Abstract
Objective:
Neuropsychological functioning of individuals with attention-deficit/hyperactivity disorder (ADHD) or heavy prenatal alcohol exposure has been well documented independently. This study examined the interaction between both factors on cognitive performance in children.
Method:
As part of a multisite study, 344 children (8-16y, M=12.28, SD=2.52) completed a comprehensive neuropsychological battery. Four subject groups were tested: children with histories of heavy prenatal alcohol exposure (AE) and ADHD (AE+, n=90), alcohol-exposed without ADHD, (AE−, n=38), non-exposed with ADHD (ADHD, n=80), and non-exposed without ADHD (CON, n=136).
Results:
Separate 2(AE) × 2(ADHD) MANCOVAs revealed significant main and interactive effects of ADHD and AE on overall WISC-IV, D-KEFS, and CANTAB performance. Individual ANOVAs revealed significant interactions on 2 WISC-IV indices [Verbal Comprehension (VCI), Perceptual Reasoning (PRI)], and four D-KEFS and CANTAB subtests [Design Fluency, Verbal Fluency, Trail Making, Spatial Working Memory]. Follow-up analyses demonstrated no difference between AE+ and AE− groups on any measures. The combined AE+/− group demonstrated more severe impairment than the ADHD group on VCI and PRI, but there were no other differences between clinical groups.
Conclusions:
These results support a combined AE+/− group for neuropsychological research and indicate that, in some cases, the neuropsychological effects seen in ADHD are altered by prenatal alcohol exposure. The effects of alcohol exposure on verbal comprehension and perceptual reasoning were greater than those related to having ADHD without alcohol exposure, although both conditions independently resulted in cognitive impairment compared to controls. Clinically, these findings demonstrate task-dependent patterns of impairment across clinical disorders.
Keywords: fetal alcohol spectrum disorders (FASD), fetal alcohol syndrome (FAS), Attention-deficit/hyperactivity disorder (ADHD), neurobehavioral profile, specificity
Heavy prenatal alcohol exposure is a leading cause of birth defects, developmental disorders, and intellectual disability (American Academy of Pediatrics Committee on Substance Abuse and Committee on Children With Disabilities, 2000). Attention-deficit/hyperactivity disorder (ADHD) is also a significant contributor to developmental disability and the most common childhood behavioral issue (Blanchard, Gurka, & Blackman, 2006; Williams, Klinepeter, Palmes, Pulley, & Foy, 2004). Children with histories of heavy prenatal alcohol exposure or ADHD are at risk for a wide range of impairments including behavioral and neuropsychological deficits. Although alcohol-exposed children often meet diagnostic criteria for ADHD (Fryer, McGee, Matt, Riley, & Mattson, 2007), the interaction between these clinical disorders on neuropsychological function is unclear.
The devastating neuropsychological, behavioral and physical effects of heavy prenatal alcohol exposure occur on a continuum. Fetal alcohol spectrum disorders (FASD) encompass the wide range of outcomes resulting from heavy prenatal alcohol exposure, including neuropsychological and neurobehavioral deficits, and pertains to affected children with and without fetal alcohol syndrome (FAS; Mattson & Riley, 1998; Mattson, Riley, Gramling, Delis, & Jones, 1997, 1998). While the presence of FAS (Bertrand, Floyd & Weber, 2005; Hoyme et al., 2005) enables identification of some children with histories of heavy prenatal alcohol exposure, most affected individuals do not exhibit obvious dysmorphology, which greatly hinders identification (Bertrand, et al., 2005; Sampson et al., 1997). Children with heavy prenatal alcohol exposure who do not meet the specific diagnostic criteria for FAS may still exhibit similar neuropsychological impairments as those with FAS and thus, current research generally combines heavily exposed individuals with and without FAS in order to study the full range of deficits resulting from alcohol’s effects (e.g., Mattson, Crocker, & Nguyen, 2011; Mattson & Riley, 1998; Mattson, Riley, Gramling, Delis, & Jones, 1997, 1998). Current estimated prevalence rates are 0.2-0.7% for FAS, and 2-5% for FASD (May et al., 2009). Higher rates of FAS and FASD have been documented internationally (May et al., 2000).
Variability in degrees of impairment, and the lack of fully encompassing diagnostic physical features complicate accurate clinical identification of alcohol-exposed children (Mattson & Riley, 2011). In an attempt to improve identification of non-dysmorphic alcohol-exposed children across the spectrum of effects, research has increasingly focused on the development of a differential profile of neuropsychological and behavioral abilities in children with FASD. If successful, the profile would facilitate more precise diagnostic criteria for identification and improve treatment by specifically defining the nature of alcohol-related deficits (Mattson & Riley, 2011). Neuropsychological impairments of children with heavy prenatal alcohol exposure occur across a wide range of cognitive domains (for review see Mattson, Crocker, et al., 2011). These impairments can be devastating and lifelong, (for review, see Bay & Kesmodel, 2011; Coles, Lynch, Kable, Johnson, & Goldstein, 2010; Kable & Coles, 2004; Mattson & Riley, 1998). Even with continued effort to refine the neurobehavioral profile of FASD, neither the range of deficits nor their specificity, is fully understood.
The overlap with other distinct clinical conditions with similar clinical presentations, such as ADHD, further limits the ability to specifically identify alcohol-exposed individuals, especially those without FAS (Mattson & Riley, 2011). Rates of ADHD in FASD are much higher than the rates in the general population (for review see O'Connor & Paley, 2009), and exceed 60% in the U.S. (Fryer, et al., 2007) and Canada (Rasmussen et al., 2010), including Native American populations (Burd, Klug, Martsolf, & Kerbeshian, 2003), and Eastern European adoptees (Landgren, Svensson, Strömland, & Grönlund, 2010). Children with a diagnosis of ADHD present with neuropsychological impairments similar to those apparent in alcohol-exposed children (e.g., executive functioning deficits and attention impairment (for review see Mattson, Crocker, et al., 2011), which further hinders differentiation between the disorders. Subsequently, a critical area of research has emerged, focusing on differentiation of neurobehavioral impairments in alcohol-exposed children from impairments that are typical of non-exposed children with ADHD (Coles et al., 1997; Crocker, Vaurio, Riley, & Mattson, 2009; Crocker, Vaurio, Riley, & Mattson, 2011; Greenbaum, Stevens, Nash, Koren, & Rovet, 2009; Kooistra, Crawford, Gibbard, Ramage, & Kaplan, 2010; Kooistra et al., 2009; Vaurio, et al., 2008). Studies have also compared children with ADHD with and without prenatal alcohol exposure (Burden et al., 2010). A small number of studies have been recently conducted comparing alcohol-exposed children with and without concomitant ADHD focused on behavioral functioning, including sluggish cognitive tempo, adaptive behavior, psychopathology, and problem behaviors (Graham et al., 2012; Ware, Crocker, et al., 2012; Ware, O'Brien, et al., 2012). These behavioral studies found exacerbated effects of having both prenatal alcohol exposure and ADHD compared to alcohol exposure alone. In contrast, neuropsychological function has not been adequately examined with this design. Although one small study found minimal differences between alcohol-exposed children with and without ADHD on measures of attention, cognition, communication, memory, executive function and adaptive behavior (Rasmussen, et al., 2010), there was no control group against which to gauge performance of the alcohol-exposed subjects. Given these results, research addressing the combined effect of alcohol exposure and ADHD on neuropsychological abilities is necessary to understand possible exacerbated effects, facilitate identification, and inform therapeutic interventions.
This study aimed to extend current knowledge on understanding the effects of heavy prenatal alcohol exposure and ADHD, both in conjunction and independently, on three major assessment measures of neuropsychological functioning. Based on the previous behavioral studies showing exacerbated deficits in children with prenatal alcohol exposure and ADHD, beyond the impairment expected for each disorder individually, we hypothesized that (1) children in the clinical groups (e.g., those with either heavy prenatal alcohol exposure or an ADHD diagnosis or both), would show deficits in neuropsychological functioning compared to controls, and (2) alcohol-exposed children with ADHD would exhibit more severe neuropsychological impairments compared to alcohol-exposed children without ADHD or non-exposed children with ADHD.
Method
Subjects
Children (N = 344) between the ages of 8-16 years (M = 12.28, SD = 2.52) were recruited for an ongoing multisite study conducted by the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD). The clinical projects included in the CIFASD have been described previously (Mattson et al., 2010). Recruitment methods differed by site location. While the CIFASD has ongoing international recruitment sites, only data collected in United States testing centers were considered in this study to minimize the potential impact of cultural or language bias on neuropsychological tests. Data were analyzed across several sites: Atlanta, Los Angeles, Northern Plains States (seven different communities throughout North Dakota, South Dakota, and Montana), Albuquerque, and San Diego.
Primary caregivers completed specific modules from the clinician-assisted National Institute of Mental Health Diagnostic Interview Schedule for Children-IV on the same day as the neuropsychological battery [C-DISC-4.0; (Shaffer, Fisher, Lucas, Dulcan, & Schwab-Stone, 2000)]. The C-DISC-4.0 is a 90-120 minute computerized structured diagnostic interview based on the DSM-IV, administered to caregivers of children between the ages of 8-17 years old (American Psychiatric Association, 2000; Shaffer, et al., 2000). The C-DISC-4.0 assesses the presence of psychiatric diagnoses, including ADHD, as defined by the DSM-IV by evaluating clinical symptoms experienced by their child during the past month, six months, year, and whole life. For the purposes of this research study, diagnoses were derived via computerized algorithms, and the symptoms over the past six months were used to designate a positive ADHD diagnosis.
Children with histories of heavy prenatal alcohol exposure were recruited retrospectively and had confirmed histories of heavy prenatal alcohol exposure defined as maternal consumption of more than four alcoholic drinks per occasion at least once per week or at least 14 drinks per week on average repeatedly during pregnancy. Several standard methods were used to confirm prenatal alcohol exposure, including medical history, birth records, social services records, and, when available, maternal report (Mattson, et al., 2010). In order to determine alcohol-related diagnoses, a member of the CIFASD Dysmorphology Core evaluated each study subject using a standardized assessment following the CIFASD Dysmorphology Core diagnostic criteria, which have been published elsewhere (Jones et al., 2006).
Four subject groups were included in this study, two with histories of heavy prenatal alcohol exposure and two without such history. Alcohol-exposed (AE) children were divided into two groups: those who met DSM-IV criteria for an ADHD diagnosis according to the C-DISC-4.0 (AE+; n = 90) and those who did not (AE−; n = 38). Of the children with histories of heavy prenatal alcohol exposure, 34 (26.5%) met diagnostic criteria for FAS [AE+; n = 23 (25.6%), AE−; n = 11 (28.9%)]. Children without prenatal alcohol exposure were also divided into two groups. The ADHD clinical comparison group (ADHD; n = 80) consisted of children who met DSM-IV diagnostic criteria for ADHD per the C-DISC-4.0. The control group (CON; n = 136) consisted of children who did not meet C-DISC-4.0 diagnostic criteria for ADHD. Children who had subclinical symptoms of ADHD, per the C-DISC-4.0, defined as having more than minimal symptoms, (greater than 3), yet not meeting the threshold of 6 or more symptoms, were excluded from all groups.
The non-exposed comparison groups (ADHD, CON) were screened for prenatal alcohol exposure and were included only if there was less than minimal exposure (defined as one drink per week on average and never more than two drinks on a single occasion) throughout gestation. In addition to group-specific criteria, all subjects had the following exclusion criteria: non-fluent in English, history of significant head injury or loss of consciousness > 30 minutes, adopted from abroad after age 5 or < 2 years before assessment, evidence of other known causes of mental deficiency (e.g. congenital hypothyroidism, chromosomal abnormalities, neurofibromatosis), or psychiatric or physical disability that prevented study completion.
Subjects were administered a comprehensive standardized neuropsychological test battery in a single session by a trained examiner who was blind to subject group. Informed written consent and assent were obtained from all subjects and primary caregivers prior to testing. All children were asked to abstain from medication on the day of testing, however not all subjects were medication naïve and not all were able to abstain during testing. Incentive was provided to both children and caregivers for participation. The Institutional Review Board (IRB) at San Diego State University and other CIFASD sites approved this study.
Procedure
This study utilized three well-regarded and psychometrically sound neuropsychological measures to assess cognitive functioning. See Table 1 for subtest descriptions. Scaled or z-scores based on age were used for all dependent measures.
Table 1.
Descriptions of Neuropsychological Measures
| Measure | Subtest | Description |
|---|---|---|
| WISC-IV | Verbal comprehension | Index score of three components (vocabulary, similarities, comprehension) assessing verbal concept formation and reasoning. |
| Perceptual reasoning | Index score of four components (block design, picture concepts, matrix reasoning, picture completion) assessing visuospatial processing, cognitive flexibility, and nonverbal reasoning. |
|
| Working memory | Index score of three components (digit span, letter-number sequencing, arithmetic) assessing attention, concentration, mental control and working memory. | |
| Processing speed | Index score of three components (coding, symbol search, cancellation) assessing speed of processing visual stimuli. | |
| D-KEFS | Design fluency switching | Scaled score of switching condition requiring rule change between production of design using a series of dots assessing fluency in generating visual designs, rule following and problem solving. |
| Color-word interference inhibition |
Scaled score of time taken to read the ink color a word is printed in (different from the actual word). while inhibiting the natural verbal reading response assessing cognitive flexibility and inhibitory control. |
|
| Color-word interference inhibition/switching |
Scaled score of time taken to complete switching between inhibitory and noninhibites responses of color naming and word reading assessing cognitive flexibility and inhibitory control. |
|
| Twenty questions | Scaled score of the quality of an intial question asked to reduce potential response options, assessing logical thinking, hypothesis testing and deduction. |
|
| Tower test | Scaled score of the total number of rule violations per item ratio, assessing spatial planning, rule learning, inhibition and the maintenance of a cognitive set. |
|
| Verbal fluency category switching |
Scaled score of total number of correct words produced during switching task, regardless of switching accuracy assessing verbal production, simultaneous processing, retrival and expressive language. |
|
| Trail making switching | Scaled score of time taken to properly connect alternating sequences of numbers and letters, assessing for visual-motor cognitive flexibility. |
|
| CANTAB | Delayed matching to sample | Z-score of percent correct matching of a novel pattern shown to one of four response options shown at 4000 ms or 12,000 ms delay assessing short-term and long-term visual and spatial memory. |
| Intra-extra dimensional shift total errors |
Z-score total numbers of errors made by failure to adjust to the novel conditions and properly attend to the correct features of shapes and lines assessing cognitive flexibility, discrimination, and attention shifting. |
|
| Intra-extra dimensional shift stages completed |
Z-score of number of rule change stages completed on a measure requiring adaptation to a series changing conditions assessing cognitive flexibility, visual discrimination, and attention shifting. |
|
| Spatial workin memory | Z-score of total number of errors counted as returning to a loction where a stimulus was previously found on a screen where one goes to novel locations to determine the correst responses assessing cognitive flexibility, rule learning, and spatial working memory. |
Note. WISC-IV = Wechsler Intellignece Scale For Children, Fourth Edition; D-KEFS = Delis-Kaplan Executive Function System; CANTAB = Cambridge Neuropsychological Test Automated Battery.
Wechsler Intelligence Scale for Children–Fourth Edition (WISC-IV).
The WISC-IV (Wechsler, 2003) was used to obtain a full-scale IQ (FSIQ) score as well as composite index scores for individual cognitive domains [Verbal Comprehension Index (VCI), Perceptual Reasoning Index (PRI), Working Memory Index (WMI), and Processing Speed Index (PSI)].
Delis-Kaplan Executive Functioning System (D-KEFS).
The D-KEFS (Delis, Kaplan, & Kramer, 2001) tasks provide a sensitive and valid approach for evaluating multiple components of executive functioning. The following scaled scores were used as the dependent variables: Design Fluency Switching (DF), Color-Word Interference Inhibition (CWI), Color-Word Interference Inhibition/Switching (CWS), Twenty Questions Test Total Initial Abstraction (TQ), Tower Test Total Achievement (TT), Verbal Fluency Switching (VF) and Trail Making Test Number-Letter Switching (TMT).
Cambridge Neuropsychological Test Automated Battery (CANTAB).
The CANTAB (Cambridge Cognition Limited, 2005) measures various nonverbal components of neuropsychological functioning with an emphasis on executive functioning and has high internal consistency and construct validity (Luciana, 2003). The CANTAB variables were the z-scores from the higher order cognitive subtests [(Delayed Matching to Sample (DMS), Intra-Extra Dimensional Shift Total Errors (IED-TE), IED Stages Completed (IED-SC), and Spatial Working Memory (SWM)].
Statistical Analysis
Statistical analyses were conducted using the SPSS statistical package version 19.0 (SPSS, 2010). Demographic data were analyzed using chi-square [sex, race/ethnicity, handedness] and standard analysis of variance (ANOVA) techniques [age and FSIQ]. The following a priori data analysis strategy was employed: three separate 2 (AE) × 2 (ADHD) between-subjects MANCOVAs would be performed on variables from the WISC-IV, D-KEFS, and CANTAB, respectively, using an alpha of p = .05. Pillai’s trace criterion would be used as the omnibus test statistic. We planned to follow up significant interactions of AE and ADHD using a three-step process: (1) an interaction contrast (Maxwell & Delaney, 2004) would determine whether the effect of ADHD in exposed children differed from non-exposed children [(AE+ − AE−) – (ADHD – CON)]; (2) if significant, the interaction contrast would be followed up using simple effects to reveal the source of the interaction; (3) finally, because our research question relates to neuropsychological function of alcohol-exposed children compared to other non-exposed groups, if the source of the interaction was a difference between AE groups, they would be compared independently to non-exposed groups (AE+ vs. ADHD, AE− vs. ADHD, AE+− vs. CON, AE− vs. CON); alternatively, if there was no significant difference between the AE groups, they would be combined (AE+/−) and compared to non-exposed groups (AE+/− vs. ADHD, AE+/− vs. CON). These comparisons would utilize a Bonferroni correction at α =.05/total number of planned contrasts) to control for Type I error. Marginal significance would be defined as p < .05 for the contrasts.
Results
Demographic Information
There were no group differences on age [F (3, 343) = 1.94, p = .122], handedness [χ2 (df = 3) = 2.38, p = .497] and ethnicity [χ2 (df = 6) = 9.80, p = .133]. However, groups differed significantly on sex [χ2 (df = 3) = 15.69, p = .001], race [χ2 (df = 18) = 31.53, p = .025], and FSIQ [F (3, 341) = 66.07, p < .001]. Pairwise comparisons indicated that the ADHD group had significantly more males than the CON (p = .001), AE− (p < .001), and the AE+ groups (p = .014), which did not differ significantly from each other (p > .112). As expected, the ADHD group had significantly more male subjects compared to the other groups, which is representative of the ADHD clinical population as there is an expected ratio of 1:3 males in non-exposed children with ADHD (Cantwell, 1996; Graetz, Sawyer, Hazell, Arney, & Baghurst, 2001; Merikangas et al., 2010). The AE− group had significantly fewer White subjects compared to the CON (p < .001), ADHD (p = .047), and AE+ groups (p = .047), which did not differ from each other (p > .232). For FSIQ, using Tukey’s HSD, the CON group had significantly higher (p < .001) scores than the AE+, AE−and ADHD groups. The AE+ group had significantly lower FSIQ than the ADHD group (p < .001) but there was no significant difference between AE groups (p = .152), or the AE− and ADHD groups (p = .540). Demographic information is presented in Table 2.
Table 2.
Participant Characteristics
| Exposed |
Nonexposed |
||||
|---|---|---|---|---|---|
| Demographic variable | AE+ (n = 90) | AE− (n = 38) | ADHD (n = 80) | CON (n = 136) | Parametric statistics |
| Handedness [n (% Right)] | 78(86.7) | 35(92.1) | 72(90.0) | 126(92.6) | |
| FAS [n (%)] | 23(25.6) | 11(28.9) | 0(0.0) | 0(0.0) | |
| Sex [n (% Females)]* | 36(40.0) | 21(55.3) | 18(22.5) | 62(45.6) | ADHD < AE+, AE−, CON |
| Race [n (%White)]* | 57(63.3) | 14(36.8) | 53(66.3) | 94(69.1) | AE− < CON, ADHD |
| Ethnicity [n (% Hispanic)] | 8(8.9) | 7(18.4) | 20(25.0) | 28(20.6) | |
| Age [M (SD)] | 12.4(2.4) | 12.6(2.6) | 11.7(2.5) | 12.5(2.6) | |
| FSIQ [M (SD)]* | 82.44(17.497) | 88.71(14.108) | 92.77(18.535) | 110.31(11.860) | ADHD, AE−, AE+, < CON; AE+ < ADHD |
| CIFASD Site [n (%)] | |||||
| Albuquerque | 7(7.8) | 2(5.3) | 11(13.8) | 20(14.7) | |
| Atlanta | 15(16.7) | 12(18.5) | 19(23.8) | 19(14.0) | |
| Los Angeles | 17(18.9) | 10(26.3) | 2(2.5) | 18(13.2) | |
| Northern Plains States | 13(14.4) | 5(13.2) | 9(11.3) | 18(13.2) | |
| San Diego | 38(42.2) | 9(23.7) | 39(48.8) | 61(44.9) | |
Note. Demographic information for the four groups: alcohol-exposed children with ADHD(AE+), and without ADHD(AE−), nonexposed children with ADHD (ADHD), and typically developing controls (CON). CIFASD = Collaborative Initiative on Fetal Alcohol Spectrum Disorders.
Significant differences between groups, p < .05.
Additionally, as this was a multi-site study, we assessed if there were site differences for demographic variables. We found that there were significant site differences for age and race (ps < .034). Further, to ensure that site did not affect our results, we re-ran the analyses using a 2 (AE: Exposed, Non-Exposed) × 2 (ADHD status: Diagnosed, Not Diagnosed) × 5 (Site: Albuquerque, Atlanta, Los Angeles, Northern Plains States, San Diego). The three-way interaction was not significant (p = .644) and the main effects and interaction effects remained the same. Therefore, we are able to confirm that there was no difference for the main effects of exposure or ADHD across sites.
Additional Covariates
Given the possible influence of demographic differences (i.e., sex, race, IQ, age) on the dependent variables, the inclusion of potential covariates was considered. Using bivariate correlations, we found that race was not significantly associated with any of the outcome variables, ps =.224, and therefore was not considered an appropriate model covariate. Sex was significantly related to outcome variables (p <.001). Follow-up bivariate correlations revealed that sex was not associated with any CANTAB variables (p = .330) but was significantly correlated with the WISC-IV and D-KEFS variables (p < .001) and therefore was considered a covariate in those analyses. Age was significantly associated with all outcome variables (p < .001). Follow-up bivariate correlations revealed that age was negatively associated with WISC (p =.032) and D-KEFS and positively associated with the CANTAB (p < .001) variables and was used as a covariate in those analyses. Although groups also differed on IQ, the extant literature illustrates the analytical, statistical and theoretical problems of covarying for IQ for neurodevelopmental disorders (Dennis et al., 2009). Furthermore, our interest in the components of IQ as variables in this study, supports not using it as a covariate.
Neuropsychological Measures
Three 2 (AE) × 2 (ADHD) between-subjects MANCOVAs were performed separately for WISC-IV, D-KEFS, and CANTAB variables. Average WISC-IV, D-KEFS, and CANTAB scores for each group are displayed in Table 3.
Table 3.
Performance Across Groups
| Exposed |
Nonexposed |
||||
|---|---|---|---|---|---|
| Measure [m (SD)] | AE+ (n = 90) | AE− (n = 38) | ADHD (n = 80) | CON (n = 136) | Planned comparisons |
| WISC-IV, Composite scores | |||||
| Verbal comprehension* | 88.07(17.75) | 92.08(13.82) | 98.92(19.77) | 113.98(14.18) | AE+/− < ADHD < CON |
| Perceptual resoning* | 88.89(16.91) | 91.87(13.82) | 96.28(17.96) | 110.26(12.50) | AE+/− < ADHD < CON |
| Working memory | 82.25(15.85) | 92.00(13.67) | 91.10(16.19) | 104.63(12.04) | |
| Processing speed | 81.18(15.69) | 87.18(14.18) | 86.77(15.13) | 98.33(13.54) | |
| D-KEFS, Scaled scores | |||||
| Design fluency* | 7.89(2.80) | 8.55(2.51) | 8.78(3.28) | 11.10(3.08) | AE+/−, ADHD < CON |
| Color-word inhibition | 7.45(3.60) | 8.82(3.04) | 9.00(3.67) | 10.80(2.41) | |
| Color-word inhibition/switching | 8.05(3.90) | 8.86(3.09) | 9.47(3.20) | 11.03(2.37) | |
| Twenty questions | 7.88(2.62) | 8.29(2.40) | 9.28(3.54) | 11.12(3.25) | |
| Tower task | 8.03(2.98) | 9.18(2.77) | 8.65(3.28) | 10.24(2.48) | |
| Verbal fluency* | 7.89(3.60) | 7.74(2.91) | 8.89(3.020 | 11.61(3.17) | AE+/−, ADHD < CON |
| Trail making* | 6.63(4.03) | 7.58(3.49) | 7.69(4.58) | 10.71(2.60) | AE+/−, ADHD < CON |
| CANTAB, z-scores | |||||
| Delayed matching to sample | −0.18(1.13) | 0.37(1.04) | 0.11(0.97) | 0.76(0.76) | |
| IED total errors | −0.20(1.14) | −0.20(0.92) | −0.04(1.08) | 0.39(1.08) | |
| IED stages completed | 0.17(0.75) | 0.03(1.08) | 0.21(0.87) | 0.48(0.66) | |
| Spatial working memory* | −0.16(0.76) | −0.07(0.92) | 0.02(0.77) | 0.72(0.69) | AE+/−, ADHD < CON |
Note. Mean scores are reported above for the four groups [children with heavy prenatal alcohol exposure and ADHD(AE+), chilfren with heavy prenatal alcohol exposure without ADHD(AE−), nonexposed children with ADHD (ADHD), and controls (CON)]. WISC-IV = Wechsler Intelligence Scale for Children, Fourth Edition; D-KEFS = Delis-Kaplan Executive Function System; CANTAB = Cambridge Neuropsychological Test Automated Battery; IED = Intra-Extra Dimensional Shift. AE+/− = combined alcohol-exposed group.
Subsets with significant AE × ADHD interactions.
WISC-IV
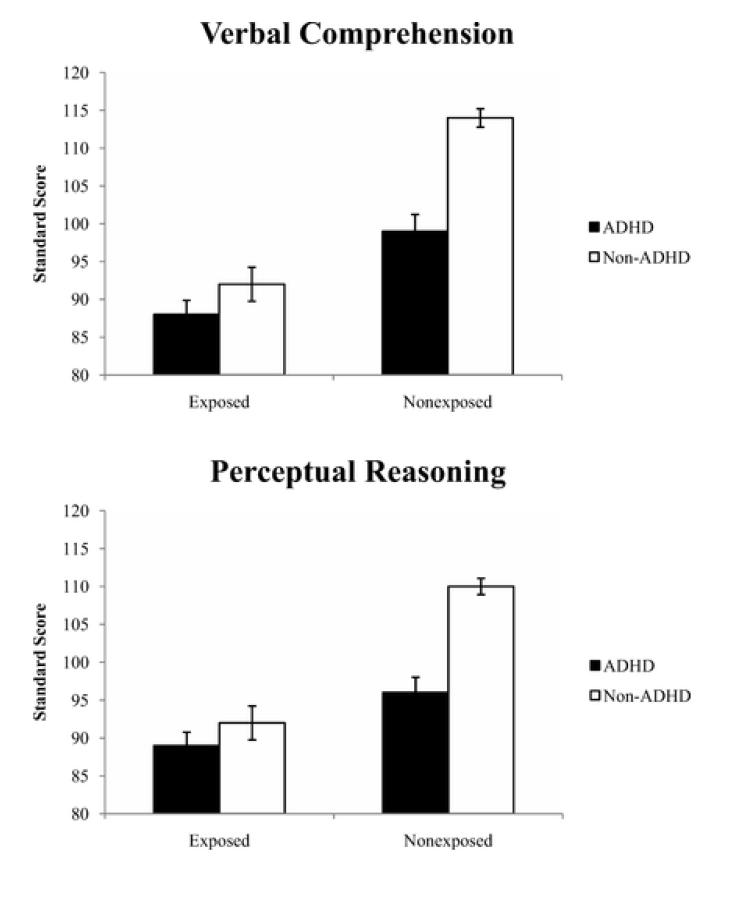
For WISC-IV variables, there were significant multivariate effects for both independent variables: AE [F (4, 333) = 17.78, p < .001, partial η2 = 0.176] and ADHD [F (4, 333) = 11.96, p < .001, partial η2 = .126], and the covariates of Sex [F (4,333) = 8.88, p < .001, partial η2 = .096] and Age [F (4,333) = 2.68, p =.032), partial η2 = .031]. The AE × ADHD interaction was also significant [F (4, 333) = 3.33, p = .011, partial η2 = .038]. To probe the significant multivariate effects, we examined the between-subjects effects for each individual dependent variable. There were significant main effects of AE and ADHD diagnosis (ps < .001) on each of the WISC-IV index composite scores; Verbal Comprehension, Perceptual Reasoning, Working Memory, and Processing Speed indices (VCI, PRI, WMI, PSI). Heavy prenatal alcohol exposure was associated with lower WISC-IV scores, regardless of ADHD diagnosis. Also, children with ADHD exhibited lower scores on WISC-IV scores than children without ADHD, across levels of AE. The AE × ADHD diagnosis interaction effects were statistically significant for VCI (p = .005) and PRI (p =. 001), indicating that the effect of AE on VCI and PRI differed depending on the presence or absence of ADHD. See Table 4 and Figure 1.
Table 4.
Main and Interaction Effects of ADHD and Alcohol Exposure (AE, ADHD, AE × ADHD)
| AE main effect |
ADHD main effect |
AE × ADHD interaction |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | ||||
| WISC-IV (overall) F(4,333) |
17.78 | <.001 | .176 | 11.96 | <.001 | .126 | 3.33 | .011 | .038 |
| Verbal comprehension F(1,336) |
63.73 | <.001 | .159 | 23.50 | <.001 | .065 | 8.11 | .005 | .024 |
| Perceptual reasonig F(1,336) |
44.18 | <.001 | .116 | 25.02 | <.001 | .069 | 10.31 | .001 | .030 |
| Working memory F(1,336) |
37.58 | <.001 | .101 | 44.81 | <.001 | .118 | 1.29 | .257 | .004 |
| Processing speed F(1,336) |
26.97 | <.001 | .074 | 20.22 | <.001 | .057 | 2.84 | .093 | .008 |
| D-KEFS overall F(7,311) |
11.02 | <.001 | .199 | 3.43 | .001 | .072 | 2.52 | .016 | .054 |
| Design fluency F(1,317) |
22.76 | <.001 | .067 | 8.60 | .004 | .026 | 4.44 | .036 | .014 |
| Color-word inhibition F(1,317) |
19.35 | <.001 | .058 | 11.70 | .001 | .036 | 0.64 | .424 | .002 |
| Color-word switching F(1,317) |
21.90 | <.001 | .065 | 7.83 | .005 | .024 | 1.25 | .264 | .004 |
| Twenty question F(1,317) |
31.68 | <.001 | .091 | 5.24 | .023 | .046 | 2.34 | .127 | .007 |
| Tower task F(1,317) |
2.92 | .088 | .009 | 9.73 | .002 | .030 | 0.62 | .430 | .002 |
| Verbal fluency F(1,317) |
40.51 | <.001 | .113 | 5.35 | .021 | .017 | 13.15 | <.001 | .040 |
| Trail making F(1,317) |
19.78 | <.001 | .059 | 14.31 | <.001 | .043 | 6.87 | .009 | .021 |
| CANTAB overall F(4,302) |
11.36 | <.001 | .131 | 6.90 | <.001 | .084 | 3.40 | .010 | .043 |
| Delay matching to sample F(1,306) |
13.45 | <.001 | .042 | 21.60 | <.001 | .066 | 0.04 | .835 | <.001 |
| IED total errors F(1,306) |
8.48 | .004 | .027 | 3.10 | .079 | .010 | 0.46 | .497 | .002 |
| IED stages completed F(1,306) |
9.79 | .002 | .031 | 0.17 | .895 | <.001 | 3.55 | .061 | .012 |
| Spatial working memory F(1,306) |
41.57 | <.001 | .120 | 11.97 | .001 | .038 | 10.42 | .001 | .033 |
Note. = Partial eta squared effect sizt; WISC-IV = Wechsler Intelligence Scale for Children, Fourth Edition; D-KEFS = Delis-Kaplan Executive Function System; CANTAB = Cambridge Neuropsychological Test Automated Battery; IED = Intra-Extra Dimensional Shift.
Figure 1.
Example of neuropsychological subtests with an Alcohol Exposure (AE) × Attention-Deficit/Hyperactivity Disorder status (ADHD) interaction. Errors bars indicate standard error of the mean. The top panel illustrates the AE × ADHD interaction for WISC-IV Verbal Comprehension Index and the bottom panel illustrates the AE × ADHD interaction for WISC-IV Perceptual Reasoning Index. For both measures, the AE+/− group performed significantly worse than the ADHD group and both clinical groups perform significantly worse than controls. There are four other significant interactions of AE and ADHD, which are not pictured, where the AE+/− group was not significantly different from the ADHD group (see Table 3).
The planned interaction contrast indicated a significant difference (ps < .006) between the differences of (AE+ and AE−) and (ADHD and CON) for both VCI and PRI. Simple effects indicated that the interaction contrast was driven by the difference of ADHD and CON (ps <.001; ADHD < CON); there were no differences between the AE+ and AE− group on either VCI or PRI (ps > .216), which supports the use of the combined AE+/− group in subsequent analyses. Importantly, the proportion of children with ADHD (70%) in this combined group was consistent with previous studies of children with FASD (Fryer, et al., 2007; Landgren, et al., 2010). This combined AE+/− group performed significantly worse than the ADHD group on both VCI (p < .001) and PRI (p = .009). Additionally, the combined AE+/− performed significantly worse than the control group on both measures (ps < .001). See Table 5.
Table 5.
Interaction Contrast, Simple Effects, and Planned Comparison Contrasts Between Groups
| Interaction contrast |
Simple effects |
Planned comparisons |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (AE+ vs. AE−) − (ADHD vs. CON) |
AE+ vs. AE− |
ADHD vs. CON |
AE+/− vs. ADHD |
AE+/− vs. CON |
|||||||
| Variable | Contrast estimate | F | p | F | p | F | p | t | p | t | p |
| Verbal comprehension | 11.042 | 7.758 | .006 | 1.543 | .216 | 41.854 | <.001 | 3.607 | <.001* | 11.180 | <.001* |
| Perceptual reasoning | 11.007 | 9.077 | .003 | .919 | .339 | 45.003 | <.001 | 2.608 | .009* | 10.092 | <.001* |
| Design fluency | 1.616 | 5.142 | .024 | 1.598 | .209 | 26.927 | <.001 | 1.268 | .206 | 7.427 | <.001* |
| Verbal fluency | 2.772 | 13.028 | <.001 | .053 | .818 | 38.118 | <.001 | 2.239 | .026 | 9.095 | <.001* |
| Trail making | 2.047 | 5.529 | .019 | 1.610 | .207 | 37.742 | <.001 | 1.085 | .279 | 7.711 | <.001* |
| Spatial working memory | .613 | 11.240 | .001 | .305 | .582 | 45.573 | <.001 | 1.218 | .224 | 8.504 | <.001* |
Note. Alcohol-exposed children with ADHD (AE+), and without ADHD (AE−), combined alcohol-exposed group (AE+/−), nonexposed children with ADHD (ADHD), and typically developing controls (CON) for the six subtests for which there was a signficant AE × ADHD interaction.
Significant planned comparisons using a Bonferroni correction at α < .025 (.05/2).
D-KEFS
There were significant multivariate effects for AE [F (7, 311) = 11.02, p < .001, partial η2 = .199], ADHD [F (7, 311) = 3.43, p = .001, partial η2 = .072], and the covariates of Sex [F (7, 313) = 3.96, p < .001, partial η2 = .082] and Age [F (7,311) = 4.72, p < .001, partial η2 = .096) for D-KEFS subtest scores. The AE × ADHD diagnosis interaction was also significant [F (7, 311) = 2.52, p = .016, partial η2 = .054]. To probe the significant multivariate effects, we examined between-subjects effects on each individual dependent variable. There were statistically significant main effects for AE and ADHD on all D-KEFS subtest scores with the exception of Tower Test (TT), which, while significant for ADHD (p = .002), was not significant for AE (p = .088). Thus, children with histories of prenatal alcohol exposure had significantly lower scores than the non-exposed groups on all executive functioning measures, except on TT, regardless of ADHD diagnosis. Also, children with ADHD exhibited lower scores on the D-KEFS than children without ADHD, regardless of AE history. The AE × ADHD diagnosis interaction effects were statistically significant (p < .036) for Design Fluency, Verbal Fluency, and Trail Making (DF, VF, and TM) scaled scores. See Table 4. The interaction contrast, (AE+ vs. AE−) vs. (ADHD vs. CON), was statistically significant (ps < .024) for all three D-KEFS subtests with significant interactions. Simple effects revealed that there was a significant difference between ADHD and CON on all three D-KEFS tasks (ps < .001; ADHD < CON), however there was no difference between the alcohol-exposed groups (ps > .207). This indicated the use of a combined AE+/− group that performed marginally worse than the ADHD group on VF (p = .026) and did not differ significantly from the ADHD group on any other D-KEFS task (p > .206). The AE+/− group performed significantly worse (p < .001) than the CON group for DF, VF and TM. See Table 5.
CANTAB
There were significant multivariate effects for AE [F (4, 302) = 11.36, p < .001, partial η2 = .131], ADHD [F (4, 302) = 6.90, p < .001, partial η2 = .084], and the covariate of Age [F (4, 302) = 22.19, p < .001, partial η2 = .227]. The AE × ADHD interaction was also significant [F (4, 302) = 3.40, p = .010, partial η2 = .043]. To probe the significant multivariate effects, we examined the between-subjects effects for each individual dependent variable.. There were statistically significant (p < .004) main effects of AE on all CANTAB subtest scores (AE< Non-exposed). There were also statistically significant main effects of ADHD (ADHD < non-ADHD) on SWM and DMS (p ≤ .001), but not on IED-SC or IED-TE (p > .079). The AE × ADHD interaction effect was statistically significant (p = .001) for SWM. See Table 4. The interaction contrast, (AE+ vs. AE−) vs. (ADHD vs. CON), was significant (p = .001) for SWM. Simple effects showed that there was no significant difference between the AE+ and AE− (p = .582), and that the interaction contrast significant was driven by the difference of ADHD and CON (p < .001; ADHD < CON). There was no significant difference (p = .224) between the combined AE+/− group and ADHD, however the combined AE+/− group performed significantly worse than the CON group (p < .001) for SWM. See Table 5.
Discussion
This study assessed neuropsychological performance in controls and three clinical groups of children: (1) children with histories of heavy prenatal alcohol exposure, (2) non-exposed children diagnosed with ADHD, and (3) children with both prenatal alcohol exposure and ADHD. We sought to examine the relationship between prenatal alcohol exposure and ADHD on neuropsychological performance. Neuropsychological abilities were assessed using a test battery designed to measure general intellectual abilities and executive functioning. As hypothesized, across all neuropsychological domains, the alcohol-exposed children were more impaired than controls, regardless of ADHD diagnosis, and children with ADHD were impaired relative to controls, regardless of exposure history. These findings are consistent with previous studies in AE (Nigg, Blaskey, Huang-Pollock, & Rappley, 2002). Furthermore, six tasks showed significant interaction effects of ADHD and prenatal alcohol exposure, including two indices of general intellectual performance (WISC-IV Verbal Comprehension and Perceptual Reasoning), and four measures of executive functioning (D-KEFS Design Fluency, Verbal Fluency and Trail Making; CANTAB Spatial Working Memory).
While we had hypothesized that the combination of multiple risk factors in the AE+ group would result in more severe deficits in the neuropsychological domain, as in recent behavioral studies (Graham, et al., 2012; Ware, O'Brien, et al., 2012), results revealed no significant differences between the AE+ and AE− groups on any neuropsychological measure. Thus, the presence of an ADHD diagnosis did not have the same impact in the exposed sample as it did in the non-exposed sample. The lack of exacerbated impairments suggests that different mechanisms or risk factors may underlie the behavioral and neuropsychological deficits in children with concomitant AE and ADHD. For example, neural changes resulting from prenatal alcohol exposure may lead to such severe impairment that the co-occurrence of changes associated with concomitant ADHD does not further exacerbate neuropsychological performance (Sonuga-Barke, 2005). Differences could also be due to methodological differences as the behavioral studies were based on parent report measures, whereas the current study assessed neuropsychological performance through direct standardized assessment measures. Possible differences between the groups in access to services, medication, and school placements also may have contributed to the discrepancy.
In support of the previous literature, verbal comprehension and perceptual reasoning performance was impaired in both AE (Aragón et al., 2008; Kaemingk & Halverson, 2000; Kodituwakku, 2007; Mattson, Crocker, et al., 2011; Willoughby, Sheard, Nash, & Rovet, 2008) and ADHD (Andreou, Agapitou, & Karapetsas, 2005; Frazier, Demaree, & Youngstrom, 2004; Pineda, Puerta, Aguirre, García-Barrera, & Kamphaus, 2007). However, we found that alcohol-exposed children, regardless of ADHD diagnosis, presented with more severe verbal comprehension and perceptual reasoning deficits than non-exposed children with ADHD. This pattern of impairment (AE+/− < ADHD < CON) has not been specifically reported for these measures but is consistent with previous comparisons of visuospatial reasoning (Coles, et al., 1997) and verbal learning (Crocker, et al., 2011), and both domains have been consistently found to be severely impaired in alcohol-exposed children (for review, see Mattson, Crocker, et al., 2011). Spatial processing is considered a central component of the FASD neurobehavioral profile (Mattson et al., 2010) and is deficient in children with FASD even when compared to an IQ-matched comparison group (Carmichael Olson, Feldman, Streissguth, Sampson, & Bookstein, 1998). There is also convergent evidence for diffuse verbal deficits in the FASD population including deficits in expressive and receptive language (Adnams et al., 2007; McGee, Bjorkquist, Riley & Mattson, 2009; Wyper & Rasmussen, 2011), word comprehension (Conry, 1990; LaDue, Streissguth, & Randels, 1992; Mattson & Riley, 1998) as well as grammatical and semantic impairments (Becker, Warr-Leeper, & Leeper, 1990). Conversely, verbal comprehension and perceptual reasoning are not considered core deficits for subjects with ADHD, although they are impaired on all of these domains compared to controls (Ek et al., 2007; Frazier, et al., 2004; Mayes & Calhoun, 2004; Wechsler, 2003).
Children with alcohol exposure and children with ADHD were similarly impaired compared to controls on all other neuropsychological measures related to executive functioning (WISC-IV Working Memory and Processing Speed, all D-KEFS and CANTAB subtests). Executive dysfunction represents a hallmark deficit for both children with FASD (Mattson, Crocker, et al., 2011) and non-exposed children with ADHD (Barkley, 1997; Nigg, 2005). The similar deficits demonstrated by the clinical groups might be a result of a shared core deficit of higher order processing (Mattson, Crocker, et al., 2011). Relative weaknesses in executive functioning in non-exposed children with ADHD may bring them into the range of deficits exhibited by the alcohol-exposed children. In spite of the similar deficits noted in the clinical groups, Mattson, et al. (2013) reported that variables from the CANTAB and D-KEFS could be used to differentiate subjects with prenatal alcohol exposure from ADHD with 73.9% clarification accuracy. Thus, while the within-group variability of executive function performance is sufficient to accurately distinguish these clinical conditions (Mattson, et al., 2013), the current results support the use of other, potentially more sensitive measures for heavy prenatal alcohol exposure, such as verbal comprehension and perceptual reasoning performance, to differentiate the clinical groups. Importantly, the lack of significant group differences in executive function ability does not obviate the need for intervention and remediation as they are both impaired compared to controls. Of note, verbal fluency was an important variable in distinguishing AE from ADHD in the 2013 study (Mattson, et al., 2013). In the current study, while EF measures overall were not significantly different between the clinical groups, performance on verbal fluency was marginally significant, given our adjusted p-value. This provides further evidence that verbal measures may be more sensitive to heavy prenatal alcohol exposure.
Strengths and Limitations
Despite having clinically relevant and significant findings, our results should be considered in the context of important limitations. We did not examine the relationship between neuropsychological performance and IQ scores. Unfortunately, the options for dealing with expected IQ differences (e.g., matching groups or covarying IQ in analyses), result in additional analytical and theoretical problems, such as producing meaningless results if the covariate is intrinsic to group membership, creating statistically overcorrected results, and decreasing the generalizability of results (Dennis, et al., 2009).
Furthermore, our analyses included index scores that contribute to an IQ score, which obviates the issue of covarying. An additional limitation to our study was the relatively large age range. While a large age range may increase variability and perhaps oversimplify important developmental changes, it allowed us to include a larger sample size, adding statistical power to our study. We addressed this limitation thoroughly, as our groups were equivalent on age, all measures had age-standardized scaled scores, and age-related variance was accounted for by including age as a covariate in the analyses. Demographic variables related to neuropsychological and executive function such as race, ethnicity and sex were evaluated as covariates in the current analysis, however socioeconomic status (SES) was not as it was not included during the implementation of the international CIFASD methodology. Further research would benefit from an investigation of home environment and SES on neurocognition in children with FASD.
Children were asked to abstain from medication during the testing, however not all children were able to and medication effects may have contributed to the results. Not excluding children based on medication status or concurrent psychopathology facilitates the generalizability of these findings across the population of children with heavy prenatal alcohol exposure. Nevertheless, the current study has notable strengths including its comparisons between alcohol-exposed children with and without ADHD and the use of a clinical contrast group. Furthermore, the sample size, collected from centers across the United States, is quite large and representative, allowing for greater generalizability of our results.
Implications and Future Directions
These data demonstrate differential behavioral and neuropsychological outcomes resulting from concomitant heavy prenatal alcohol exposure and ADHD. Although, as evidenced in our sample, alcohol-affected children are more likely to have ADHD than the general population (Fryer, et al., 2007; Landgren, et al., 2010; Rasmussen, et al., 2010), there was no evidence of exacerbated cognitive deficits for alcohol-exposed children with ADHD compared to those without ADHD as seen in previous studies of behavior ratings. Therefore, this study supports the current methodological standard of combining AE+ and AE− into one alcohol-exposed group regardless of ADHD status. Results further indicate that cognitive findings from the last several decades of research in heavy prenatal alcohol exposure apply to children across the spectrum of FASD, regardless of concomitant ADHD.
Our results support and extend the prior literature demonstrating numerous shared deficits for both FASD and ADHD compared to controls (set shifting, complex motor skills, social skills, static balance, communication skills, parent reports of behavior), which hinders diagnostic specificity (Mattson, Crocker, et al., 2011; Mattson & Riley, 2011). However, in spite of these similarities, the presence of an ADHD diagnosis did not have the same impact on neuropsychological functioning in the exposed sample as it did in the non-exposed sample emphasizing that these two clinical conditions are not identical. We found that certain measures may be more sensitive in differentiating between ADHD and FASD. These results add to previous reports demonstrating differences between FASD and ADHD on attention (Coles, et al., 1997; Kooistra, Crawford, Gibbard, Kaplan, & Fan, 2011), response inhibition (Burden, et al., 2010), motor response time and balance (Kooistra, et al., 2010), overall deficits in letter fluency (Vaurio, et al., 2008), verbal learning and memory (Crocker, et al., 2011), and mathematics (Jacobson, Dodge, Burden, Klorman, & Jacobson, 2011). Further, this study indicates that measures of verbal comprehension and perceptual reasoning are also sensitive assessment measures for distinguishing alcohol-exposed children from non-exposed children with ADHD.
There are definite clinical benefits to accurately distinguishing children with FASD from non-exposed children with ADHD. To this end, successful identification of distinct clinical populations requires greater understanding of the complex relationship between ADHD and neuropsychological performance in children with and without prenatal exposure. There is evidence of greater positive outcomes for children with prenatal alcohol exposure who are identified and treated early (Adnams, et al., 2007; Paley & O'Connor, 2009; Yazdani, Motz, & Koren, 2009) and certain medications have different treatment efficacies between alcohol-exposed and non-exposed children with ADHD (Coles, et al., 1997; Oesterheld et al., 1998). Clinically, these findings demonstrate task-dependent patterns of impairment across clinical groups, with alcohol-exposed children demonstrating more severe verbal and perceptual deficits compared to children with ADHD. Both groups demonstrated executive function deficits. Further investigation of the underlying mechanisms of these domains may facilitate better characterization of the clinical similarities and differences between groups and lead to targeted and specialized interventions for these highly prevalent clinical groups.
Acknowledgments
Research described in this paper was supported by NIAAA grant numbers U01 AA014834 (Mattson), U24 AA014811 (Riley), T32 AA013525 (Riley), U24 AA014818 (Barnett), and U24 AA014815 (Jones).
Footnotes
The authors have no financial or other conflicts of interest.
References
- 1.Adnams CM, Sorour P, Kalberg WO, Kodituwakku P, Perold MD, Kotze A, May PA. Language and literacy outcomes from a pilot intervention study for children with FASD in South Africa. Alcohol. 2007;41(6):403–414. doi: 10.1016/j.alcohol.2007.07.005. doi: 10.1016/j.alcohol.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Academy of Pediatrics Committee on Substance Abuse and Committee on Children With Disabilities Fetal alcohol syndrome and alcohol-related neurodevelopmental disorders. Pediatrics. 2000;106(2):358–361. [PubMed] [Google Scholar]
- 3.American Psychiatric Association . Diagnostic and statistical manual of mental disorders: DSM-IV-TR. American Psychiatric Publishing, Inc.; Washington, DC: 2000. [Google Scholar]
- 4.Andreou G, Agapitou P, Karapetsas A. Verbal skills in children with ADHD. European Journal of Special Needs Education. 2005;20(2):231–238. [Google Scholar]
- 5.Aragón AS, Coriale G, Fiorentino D, Kalberg WO, Buckley D, Gossage JP, May PA. Neuropsychological characteristics of Italian children with fetal alcohol spectrum disorders. Alcoholism: Clinical and Experimental Research. 2008;32(11):1909–1919. doi: 10.1111/j.1530-0277.2008.00775.x. doi: 10.1111/j.1530-0277.2008.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121(1):65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- 7.Bay B, Kesmodel US. Prenatal alcohol exposure - A systematic review of the effects on child motor function. Acta Obstetricia et Gynecologica Scandinavica. 2011;90(3):210–226. doi: 10.1111/j.1600-0412.2010.01039.x. doi: 10.1111/j.1600-0412.2010.01039.x. [DOI] [PubMed] [Google Scholar]
- 8.Becker M, Warr-Leeper GA, Leeper HA. Fetal alcohol syndrome: A description of oral motor, articulatory, short-term memory, grammatical, and semantic abilities. Journal of Communication Disorders. 1990;23(2):97–124. doi: 10.1016/0021-9924(90)90016-r. doi: 10.1016/0021-9924.90.90016-R. [DOI] [PubMed] [Google Scholar]
- 9.Bertrand J, Floyd RL, Weber MK. Guidelines for identifying and referring persons with fetal alcohol syndrome. Morbidity and Mortality Weekly Report Recommendations and Reports. 2005;54(RR-11):1–14. [PubMed] [Google Scholar]
- 10.Blanchard LT, Gurka MJ, Blackman JA. Emotional, developmental, and behavioral health of American children and their families: A report from the 2003 National Survey of Children's Health. Pediatrics. 2006;117(6):e1202–1212. doi: 10.1542/peds.2005-2606. doi: 10.1542/peds.2005-2606. [DOI] [PubMed] [Google Scholar]
- 11.Burd L, Klug MG, Martsolf JT, Kerbeshian J. Fetal alcohol syndrome: Neuropsychiatric phenomics. Neurotoxicology and Teratology. 2003;25(6):697–705. doi: 10.1016/j.ntt.2003.07.014. doi: 10.1016/j.ntt.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 12.Burden MJ, Jacobson JL, Westerlund A, Lundahl LH, Morrison A, Dodge NC, Jacobson SW. An event-related potential study of response inhibition in ADHD with and without prenatal alcohol exposure. Alcoholism: Clinical and Experimental Research. 2010;34(4):617–627. doi: 10.1111/j.1530-0277.2009.01130.x. doi: 10.1111/j.1530-0277.2009.01130.x. [DOI] [PubMed] [Google Scholar]
- 13.Cambridge Cognition Limited . CANTAB Eclipse Test Administration Guide. Cambridge, UK: 2005. [Google Scholar]
- 14.Cantwell DP. Attention deficit disorder: A review of the past 10 years. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35(8):978–987. doi: 10.1097/00004583-199608000-00008. doi: 10.1097/00004583-199608000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Carmichael Olson H, Feldman JJ, Streissguth AP, Sampson PD, Bookstein FL. Neuropsychological deficits in adolescents with fetal alcohol syndrome: Clinical findings. Alcoholism: Clinical and Experimental Research. 1998;22(9):1998–2012. doi: 10.1111/j.1530-0277.1998.tb05909.x. [PubMed] [Google Scholar]
- 16.Coles CD, Lynch ME, Kable JA, Johnson KC, Goldstein FC. Verbal and nonverbal memory in adults prenatally exposed to alcohol. Alcoholism: Clinical and Experimental Research. 2010;34(5):897–906. doi: 10.1111/j.1530-0277.2010.01162.x. doi: 10.1111/j.1530-0277.2010.01162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coles CD, Platzman KA, Raskind-Hood CL, Brown RT, Falek A, Smith IE. A comparison of children affected by prenatal alcohol exposure and attention deficit, hyperactivity disorder. Alcoholism: Clinical and Experimental Research. 1997;21(1):150–161. doi: 10.1111/j.1530-0277.1997.tb03743.x. [PubMed] [Google Scholar]
- 18.Conry J. Neuropsychological deficits in fetal alcohol syndrome and fetal alcohol effects. Alcoholism: Clinical and Experimental Research. 1990;14(5):650–655. doi: 10.1111/j.1530-0277.1990.tb01222.x. doi: 10.1111/j.1530-0277.1990.tb01222.x. [DOI] [PubMed] [Google Scholar]
- 19.Crocker N, Vaurio L, Riley EP, Mattson SN. Comparison of adaptive behavior in children with heavy prenatal alcohol exposure or attention-deficit/hyperactivity disorder. Alcoholism: Clinical and Experimental Research. 2009;33(11):2015–2023. doi: 10.1111/j.1530-0277.2009.01040.x. doi: 10.1111/j.1530-0277.2009.01040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crocker N, Vaurio L, Riley EP, Mattson SN. Comparison of verbal learning and memory in children with heavy prenatal alcohol exposure or attention-deficit/hyperactivity disorder. Alcoholism: Clinical and Experimental Research. 2011;35(6):1114–1121. doi: 10.1111/j.1530-0277.2011.01444.x. doi: 10.1111/j.1530-0277.2011.01444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delis DC, Kaplan E, Kramer JH. The Delis-Kaplan Executive Function System: Examiner’s Manual. The Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- 22.Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA, Fletcher JM. Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. Journal of the International Neuropsychological Society. 2009;15(3):331–343. doi: 10.1017/S1355617709090481. doi: 10.1017/S1355617709090481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ek U, Fernell E, Westerlund J, Holmberg K, Olsson P-O, Gillberg C. Cognitive strengths and deficits in schoolchildren with ADHD. Acta Paediatrica. 2007;96(5):756–761. doi: 10.1111/j.1651-2227.2007.00297.x. doi: 10.1111/j.1651-2227.2007.00297.x. [DOI] [PubMed] [Google Scholar]
- 24.Frazier TW, Demaree HA, Youngstrom EA. Meta-analysis of intellectual and neuropsychological test performance in attention-deficit/hyperactivity disorder. Neuropsychology. 2004;18(3):543–555. doi: 10.1037/0894-4105.18.3.543. doi: 10.1037/0894-4105.18.3.543. [DOI] [PubMed] [Google Scholar]
- 25.Fryer SL, McGee CL, Matt GE, Riley EP, Mattson SN. Evaluation of psychopathological conditions in children with heavy prenatal alcohol exposure. Pediatrics. 2007;119:e733–e741. doi: 10.1542/peds.2006-1606. doi: 10.1542/peds.2006-1606. [DOI] [PubMed] [Google Scholar]
- 26.Graetz BW, Sawyer MG, Hazell PL, Arney F, Baghurst P. Validity of DSM-IV ADHD subtypes in a nationally representative sample of Australian children and adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40(12):1410–1417. doi: 10.1097/00004583-200112000-00011. doi: 10.1097/00004583-200112000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Graham DM, Crocker N, Deweese BN, Roesch SC, Coles CD, Kable JA, May PA, Kalberg WO, Sowell ER, Jones KL, Riley EP, Mattson SN, CIFASD Prenatal alcohol exposure, attention-deficit/hyperactivity disorder, and sluggish cognitive tempo. Alcoholism: Clinical and Experimental Research. 2013;37(1):e338–e346. doi: 10.1111/j.1530-0277.2012.01886.x. doi: 10.1111/j.1530-0277.2012.01886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenbaum RL, Stevens SA, Nash K, Koren G, Rovet J. Social cognitive and emotion processing abilities of children with fetal alcohol spectrum disorders: A comparison with attention deficit hyperactivity disorder. Alcoholism: Clinical and Experimental Research. 2009;33(10):1656–1670. doi: 10.1111/j.1530-0277.2009.01003.x. doi: 10.1111/j.1530-0277.2009.01003.x. [DOI] [PubMed] [Google Scholar]
- 29.Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, Robinson LK. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: Clarification of the 1996 Institute of Medicine criteria. Pediatrics. 2005;115(1):39–47. doi: 10.1542/peds.2004-0259. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobson JL, Dodge NC, Burden MJ, Klorman R, Jacobson SW. Number processing in adolescents with prenatal alcohol exposure and ADHD: Differences in the neurobehavioral phenotype. Alcoholism: Clinical and Experimental Research. 2011;35(3):431–442. doi: 10.1111/j.1530-0277.2010.01360.x. doi: 10.1111/j.1530-0277.2010.01360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones KL, Robinson LK, Bakhireva LN, Marintcheva G, Storojev V, Strahova A, Chambers CD. Accuracy of the diagnosis of physical features of fetal alcohol syndrome by pediatricians after specialized training. Pediatrics. 2006;118(6):e1734–e1738. doi: 10.1542/peds.2006-1037. doi: 10.1542/peds.2006-1037. [DOI] [PubMed] [Google Scholar]
- 32.Kable JA, Coles CD. The impact of prenatal alcohol exposure on neurophysiological encoding of environmental events at six months. Alcoholism: Clinical and Experimental Research. 2004;28(3):489–496. doi: 10.1097/01.alc.0000117837.66107.64. doi: 10.1097/01.alc.0000117837.66107.64. [DOI] [PubMed] [Google Scholar]
- 33.Kaemingk KL, Halverson PT. Spatial memory following prenatal alcohol exposure: More than a material specific memory deficit. Child Neuropsychology. 2000;6(2):115–128. doi: 10.1076/chin.6.2.115.7058. doi: 10.1076/chin.6.2.115.7058. [DOI] [PubMed] [Google Scholar]
- 34.Kodituwakku PW. Defining the behavioral phenotype in children with fetal alcohol spectrum disorders: A review. Neuroscience and Biobehavioral Reviews. 2007;31(2):192–201. doi: 10.1016/j.neubiorev.2006.06.020. doi:10.1016/j.neubiorev.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 35.Kooistra L, Crawford S, Gibbard B, Kaplan BJ, Fan J. Comparing attentional networks in fetal alcohol spectrum disorder and the inattentive and combined subtypes of attention deficit hyperactivity disorder. Developmental Neuropsychology. 2011;36(5):566–577. doi: 10.1080/87565641.2010.549978. doi: 10.1080/87565641.2010.549978. [DOI] [PubMed] [Google Scholar]
- 36.Kooistra L, Crawford S, Gibbard B, Ramage B, Kaplan BJ. Differentiating attention deficits in children with fetal alcohol spectrum disorder or attention-deficit-hyperactivity disorder. Developmental Medicine and Child Neurology. 2010;52(2):205–211. doi: 10.1111/j.1469-8749.2009.03352.x. doi: 10.1111/j.1469-8749.2009.03352.x. [DOI] [PubMed] [Google Scholar]
- 37.Kooistra L, Ramage B, Crawford S, Cantell M, Wormsbecker S, Gibbard B, Kaplan BJ. Can attention deficit hyperactivity disorder and fetal alcohol spectrum disorder be differentiated by motor and balance deficits? Human Movement Science. 2009;28(4):529–542. doi: 10.1016/j.humov.2009.01.007. doi: 10.1016/j.humov.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 38.LaDue RA, Streissguth AP, Randels SP. Clinical considerations pertaining to adolescents and adults with fetal alcohol syndrome. In: Sonderegger TB, editor. Perinatal Substance Abuse: Research Findings and Clinical Implications. The Johns Hopkins University Press; Baltimore, MD: 1992. pp. 104–131. [Google Scholar]
- 39.Landgren M, Svensson L, Strömland K, Grönlund MA. Prenatal alcohol exposure and neurodevelopmental disorders in children adopted from eastern Europe. Pediatrics. 2010;125(5):e1178–e1185. doi: 10.1542/peds.2009-0712. doi: 10.1542/peds.2009-0712. [DOI] [PubMed] [Google Scholar]
- 40.Luciana M. Practitioner review: Computerized assessment of neuropsychological function in children: Clinical and research applications of the Cambridge Neuropsychological Testing Automated Battery (CANTAB) Journal of Child Psychology and Psychiatry. 2003;44(5):649–663. doi: 10.1111/1469-7610.00152. doi: 10.1111/1469-7610.00152. [DOI] [PubMed] [Google Scholar]
- 41.Mattson SN, Crocker N, Nguyen TT. Fetal alcohol spectrum disorders: Neuropsychological and behavioral features. Neuropsychology Review. 2011;21(2):81–101. doi: 10.1007/s11065-011-9167-9. doi: 10.1007/s11065-011-9167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mattson SN, Foroud T, Sowell ER, Jones KL, Coles CD, Fagerlund Å, CIFASD Collaborative initiative on fetal alcohol spectrum disorders: Methodology of clinical projects. Alcohol. 2010;44(7-8):635–641. doi: 10.1016/j.alcohol.2009.08.005. doi: 10.1016/j.alcohol.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mattson SN, Riley EP. A review of the neurobehavioral deficits in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcoholism: Clinical and Experimental Research. 1998;22(2):279–294. doi: 10.1111/j.1530-0277.1998.tb03651.x. doi: 10.1111/j.1530-0277.1998.tb03651.x. [DOI] [PubMed] [Google Scholar]
- 44.Mattson SN, Riley EP. The quest for a neurobehavioral profile of heavy prenatal alcohol exposure. Alcohol Research and Health. 2011;34(1):51–55. [PMC free article] [PubMed] [Google Scholar]
- 45.Mattson SN, Riley EP, Gramling LJ, Delis DC, Jones KL. Heavy prenatal alcohol exposure with or without physical features of fetal alcohol syndrome leads to IQ deficits. Journal of Pediatrics. 1997;131(5):718–721. doi: 10.1016/s0022-3476(97)70099-4. doi: 10.1016/S0022-3476(97)70099-4. [DOI] [PubMed] [Google Scholar]
- 46.Mattson SN, Riley EP, Gramling LJ, Delis DC, Jones KL. Neuropsychological comparison of alcohol-exposed children with or without physical features of fetal alcohol syndrome. Neuropsychology. 1998;12(1):146–153. doi: 10.1037//0894-4105.12.1.146. [DOI] [PubMed] [Google Scholar]
- 47.Mattson SN, Roesch SC, Glass L, Deweese BN, Coles CD, Kable JA, CIFASD Further development of a neurobehavioral profile of fetal alcohol spectrum disorders. Alcoholism: Clinical and Experimental Research. 2013;37(3):517–528. doi: 10.1111/j.1530-0277.2012.01952.x. doi: 10.1111/j.1530-0277.2012.01952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.May PA, Brooke L, Gossage JP, Croxford J, Adnams C, Jones KL, Viljoen D. Epidemiology of fetal alcohol syndrome in a South African community in the Western Cape Province. American Journal of Public Health. 2000;90(12):1905–1912. doi: 10.2105/ajph.90.12.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, Hoyme HE. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Developmental Disabilities Research Reviews. 2009;15(3):176–192. doi: 10.1002/ddrr.68. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- 50.Maxwell S, Delaney H. Designing experiments and analyzing data: a model comparison approach. Lawrence Erlbaum Associates; Mahwah, NJ: 2004. [Google Scholar]
- 51.Mayes SD, Calhoun SL. Similarities and differences in Wechsler Intelligence Scale for Children-Third Edition (WISC-III) profiles: Support for subtest analysis in clinical referrals. The Clinical Neuropsychologist. 2004;18(4):559–572. doi: 10.1080/13854040490888530. doi: 10.1080/13854040490888530. [DOI] [PubMed] [Google Scholar]
- 52.McGee CL, Bjorkquist OA, Riley EP, Mattson SN. Impaired language performance in young children with heavy prenatal alcohol exposure. Neurotoxicology and Teratology. 2009;31(2):71–75. doi: 10.1016/j.ntt.2008.09.004. doi: 10.1016/j.ntt.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Merikangas KR, He J-P, Brody D, Fisher PW, Bourdon K, Koretz DS. Prevalence and treatment of mental disorders among US children in the 2001-2004 NHANES. Pediatrics. 2010;125(1):75–81. doi: 10.1542/peds.2008-2598. doi: 10.1542/peds.2008-2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nigg JT. Neuropsychologic theory and findings in attention-deficit/hyperactivity disorder: The state of the field and salient challenges for the coming decade. Biological Psychiatry. 2005;57(11):1424–1435. doi: 10.1016/j.biopsych.2004.11.011. doi: 10.1016/j.biopsych.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 55.Nigg JT, Blaskey LG, Huang-Pollock CL, Rappley MD. Neuropsychological executive functions and DSM-IV ADHD subtypes. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41(1):59–66. doi: 10.1097/00004583-200201000-00012. doi: 10.1097/00004583-200201000-00012. [DOI] [PubMed] [Google Scholar]
- 56.O'Connor MJ, Paley B. Psychiatric conditions associated with prenatal alcohol exposure. Developmental Disabilities Research Reviews. 2009;15(3):225–234. doi: 10.1002/ddrr.74. doi: 10.1002/ddrr.74. [DOI] [PubMed] [Google Scholar]
- 57.Oesterheld JR, Kofoed L, Tervo R, Fogas B, Wilson A, Fiechtner H. Effectiveness of methylphenidate in Native American children with fetal alcohol syndrome and attention deficit/hyperactivity disorder: a controlled pilot study. Journal of Child and Adolescent Psychopharmacology. 1998;8(1):39–48. doi: 10.1089/cap.1998.8.39. doi:10.1089/cap.1998.8.39. [DOI] [PubMed] [Google Scholar]
- 58.Paley B, O'Connor MJ. Intervention for individuals with fetal alcohol spectrum disorders: Treatment approaches and case management. Developmental Disabilities Research Reviews. 2009;15(3):258–267. doi: 10.1002/ddrr.67. doi: 10.1002/ddrr.67. [DOI] [PubMed] [Google Scholar]
- 59.Pineda DA, Puerta IC, Aguirre DC, García-Barrera MA, Kamphaus RW. The role of neuropsychologic tests in the diagnosis of attention deficit hyperactivity disorder. Pediatric neurology. 2007;36(6):373–381. doi: 10.1016/j.pediatrneurol.2007.02.002. doi: 10.1016/j.pediatrneurol.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 60.Rasmussen C, Benz J, Pei J, Andrew G, Schuller G, Abele-Webster L, Lord L. The impact of an ADHD co-morbidity on the diagnosis of FASD. The Canadian Journal of Clinical Pharmacology. 2010;17(1):e165–e176. [PubMed] [Google Scholar]
- 61.Sampson PD, Streissguth AP, Bookstein FL, Little RE, Clarren SK, Dehaene P, Graham JM., Jr Incidence of fetal alcohol syndrome and prevalence of alcohol-related neurodevelopmental disorder. Teratology. 1997;56(5):317–326. doi: 10.1002/(SICI)1096-9926(199711)56:5<317::AID-TERA5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 62.Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39(1):28–38. doi: 10.1097/00004583-200001000-00014. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 63.Sonuga-Barke EJ. Causal models of attention-deficit/hyperactivity disorder: From common simple deficits to multiple developmental pathways. Biological Psychiatry. 2005;57(11):1231–1238. doi: 10.1016/j.biopsych.2004.09.008. doi: 10.1016/j.biopsych.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 64.SPSS . SPSS 19.0 for Mac OS X. Chicago, IL: 2010. [Google Scholar]
- 65.Vaurio L, Riley EP, Mattson SN. Differences in executive functioning in children with heavy prenatal alcohol exposure or attention-deficit/hyperactivity disorder. Journal of the International Neuropsychological Society. 2008;14:119–129. doi: 10.1017/S1355617708080144. doi: 10.1017/S1355617708080144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ware AL, Crocker N, O’Brien JW, Deweese BN, Roesch SC, Coles CD, CIFASD Executive function predicts adaptive behavior in children with histories of heavy prenatal alcohol exposure and attention deficit/hyperactivity disorder. Alcoholism: Clinical and Experimental Research. 2012;36(8):1431–1441. doi: 10.1111/j.1530-0277.2011.01718.x. doi: 10.1111/j.1530-0277.2011.01718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ware AL, O’Brien JW, Crocker N, Deweese BN, Roesch SC, Coles CD, CIFASD The effects of prenatal alcohol exposure and attention-deficit/hyperactivity disorder on psychopathology and behavior. Alcoholism: Clinical and Experimental Research. 2013;37(3):507–516. doi: 10.1111/j.1530-0277.2012.01953.x. doi: 10.1111/j.1530-0277.2012.01953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wechsler D. Manual for the Wechsler Intelligence Scale for Children-Fourth Edition. The Psychological Corporation; San Antonio, TX: 2003. [Google Scholar]
- 69.Williams J, Klinepeter K, Palmes G, Pulley A, Foy JM. Diagnosis and treatment of behavioral health disorders in pediatric practice. Pediatrics. 2004;114(3):601–606. doi: 10.1542/peds.2004-0090. doi: 10.1542/peds.2004-0090. [DOI] [PubMed] [Google Scholar]
- 70.Willoughby KA, Sheard ED, Nash K, Rovet J. Effects of prenatal alcohol exposure on hippocampal volume, verbal learning, and verbal and spatial recall in late childhood. Journal of the International Neuropsychological Society. 2008;14(6):1022–1033. doi: 10.1017/S1355617708081368. doi: 10.1017/S1355617708081368. [DOI] [PubMed] [Google Scholar]
- 71.Wyper KR, Rasmussen CR. Language impairments in children with fetal alcohol spectrum disorders. Journal of Population Therapeutics and Clinical Pharmacology. 2011;18(2):e364–e376. [PubMed] [Google Scholar]
- 72.Yazdani P, Motz M, Koren G. Estimating the neurocognitive effects of an early intervention program for children with prenatal alcohol exposure. The Canadian Journal of Clinical Pharmacology. 2009;16(3):e453–e459. [PubMed] [Google Scholar]