamilton syringe (Hamilton, Bonadur, Switzerland). Tumour growth was measured using a digital calliper (VWR, Radnor, PA, USA) on a weekly basis. Upon development of palpable tumours, mice were switched from a standard diet to doxycycline-containing chow (S3888; Bio-Serv, San Diego, CA, USA) for the remainder of the experiment. Mice were killed when tumour volume exceeded 1000 mm3 or when bioluminescence imaging revealed metastases.
Clinical samples
The clinical sample sets and tissue microarray assembly have been previously described.52, 53
Immunohistochemistry
IHC antigen retrieval methods, antibodies and detection have been described previously.52 The protocol used to detect FER was as follows: antigen retrieval by boiling for 20 min in 10 mM citrate pH 6.0. A cooling period of 30 min, followed by incubation with anti-FER antibody (1:300; clone 5D2, Cell Signaling Technologies, Danvers, MA, USA) for 1 h at room temperature.
Scoring of immunohistochemistry
All scoring was done by two individual observers blinded to patient characteristics and results of other staining, as described.52 FER staining was scored based on the intensity of the cytosolic staining, scores of 0 and 1+ were scored as low, and 2+ and 3+ were scored as high intensity.
Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics version 18.0 (SPSS Inc., Chicago, IL, USA). For clinical samples, associations between categorical variables were examined using Pearson's χ2-test. Survival analysis was performed with the Kaplan–Meier method. Multivariate analysis was performed using Cox regression with the backward-step likelihood ratio model. In the model, tumour size, tumour grade, age, lymph node status, histology, mitotic activity index, Sorlie/Perou classification, BRCA mutation status and FER expression were taken into account.
Differences in tumour volume were evaluated using a two-way mixed model analysis of variance with time (weeks) as the repeated factor and group (control, FER (680–698) and FER (980–998)) as factor 2. Tukey's test was used for post hoc analysis. All other data were analysed with one-way analysis of variance. Data are presented as mean±s.e.m. P-values <0.05 were considered to be statistically significant.
Study approval
All animal experiments were approved by the Utrecht University Animal Experimental Committee (DEC-Utrecht number 2010.III.10.120). The use of anonymous or coded leftover material for scientific purposes is part of the standard treatment contract with patients in the Netherlands.54 Ethical approval was not required.
Acknowledgments
We thank N Heisterkamp for providing the pUHG10-3(FER) vector, E Danen and A Sonnenberg for anti-integrin antibodies and L Price for help with the Matrigel assays. Luciferase-expressing MDA-MB-231 cells were a kind gift from G van der Pluijm. We are grateful to C Pfauth, T Westphal and the Netherlands Cancer Institute for providing RAG2−/−;IL2Rγc−/− recipient mice. M van Amersfoort and D van der Giezen are acknowledged for expert technical assistance with the mouse experiments and F Morsink for performing the vimentin IHC staining. We also thank S Huveneers for critically reviewing this manuscript. This research is supported by an unrestricted educational grant from Aegon Inc. and The Netherlands Organization for Scientific Research Grant (NWO-VIDI 917.96.318). IA Ivanova is a Research Fellow of the Terry Fox Foundation (Award #700041).
The authors declare no conflicts of interest.
Footnotes
Supplementary Information accompanies this paper on the Oncogene website (http://www.nature.com/onc)
Supplementary Material
References
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2008;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J Clin. 2010;2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- Hudis CA. Trastuzumab: mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- Heath RJ, Insall RH. F-BAR domains: multifunctional regulators of membrane curvature. J Cell Sci. 2008;121:1951–1954. doi: 10.1242/jcs.023895. [DOI] [PubMed] [Google Scholar]
- Pawson T, Letwin K, Lee T, Hao QL, Heisterkamp N, Groffen J. The FER gene is evolutionarily conserved and encodes a widely expressed member of the FPS/FES protein-tyrosine kinase family. Mol Cell Biol. 1989;9:5722–5725. doi: 10.1128/mcb.9.12.5722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirngibl R, Schulze D, Mirski SE, Cole SP, Greer PA. Subcellular localization analysis of the closely related Fps/Fes and Fer protein-tyrosine kinases suggests a distinct role for Fps/Fes in vesicular trafficking. Exp Cell Res. 2001;266:87–94. doi: 10.1006/excr.2001.5217. [DOI] [PubMed] [Google Scholar]
- Kim L, Wong TW. The cytoplasmic tyrosine kinase FER is associated with the catenin-like substrate pp120 and is activated by growth factors. Mol Cell Biol. 1995;15:4553–4561. doi: 10.1128/mcb.15.8.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim L, Wong TW. Growth factor-dependent phosphorylation of the actin-binding protein cortactin is mediated by the cytoplasmic tyrosine kinase FER. J Biol Chem. 1998;273:23542–23548. doi: 10.1074/jbc.273.36.23542. [DOI] [PubMed] [Google Scholar]
- Xu G, Craig AW, Greer P, Miller M, Anastasiadis PZ, Lilien J, et al. Continuous association of cadherin with beta-catenin requires the non-receptor tyrosine-kinase Fer. J Cell Sci. 2004;117:3207–3219. doi: 10.1242/jcs.01174. [DOI] [PubMed] [Google Scholar]
- El Sayegh TY, Arora PD, Fan L, Laschinger CA, Greer PA, McCulloch CA, et al. Phosphorylation of N-cadherin-associated cortactin by Fer kinase regulates N-cadherin mobility and intercellular adhesion strength. Mol Biol Cell. 2005;16:5514–5527. doi: 10.1091/mbc.E05-05-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosato R, Veltmaat JM, Groffen J, Heisterkamp N. Involvement of the tyrosine kinase fer in cell adhesion. Mol Cell Biol. 1998;18:5762–5770. doi: 10.1128/mcb.18.10.5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arregui C, Pathre P, Lilien J, Balsamo J. The nonreceptor tyrosine kinase fer mediates cross-talk between N-cadherin and beta1-integrins. J Cell Biol. 2000;149:1263–1274. doi: 10.1083/jcb.149.6.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangrar W, Gao Y, Scott M, Truesdell P, Greer PA. Fer-mediated cortactin phosphorylation is associated with efficient fibroblast migration and is dependent on reactive oxygen species generation during integrin-mediated cell adhesion. Mol Cell Biol. 2007;27:6140–6152. doi: 10.1128/MCB.01744-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AW, Greer PA. Fer kinase is required for sustained p38 kinase activation and maximal chemotaxis of activated mast cells. Mol Cell Biol. 2002;22:6363–6374. doi: 10.1128/MCB.22.18.6363-6374.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T, Hasegawa J, Tsujita K, Kanaho Y, Takenawa T. The tyrosine kinase Fer is a downstream target of the PLD-PA pathway that regulates cell migration. Sci Signal. 2009;2:ra52. doi: 10.1126/scisignal.2000393. [DOI] [PubMed] [Google Scholar]
- Yoneyama T, Angata K, Bao X, Courtneidge S, Chanda SK, Fukuda M. Fer kinase regulates cell migration through alpha-dystroglycan glycosylation. Mol Biol Cell. 2012;23:771–780. doi: 10.1091/mbc.E11-06-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasder O, Shpungin S, Salem Y, Makovsky A, Vilchick S, Michaeli S, et al. Downregulation of Fer induces PP1 activation and cell-cycle arrest in malignant cells. Oncogene. 2006;25:4194–4206. doi: 10.1038/sj.onc.1209695. [DOI] [PubMed] [Google Scholar]
- Voisset E, Lopez S, Chaix A, Georges C, Hanssens K, Prebet T, et al. FES kinases are required for oncogenic FLT3 signaling. Leukemia. 2010;24:721–728. doi: 10.1038/leu.2009.301. [DOI] [PubMed] [Google Scholar]
- Allard P, Zoubeidi A, Nguyen LT, Tessier S, Tanguay S, Chevrette M, et al. Links between Fer tyrosine kinase expression levels and prostate cell proliferation. Mol Cell Endocrinol. 2000;159:63–77. doi: 10.1016/s0303-7207(99)00205-1. [DOI] [PubMed] [Google Scholar]
- Zoubeidi A, Rocha J, Zouanat FZ, Hamel L, Scarlata E, Aprikian AG, et al. The Fer tyrosine kinase cooperates with interleukin-6 to activate signal transducer and activator of transcription 3 and promote human prostate cancer cell growth. Mol Cancer Res. 2009;7:142–155. doi: 10.1158/1541-7786.MCR-08-0117. [DOI] [PubMed] [Google Scholar]
- Li H, Ren Z, Kang X, Zhang L, Li X, Wang Y, et al. Identification of tyrosine-phosphorylated proteins associated with metastasis and functional analysis of FER in human hepatocellular carcinoma cells. BMC Cancer. 2009;9:366. doi: 10.1186/1471-2407-9-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C, Wu S, Li X, Wang Y, Ren R, Lai Y, et al. High expression of FER tyrosine kinase predicts poor prognosis in clear cell renal cell carcinoma. Oncol Lett. 2013;5:473–478. doi: 10.3892/ol.2012.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata Y, Kanda S, Sakai H, Greer PA. Fer expression correlates with malignant aggressiveness and poor prognosis in renal cell carcinoma. Cancer Sci. 104:681–686. doi: 10.1111/cas.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Stark GR. FER tyrosine kinase (FER) overexpression mediates resistance to quinacrine through EGF-dependent activation of NF-kappaB. Proc Natl Acad Sci USA. 2011;108:7968–7973. doi: 10.1073/pnas.1105369108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae JM, Creighton CJ, Meck JM, Haddad BR, Johnson MD. MDA-MB-435 cells are derived from M14 melanoma cells: a loss for breast cancer, but a boon for melanoma research. Breast Cancer Res Treat. 2007;104:13–19. doi: 10.1007/s10549-006-9392-8. [DOI] [PubMed] [Google Scholar]
- Kenny PA, Lee GY, Myers CA, Neve RM, Semeiks JR, Spellman PT, et al. The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression. Mol Oncol. 2007;1:84–96. doi: 10.1016/j.molonc.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derksen PW, Liu X, Saridin F, van der Gulden H, Zevenhoven J, Evers B, et al. Somatic inactivation of E-cadherin and p53 in mice leads to metastatic lobular mammary carcinoma through induction of anoikis resistance and angiogenesis. Cancer Cell. 2006;10:437–449. doi: 10.1016/j.ccr.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Gimeno R, Weijer K, Voordouw A, Uittenbogaart CH, Legrand N, Alves NL, et al. Monitoring the effect of gene silencing by RNA interference in human CD34+ cells injected into newborn RAG2−/− gammac−/− mice: functional inactivation of p53 in developing T cells. Blood. 2004;104:3886–3893. doi: 10.1182/blood-2004-02-0656. [DOI] [PubMed] [Google Scholar]
- Leone BA, Romero A, Rabinovich MG, Vallejo CT, Bianco A, Perez JE, et al. Stage IV breast cancer: clinical course and survival of patients with osseous versus extraosseous metastases at initial diagnosis. The GOCS (Grupo Oncologico Cooperativo del Sur) experience. Am J Clin Oncol. 1988;11:618–622. [PubMed] [Google Scholar]
- Rosen PR, Groshen S, Saigo PE, Kinne DW, Hellman S. A long-term follow-up study of survival in stage I (T1N0M0) and stage II (T1N1M0) breast carcinoma. J Clin Oncol. 1989;7:355–366. doi: 10.1200/JCO.1989.7.3.355. [DOI] [PubMed] [Google Scholar]
- Braun S, Cevatli BS, Assemi C, Janni W, Kentenich CR, Schindlbeck C, et al. Comparative analysis of micrometastasis to the bone marrow and lymph nodes of node-negative breast cancer patients receiving no adjuvant therapy. J Clin Oncol. 2001;19:1468–1475. doi: 10.1200/JCO.2001.19.5.1468. [DOI] [PubMed] [Google Scholar]
- Giampieri S, Manning C, Hooper S, Jones L, Hill CS, Sahai E. Localized and reversible TGFbeta signalling switches breast cancer cells from cohesive to single cell motility. Nat Cell Biol. 2009;11:1287–1296. doi: 10.1038/ncb1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10:515–527. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao J, Salari K, Bocanegra M, Choi YL, Girard L, Gandhi J, et al. Molecular profiling of breast cancer cell lines defines relevant tumor models and provides a resource for cancer gene discovery. PLoS One. 2009;4:e6146. doi: 10.1371/journal.pone.0006146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew CT, Aronchik I, Kosco K, McCammon J, Bjeldanes LF, Firestone GL. Indole-3-carbinol inhibits MDA-MB-231 breast cancer cell motility and induces stress fibers and focal adhesion formation by activation of Rho kinase activity. Int J Cancer. 2009;124:2294–2302. doi: 10.1002/ijc.24210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadwaj S, Thanawala R, Bon G, Falcioni R, Prasad GL. Resensitization of breast cancer cells to anoikis by tropomyosin-1: role of Rho kinase-dependent cytoskeleton and adhesion. Oncogene. 2005;24:8291–8303. doi: 10.1038/sj.onc.1208993. [DOI] [PubMed] [Google Scholar]
- Greer P. Closing in on the biological functions of Fps/Fes and Fer. Nat Rev Mol Cell Biol. 2002;3:278–289. doi: 10.1038/nrm783. [DOI] [PubMed] [Google Scholar]
- MacGrath SM, Koleske AJ. Cortactin in cell migration and cancer at a glance. J Cell Sci. 2012;125:1621–1626. doi: 10.1242/jcs.093781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung BH, Zhu X, Kaverina I, Weaver AM. Cortactin controls cell motility and lamellipodial dynamics by regulating ECM secretion. Curr Biol. 2011;21:1460–1469. doi: 10.1016/j.cub.2011.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caswell PT, Chan M, Lindsay AJ, McCaffrey MW, Boettiger D, Norman JC. Rab-coupling protein coordinates recycling of alpha5beta1 integrin and EGFR1 to promote cell migration in 3D microenvironments. J Cell Biol. 2008;183:143–155. doi: 10.1083/jcb.200804140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills GB, Jurisica I, Yarden Y, Norman JC. Genomic amplicons target vesicle recycling in breast cancer. J Clin Invest. 2009;119:2123–2127. doi: 10.1172/JCI40256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei F, Kweon SM, Haataja L, De Sepulveda P, Groffen J, Heisterkamp N. The Fer tyrosine kinase regulates interactions of Rho GDP-dissociation inhibitor alpha with the small GTPase Rac. BMC Biochem. 2010;11:48. doi: 10.1186/1471-2091-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander EE, ten Klooster JP, van Delft S, van der Kammen RA, Collard JG. Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J Cell Biol. 1999;147:1009–1022. doi: 10.1083/jcb.147.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santner SJ, Dawson PJ, Tait L, Soule HD, Eliason J, Mohamed AN, et al. Malignant MCF10CA1 cell lines derived from premalignant human breast epithelial MCF10AT cells. Breast Cancer Res Treat. 2001;65:101–110. doi: 10.1023/a:1006461422273. [DOI] [PubMed] [Google Scholar]
- Wetterwald A, van der Pluijm G, Que I, Sijmons B, Buijs J, Karperien M, et al. Optical imaging of cancer metastasis to bone marrow: a mouse model of minimal residual disease. Am J Pathol. 2002;160:1143–1153. doi: 10.1016/S0002-9440(10)64934-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AW, Zirngibl R, Williams K, Cole LA, Greer PA. Mice devoid of fer protein-tyrosine kinase activity are viable and fertile but display reduced cortactin phosphorylation. Mol Cell Biol. 2001;21:603–613. doi: 10.1128/MCB.21.2.603-613.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schackmann RC, van Amersfoort M, Haarhuis JH, Vlug EJ, Halim VA, Roodhart JM, et al. Cytosolic p120-catenin regulates growth of metastatic lobular carcinoma through Rock1-mediated anoikis resistance. J Clin Invest. 2011;121:3176–3188. doi: 10.1172/JCI41695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova IA, D′Souza SJ, Dagnino L. E2F1 stability is regulated by a novel-PKC/p38beta MAP kinase signaling pathway during keratinocyte differentiation. Oncogene. 2006;25:430–437. doi: 10.1038/sj.onc.1208999. [DOI] [PubMed] [Google Scholar]
- Vespa A, D'Souza SJ, Dagnino L. A novel role for integrin-linked kinase in epithelial sheet morphogenesis. Mol Biol Cell. 2005;16:4084–4095. doi: 10.1091/mbc.E05-02-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danen EH, Sonneveld P, Brakebusch C, Fassler R, Sonnenberg A. The fibronectin-binding integrins alpha5beta1 and alphavbeta3 differentially modulate RhoA-GTP loading, organization of cell matrix adhesions, and fibronectin fibrillogenesis. J Cell Biol. 2002;159:1071–1086. doi: 10.1083/jcb.200205014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen JF, van de Ven RA, Ercan C, van der Groep P, van der Wall E, Bult P, et al. Nuclear kaiso expression is associated with high grade and triple-negative invasive breast cancer. PLoS One. 2012;7:e37864. doi: 10.1371/journal.pone.0037864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoefnagel LD, van de Vijver MJ, van Slooten HJ, Wesseling P, Wesseling J, Westenend PJ, et al. Receptor conversion in distant breast cancer metastases. Breast Cancer Res. 2010;12:R75. doi: 10.1186/bcr2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Diest PJ. No consent should be needed for using leftover body material for scientific purposes. BMJ. 2002;325:648–651. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.