Abstract
Deficits in brain networks that support cognitive regulatory functions are prevalent in many psychiatric disorders. Findings across neuropsychology and neuroimaging point to broad-based impairments that cross traditional diagnostic boundaries. These dysfunctions are largely separate from the classical symptoms of the disorders, and manifest in regulatory problems in both traditional cognitive and emotional domains. As such, they relate to the capacity of patients to engage effectively in their daily lives and activity, often persist even in the face of symptomatically effective treatment, and are poorly targeted by current treatments. Advances in cognitive neuroscience now allow us to ground an understanding of these cognitive regulatory deficits in the function and interaction of key brain networks. This emerging neurobiological understanding furthermore points to several promising routes for novel neuroscience-informed treatments targeted more specifically at improving cognitive function in a range of psychiatric disorders.
Keywords: amygdala, anxiety, bipolar, cingulate, default mode, depression, emotion regulation, executive function, prefrontal, schizophrenia
Abstract
Los déficits en las redes cerebrales que dan soporte a las funciones de regulación cognitiva son frecuentes en muchos trastornos psiquiátricos. Los hallazgos de la neuropsicología y de las neuroimágenes apuntan a grandes deterioros que cruzan las fronteras de los diagnósticos tradicionales. Estas disfunciones son en gran medida independientes de los síntomas clásicos de los trastornos y se expresan en problemas de regulación de las áreas cognitiva y emocional. Es así como ellas se relacionan con la capacidad de los pacientes de participar efectivamente en la vida y en la actividad cotidiana, y a menudo persisten a pesar de los tratamientos sintomáticos efectivos y están mal abordadas por los tratamientos actuales. Los avances en las neurociencias cognitivas nos permiten aterrizar una comprensión de estos déficits de regulación cognitiva en la función e interacción de las principals redes cerebrales. Esta comprensión neuro-biológica emergente además señala algunas rutas promisorias para nuevos tratamientos en base a las neurociencias, orientados más específicamente a mejorar la función cognitiva en varios trastornos psiquiátricos.
Abstract
Les déficits des réseaux cérébraux à la base des fonctions régulatrices cognitives sont prévalents dans de nombreux troubles psychiatriques. Les données de neuropsychologie et de neuro-imagerie mettent en évidence des atteintes importantes qui dépassent les frontières diagnostiques traditionnelles. Ces atteintes sont bien séparées des symptômes classiques des troubles psychiatriques ; ce sont des problèmes de régulation dans les domaines émotionnel et cognitif traditionnels. À ce titre, ces troubles se reflètent dans l'aptitude des patients à participer efficacement à leurs activités et à leur vie quotidiennes ; ils persistent souvent malgré un traitement efficace sur les symptômes et sont mal pris en charge par les traitements actuels. Les progrès des neurosciences cognitives nous permettent maintenant de comprendre comment ces déficits de la régulation cognitive trouvent leur origine dans la fonction et l'interaction de réseaux cérébraux clés. Cette compréhension neurobiologique récente ouvre plusieurs voies prometteuses pour de nouveaux traitements basés sur les neurosciences et s'intéressant plus particulièrement à l'amélioration de la fonction cognitive dans de nombreux troubles mentaux.
Introduction
Cognition refers to a broad range of mental processes including attention, decision-making, behavioral-, thought- and self-regulation, problem solving, language, and memory. Dysfunctions in these processes have wide-ranging correlates and are related to problems in general adjustment, emotional and social functioning, and well-being. The prefrontal cortex (PFC) has been implicated in this array of functions, with multiple neural subsystems within the PFC subserving and coordinating different aspects of these processes. Most psychiatric disorders include disruption of some aspect of cognition. Increasing evidence indicates that these deficits may predispose individuals to developing the psychiatric disorder, may be an early marker of subsequent illness, may help maintain the disorder, and may predict the likelihood of recovery. Indeed, cognitive functioning in some psychiatric disorders predicts long-term illness course independent of symptoms that may be more characteristic or diagnostic of the illness (eg, hallucinations in schizophrenia, mood regulatory problems and rumination in depression). As such, cognition and associated neural circuitry is increasingly recognized as an important target for new treatments in psychiatry.
The most consistently documented cognitive deficits in psychiatric disorders involve executive functioning (EF). Executive functions, often referred to as cognitive regulatory or executive control systems, include three broad categories of functions: (i) inhibitory functions (the ability to suppress one response in favor of another); (ii) working memory (the ability to maintain and manipulate multiple pieces of information at the same time); and (iii) cognitive flexibility (the ability to adjust response or attention quickly in the face of changing demands).1,2 Higher-order EF, such as problem solving and planning, typically builds upon a combination of these three components. As a regulatory capacity, EF is central to a range of normal and abnormal behavior particularly relevant for psychiatric illness, and has been suggested to impact psychiatric functioning through involvement in, and overlap with, emotional regulation (ER) processes. Indeed, both EF and ER deficits are pervasive throughout psychiatric disorders, to varying degrees of severity and specificity, and hence may be of significant transdiagnostic importance. There is evidence that the neural circuitry that supports EF and ER is largely overlapping. In this review we will focus specifically on the contribution of circuit abnormalities relevant to EF and ER to psychiatric disorders. We restrict our focus to patients aged 60 and below to insure that the relationship of cognitive deficits to psychiatric disorders is not primarily due to age-related changes in cognition. We will begin with an overview of the neural systems underlying EF and ER, followed by a description of how deficits in these systems, or their behavioral output, subserve a range of psychiatric disorders. Finally, we will examine the relationship between EF and ER capacities and current treatments, as well as avenues for novel treatments through a neurobiological understanding of EF and ER.
Neural systems supporting EF and ER
Cognitive regulation of behavior and emotions is supported by several circuits in the PFC. While the PFC is typically not necessary for the learning or performance of simple tasks, when task demands change, the PFC is required for proper adjustments in behavior to maintain accuracy and goal-directed behavior. This capacity of the PFC is conserved across mammalian species.3-5 Viewed this way, the PFC is responsible for maintaining an internal representation of current goals and modulating activity in brain regions responsible for perception or action in order to flexibly achieve these goals. In order to accomplish this, the PFC must be able to maintain a representation of goals in the face of distraction, update these representations as new information is received through multiple sensory modalities, and provide a feedback signal that can select the neural pathways appropriate for the current task context.6
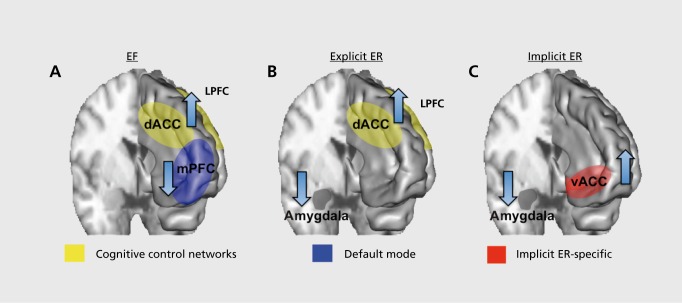
Within this broad capacity for EF, several more specific subgroupings of functions are possible, commonly considered to be inhibition, working memory, and cognitive flexibility. Nonetheless, any individual task paradigm aimed at one of these domains will, to some degree, involve one or both of the others. Working memory in particular is a function common to most tests of EF.1,2 Consistent with this, neuroimaging studies have identified a set of regions frequently implicated in EF across a range of tasks.7,8 Moreover, these regions can be further parcellated into two well-conserved cognitive control networks—a frontoparietal network containing the dorsolateral PFC (DLPFC) and posterior parietal cortices, and a cingulo-opercular network containing the dorsal anterior cingulate cortex (dACC), anterior insula, and the anterior PFC (Figure 1A).Figure 1A7,8 In addition to these common cognitive control networks, a set of regions including the inferior frontal gyrus, supplementary motor area, and subthalamic nucleus have been implicated in response inhibition specifically.9 Beyond the cognitive control networks, the default mode network has also been importantly implicated in EF. This network is comprised of medial prefrontal, medial, and lateral parietal, and medial temporal regions. Concomitant with engagement of the frontoparietal and cingulo-opercular network, the default mode network deactivates (Figure 1A). Deactivation is associated with a negative blood oxygen level-dependent response on functional magnetic resonance imaging (fMRI) scans, and suppression of gamma band activity on intracranial recordings.10,11 Momentary impairments in this coordination between activation in the frontoparietal and cingulo-opercular networks and deactivation in the default mode network are associated with lapses in attention and behavioral performance.12-14 Conversely, internally oriented mentation, such as self-reflection and autobiographical memory, activates the default mode network,10 further suggesting that the balance between the cognitive control networks and the default mode network is important for flexible transitioning from an internal focus of attention to externally focused attention demanding tasks.
Figure 1. Key regions important for emotion regulation and executive functioning. EF, executive function; ER, emotional regulation; dACC, dorsal anterior cingulate cortex; mPFC, medial prefrontal cortex; LPFC lateral prefrontal cortex; vACC ventral anterior cingulate cortex.
Evidence indicates that the above described EF circuitry is also crucially implicated in the regulation of emotions. Emotions themselves are complex, coordinated phenomena that involve behavioral, cognitive, and physiological changes, activate action tendencies, and create subjective feelings.15 ER includes an array of processes, ranging from the deliberate and effortful deployment of cognitive resources to alter an emotional reaction,15 to the uncued, spontaneous, use of “automatic” (ie, implicit) processes that occur entirely outside of awareness.16 Consistent with the fact that explicit ER requires deliberate and effortful deployment of cognitive resources, neuroimaging studies have found that it is also associated with activation in the frontoparietal and cingulo-opercular cognitive control networks implicated in EF more broadly,17,18 along with decreased activation in the amygdala (Figure 1B). As such, understanding and remediating deficits in core components of EF has bearing on both EF and explicit ER. Implicit ER, on the other hand, has only recently begun to be understood at the neurobiological level. We have reported on a task wherein subjects spontaneously regulate emotional conflict, a salient emotional stimulus, adaptively from trial to trial. In this task, the ventral anterior cingulate cortex (vACC) regulates emotional conflict on a trial-by-trial basis by dampening amygdala activity, but without involvement of activation in EF-related cognitive control networks (Figure 1C).19,20 Moreover, activation of the vACC during regulation in this task is specific, and not seen during similar regulation of nonemotional conflict.19 The causal role of the vACC has been demonstrated in a recent lesion study, in which subjects with vACC lesions were impaired only in the regulation of emotional conflict, but not non-emotional conflict.21 Thus, ER and EF involve a set of overlapping brain circuits for attention and behavioral adjustment, with ER having additional circuit-level specificity with respect to explicit versus implicit ER.
Perturbations in executive functioning in psychiatric disorders
Schizophrenia, psychosis, and bipolar disorders
Neuropsychological findings
While psychosis is a hallmark symptom of schizophrenia and dominates its acute clinical presentation, cognitive dysfunction both predates onset of psychosis and is present in the absence of psychotic symptoms.22 A vast body of work has found that patients with schizophrenia typically perform 0.8 to 1.5 standard deviations worse than control subjects in most neuropsychological tests subserved by PFC function.23 Impaired capacities include the domains of EF outlined above, including verbal memory, and verbal fluency. Cognitive dysfunction is more chronic, predicts poor outcome (including impairments in functional capacity), and is not substantially helped by available pharmacotherapies.24,25 Impairments across these domains are found in individuals with prodromal psychosis, which worsens further in those who transition to psychosis.26 These data therefore support a neurodevelopmental view of schizophrenia, such that core and pervasive cognitive impairments are present early on, long before a clear clinical picture emerges. Moreover, deficits in many of these cognitive domains are seen in unaffected first-order relatives of patients with schizophrenia, consistent with a strong genetic contribution to the risk of schizophrenia.27 Further, studies of monozygogic and dizygotic twins concordant and discordant for schizophrenia found that additive genetic factors were the main source of phenotypic correlations between schizophrenia and measures of executive function.28 Together, these data have led the US Food and Drug Administration (FDA) to allow approval of medications aimed at improving specifically the cognitive symptoms in schizophrenia, separate to psychotic and other symptoms.
Recent work has also implicated a similar range of cognitive deficits in other disorders that include psychosis. Even when euthymic, patients with bipolar disorder show cognitive impairments relative to healthy controls with medium to large effect sizes, especially within EF.29 As in schizophrenia, their first-degree relatives also have impairments in EF, albeit with small to medium effect sizes.29 Similarly, cognitive impairments in bipolar disorder correlate with poor functional capacity.30 More broadly within the category of affective disorders, patients with Major Depressive Disorder (MDD) who have psychotic symptoms are also cognitively impaired to a similar degree to bipolar patients.31
Neuroimaging findings
Consistent with the neuropsychological literature, imaging studies of schizophrenia have used a variety of tasks that probe elements of EF with fMRI or positron emission tomography (PET) imaging. A meta-analysis that included all of these studies found that across all of the EF domains tested, patients consistently hypoactivated a set of largely lateral and medial prefrontal regions.32 Specifically, patients hypoactivated the DLPFC, ventrolateral PFC (VLPFC), dACC, and thalamus (in the region of the mediodorsal nucleus). This is consistent with the failure to engage normal cognitive control circuitry in the prefrontal cortex. Interestingly, greater activation was found in patients in a more posterior region of the dACC, along with a portion of the VLPFC, which may reflect network inefficiency or efforts to compensate for impaired activation of prefrontal cognitive control regions. In part, however, whether hypoactivation or hyperactivation is observed reflects the difficulty of the task. DLPFC activity in healthy subjects, for example, decreases from its peak as working memory is stressed beyond its maximal capacity.33 In line with the interpretation that the DLPFC of schizophrenic patients operates less efficiently than that of controls, patients hyperactivate this region as they strain to keep up at low working memory loads that control subjects can easily handle, and hypoactivate this region at higher working memory loads that exceed patients' working memorycapacity, but not that of controls.34
The network formulation of EF circuitry outlined above argues that cognitive impairments may arise because of failure to activate prefrontal cognitive control networks, failure to deactivate the default mode network, or abnormalities in the interaction between prefrontal cognitive control networks and the default mode network. In line with this prediction, patients with schizophrenia also display a failure to suppress activity in the default mode network with cognitively engaging tasks.35 This default mode deficit is also seen even after controlling for task difficulty or performance impairment.36 The reciprocal relationship between prefrontal cognitive control networks and the default mode network is also perturbed in schizophrenia.37 As with the neuropsychological data, abnormalities in activation of cognitive control networks, deactivation of the default mode network, and interactions between these two networks are all perturbed in unaffected first-degree relatives of patients with schizophrenia.36,38,39 These impairments in network activation, connectivity, and interactions may furthermore be related to disruptions in glutamatergic signaling implicated in schizophrenia, specifically through activity at the N-methylD-aspartate (NMDA) receptor. Blockade of the NMDA receptor in healthy subjects using ketamine results in decreased cognitive control network activation, blunted default mode network activation, reductions in the reciprocal connectivity relationship between these regions, and impairment in working memory task performance.40 Imaging studies of EF in bipolar patients have yielded broadly similar results as observed in schizophrenia. During a working memory task, depressed bipolar patients fail to activate the DLPFC and deactivate the medial PFC (mPFC) component of the default mode network.“ In another study of euthymic, manic, and depressed bipolar patients, DLPFC hypoactivation was observed in all patient groups.42 Bipolar patients also show generally similar disruptions in reciprocal connectivity between the default mode network and cognitive control networks as patients with schizophrenia.37 Disruptions in more anterior lateral prefrontal regions have also been observed during working memory in unaffected first-degree relatives of bipolar patients.43,44
Depression and anxiety disorders
Neuropsychological findings
Of the affective disorders, MDD has been best studied with respect to neuropsychological measures of cognition. Indeed, so pervasive is the presence of EF in MDD, that they are considered a core symptom. Deficits in a range of EFs have been found in MDD with small to large effect sizes, depending on the test or component of EF under investigation.45 In particular, measures of inhibition, sustained attention, working memory, and task shifting are all impacted, suggesting that there is a broad disruption in EF. In a recent large meta-analysis of these studies, the authors failed to find an effect of current symptoms (ie, symptomatic versus remitted patients) on many aspects of EF task performance,45 suggesting that many of these impairments persist beyond the current mood episode, much as noted in bipolar disorder.
Components of EF function are also implicated in anxiety disorders, such as post-traumatic stress disorder (PTSD). Popular models of PTSD center around impairments in the learning and extinction of fear-based memories.46 In part due to this view, cognitive functioning has not been as well studied in PTSD relative to MDD, though PTSD has nonetheless been better studied than other anxiety disorders. Available evidence, however, demonstrates a similarly broad EF deficit in PTSD to that in MDD, including inhibition, sustained attention, working memory, and task shifting.47,48 These deficits are particularly notable when reaction times are measured for relatively simple tasks done under time pressure (eg, shifting impairments in the Trail-Making Test but there are fewer impairments in the Wisconsin card-sort test). One important potential confounding factor for this work, however, is the high rate of MDD comorbidity with PTSD. In one meta-analysis, presence of comorbid depression significantly moderated the magnitude of PTSD deficits in sustained attention, working memory, and attentional shifting.48 Given the high prevalence of comorbidity-amongst mood and anxiety disorders, it is unclear whether the common incidence of depressive symptoms—which also comprise some of the diagnostic criteria for PTSD—indicates the presence of a separate depression-related psychopathological process or if this is simply one type of clinical presentation for individuals with trauma-triggered psychopathology.
Outside of PTSD, limited neuropsychological assessment data are available on social anxiety disorder, in which the subtle deficits that have been observed in cognition appear to be at least partially related to elevated situational anxiety.49 In obsessive-compulsive disorder (OCD), deficits have been observed in shifting tasks, which have been attributed to greater perseverative errors, consistent with a view of OCD as involving an overly rigid pattern of cognition.50,51 Reports of other EF deficits in OCD have been conflicting or inconsistently observed. For generalized anxiety disorder and panic disorder, studies are limited and findings mixed. However, some investigations indicate that individuals with anxiety comorbid with another psychiatric disorder are more likely to have executive function deficits than those without comorbid anxiety.52
Neuroimaging findings
Much as with schizophrenia, individual studies have reported both hypoactivation and hyperactivation of prefrontal cognitive control regions in depression, compared with healthy controls.53 This disparity between findings across studies, however, can also be seen in light of these networks functioning inefficiently, whereby depressed patients need to recruit these regions more during less challenging task conditions, and thus are unable to sufficiently increase their activation to cope with more challenging task conditions. Though few direct comparisons have been made between patients with depression and those with schizophrenia, DLPFC inefficiency may be greater in schizophrenia than depression.54,55
Depressed patients also fail to deactivate the default mode network,56 which is also related to local glutamate concentrations.57 Perturbations have also been observed in the reciprocal relationship between prefrontal cognitive control networks and the default mode network, with the greatest abnormalities found in the most ruminative patients.58 In PTSD, most imaging studies have examined symptom provocation as well as other negative emotional processing tasks,59 with only a handful employing conventional tests of EF. Nonetheless, preliminary evidence implicates abnormalities in cognitive control network activation during working memory in PTSD.60 More recently, we have found evidence of impaired default mode connectivity and deactivation in PTSD.61 Importantly, for both connectivity and deactivation, these deficits were specific for PTSD relative to both healthy controls and patients with generalized anxiety disorder (who had similar levels of general anxiety and depression symptoms but not due to trauma).
Summary and integration
Cognitive dysfunction, and in particular impairments in EF, can be found across a wide range of psychiatric disorders. The greatest severity of impairment appears to be in chronic psychosis, but can nonetheless be seen in nonpsychotic mood and anxiety disorders. Moreover, these impairments largely persist into periods with reduced or absent expression of disorder-related symptoms, and are also largely not normalized by current antidepressant, mood-stabilizing, or antipsychotic medications.
The imaging findings from studies of EF across psychotic and affective disorders mirror the neuropsychological findings, wherein broadly similar abnormalities were observed across symptomatically disparate disorders. Specifically, deficits were observed in activation of cognitive control networks, deactivation of the default mode network, and in the reciprocal interaction between these two brain systems, all of which may contribute to cognitive dysfunction. In psychosis, where these impairments appear to be greatest, and where there is less evidence for biased emotional processing, they may be expressed primarily as severe cognitive deficits. In affective disorders, in which biased emotional processing has been well-documented (especially in terms of biases towards negative stimuli),62 these network impairments may contribute to both cognitive dysfunction and perseverative emotion-related cognition such as rumination.63 That is, impaired ability to engage EF and disengage from an internally focused default mode-dominated state, coupled with a bias to remember and attend to negative stimuli, may maintain inwardly oriented negative cognition in conditions such as depression and PTSD.
Overall, dysfunction in EF and the neurocircuits subserving these cognitive control processes, may represent a potential core endophenotype of severe mental illnesses across traditional diagnostic categories. In light of the relationship between cognitive dysfunction and worse functional capacity in various disorders, the severity of trans-diagnostic real-world functional impairment may be the primary symptomatic expression of the severity of the disturbance in cognition.
Perturbations in emotion regulation in psychiatric disorders
Given the close relationship between circuitry important for EF and ER, the expectation is that in those patients in whom there is a deficit in EF, there will also be a deficit in ER. Moreover, even for those patients in whom the EF deficit is more subtle, the combination of a mild EF deficit with robust emotional capture of attention could result in an ER deficit due to heightened reactivity. However, unlike for EF, there are no well-validated neuropsychological assessments of ER, and hence the literature developed on ER focuses on neuroimaging.
Schizophrenia, psychosis, and bipolar disorders
Though the emotion regulation literature has focused primarily on affective disorders, one study of patients with schizophrenia found that they failed to activate a VLPFC region implicated in explicit ER during efforts to downregulate negative emotion, and failed to show the expected reciprocal amygdala-prefrontal relationship.64 Interestingly, in the same study, patients with bipolar disorder hyperactivated the same region (half were euthymic and half hypomanic). The authors interpreted this as suggesting a deficit in engagement of cognitive control over emotion in schizophrenia, and inefficiency of this circuitry, once engaged, in bipolar disorder. One additional factor that may account for different ER abnormalities in schizophrenia and bipolar disorder is that bipolar patients generally overengage emotional systems in response to facial expression stimuli, while schizophrenics underengage these systems.65 Another study of only euthymic bipolar patients, however, found underactivation of the DLPFC, VLPFC, mPFC, and ACC during downregulation of negative emotion.66
Depression and anxiety disorders
Studies in affective disorders have also shown relativelysimilar deficits in explicit ER across disorders. Depressed patients generally activated cognitive control circuitry the same as, or more than, controls during explicit downregulation of negative emotion, but either did not show amygdala decreases,67,68 did not show the expected reciprocal amygdala-prefrontal relationship during regulation,69 or were unable to sustain those decreases.70 In remitted depressed patients, DLPFC hypoactivation could be seen during explicit downregulation of negative emotion, suggesting that ER deficits may also be a trait marker in depression.71 Though fewer studies of explicit ER have been conducted in anxiety disorder patients, during downregulation of negative emotion these have found DLPFC and mPFC hypoactivation in PTSD,72 generalized anxiety disorder,73 social anxiety disorder,74 and panic disorder.73
Implicit ER has only very recently been investigated with neuroimaging, and its parameters are only now being fleshed out.16 Using the emotional conflict task described above, we found that patients with generalized anxiety disorder or major depression all failed to activate the ventral ACC and failed to dampen the amygdala.75 Consistent with this finding, patients with PTSD (but not patients with social anxiety or specific phobia) hypoactivated the ventral ACC and hyperactivated the amygdala during symptom provocation and negative emotional processing.59 During fear extinction, a process that also involves ventromedial PFC activation, patients with PTSD, schizophrenia and OCD all similarly failed to activate the ventromedial PFC.76-78
Summary and integration
Despite a bias in the study of ER towards affective disorders and the relatively early stage of this literature, the available neuroimaging evidence suggests abnormalities
in both explicit and implicit ER that cross traditional diagnostic boundaries. It may be, however, that the cause of these abnormalities differ across disorder, with a primary deficit in EF and disruption of normal pathways for emotion processing accounting for ER abnormalities in schizophrenia, and more subtle EF deficits together with heightened emotional capture in affective disorders accounting for ER abnormalities in those conditions.
Towards future interventions targeting EF and ER dysfunction
Available evidence suggests that EF and ER abnormalities persist during euthymic states, are seen during periods of lower expression of psychotic symptoms, and are not normalized, even when symptoms have remitted with treatment. As such, EF and ER represent a broad domain of dysfunction in psychiatric illness that is unaddressed by current treatments. This pressing clinical and scientific need has motivated efforts to identify potential novel cognitive enhancers in schizophrenia, including Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS), Treatment Units for Research on Neurocognition and Schizophrenia (TURNS), and other related approaches. What is notable about MATRICS is that it was conceived as a process that would also involve changing expectations at the FDA to allow as a primary indication the improvement in cognition, even in the absence of effects on other symptoms of the disorder. Potential compounds emerging from this and related work target glutamatergic transmission, nicotinic acetylcholine receptors, and selective dopaminergic agents. Another emerging trend involves brain training approaches. Having already identified the neural circuits important for EF and ER, as well as demonstrated abnormalities in these circuits across multiple psychiatric disorders, it may be possible to strengthen the functioning of these neural circuits by repeatedly doing adaptive versions of the tasks that normally engage them. In other words, deficits in working memory may be ameliorated through a challenging course of working memory training. There is a growing body of evidence suggesting that this once-controversial proposition may be possible, at least in certain contexts. Moreover, adult brains, even those dysfunctional because of mental illness, still retain a surprising degree of plasticity.79-81
Brain training has been most extensively investigated in schizophrenia, where it derives from a tradition of cognitive remediation research and psychosocial rehabilitation. In schizophrenia, evidence shows that brain training alone, aimed at EF and basic sensory discrimination and gating, can yield beneficial effects on tests of EF and in terms of daily functioning.82 Very early evidence points to promise for EF-focused brain training in depression as well.83 Within the affective disorder spectrum, additional benefit may be gained through brain training methods that diminish negative biases. There is already evidence in anxiety disorders that training subjects to avert their attention from threat stimuli may modify their attentional bias and diminish symptoms.84,85 Thus, an optimal brain training approach for affective disorders may target both the EF abnormalities identified in these disorders and emotional reactivity, which together may improve their capacity for ER. More generally, computer-based brain training interventions have the advantage that they can be readily standardized and well controlled in randomized trials, do not require involvement of a therapist or even particular treatment expertise in the provider, and can be readily disseminated. Much more work, however, will be needed to optimize this training approach (eg, dose, duration, type of stimuli, ideal target populations) from where it currently is.
Finally, and in line with the concepts driving brain training, it may be possible to selectively target EF- and ERrelated circuitry using brain stimulation. Transcranial magnetic stimulation (TMS), for example, can be used to activate local superficial cortical sites, and their interconnected network partners, and when applied repetitively (rTMS) produces plastic circuit changes. rTMS directed at the DLPFC has been used for over two decades for the treatment of MDD, for which it received FDA approval in 2008. Left high-frequency DLPFC rTMS also appears to improve cognitive functioning primarily in studies of depression,86 and bilateral DLPFC rTMS improves working memory in schizophrenia.87 Despite this, relatively little is understood about the mechanism of rTMS. One recent resting-state fMRI study examined connectivity patterns of sites within the DLPFC that are in clinical studies associated with better or worse clinical outcome.88 They found that the sites associated with the best clinical outcome were also those for which the reciprocal relationship was strongest with the default mode network. We have recently used concurrent TMS and fMRI89 to examine the effects of transient activation of DLPFC subregions with single excitatory TMS pulses, as well as inhibition of each of these subregions with trains of low-frequency rTMS. We found that targeting a region in the posterior DLPFC, typically associated with the fronto-parietal network, causally inhibits in particular the mPFC component of the default mode network.90 By contrast, an anterior DLPFC regions typically associated with the cingulo-opercular network fails to do so. Taken together, these data suggest that improvements in cognition may be possible with rTMS, and that targeting rTMS based on an understanding of how specific cortical targets causally modulate key cognitive control and default mode network circuitry, such as through concurrent TMS/fMRI, may allow optimization and personalization of rTMS treatment.
Conclusion
The importance of abnormalities in EF and ER is clear across a broad range of psychiatric disorders, suggesting that they represent core and related endophenotypes of severe mental illnesses. The findings reviewed here demonstrate that a clearer neurobiological understanding of these disruptions in both EF and ER is beginning to emerge, and that this understanding has already led to promising avenues for remediation of these deficits.
Selected abbreviations and acronyms
- dACC
dorsal anterior cingulate cortex
- DLPFC
dorsolateral prefrontal cortex
- EF
executive functioning
- ER
emotional regulation
- rTMS
repetitive transcranial magnetic stimulation
- vACC
ventral anterior cingulate cortex
- VLPFC
ventrolateral prefrontal cortex
Contributor Information
Amit Etkin, Departments of Psychiatry and Behavioral Sciences and Psychology, Stanford University, Stanford, California, USA.
Anett Gyurak, Departments of Psychiatry and Behavioral Sciences and Psychology, Stanford University, Stanford, California, USA, Sierra-Pacific Mental Illness Research, Education, and Clinical Center (MIRECC), Veterans Affairs Palo Alto Health Care System, Palo Alto, California, USA.
Ruth O'Hara, Departments of Psychiatry and Behavioral Sciences and Psychology, Stanford University, Stanford, California, USA.
REFERENCES
- 1.Diamond A. Executive functions. Annu Rev Psychol. 2013;64:135–168. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miyake A., Friedman NP., Emerson MJ., Witzki AH., Howerter A., Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cogn Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- 3.Birrell JM., Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dias R., Robbins TW., Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- 5.Milner B. Effects of different brain lesions on card sorting. Arch Neurol. 1963;9 [Google Scholar]
- 6.Miller EK., Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 7.Dosenbach NU., Fair DA., Miezin FM., et al Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dosenbach NU., Visscher KM., Palmer ED., et al A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aron AR. From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biol Psychiatry. 2011;69:e55–e68. doi: 10.1016/j.biopsych.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buckner RL., Andrews-Hanna JR., Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 11.Ossandon T., Jerbi K., Vidal JR., et al Transient suppression of broadband gamma power in the default-mode network is correlated with task complexity and subject performance. J Neurosci. 2011;31:14521–14530. doi: 10.1523/JNEUROSCI.2483-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eichele T., Debener S., Calhoun VD., et al Prediction of human errors by maladaptive changes in event-related brain networks. Proc Natl Acad Sci U S A. 2008;105:6173–6178. doi: 10.1073/pnas.0708965105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prado J., Weissman DH. Heightened interactions between a key default mode region and a key task-positive region are linked to suboptimal current performance but to enhanced future performance. Neuroimage. 2011;56:2276–2282. doi: 10.1016/j.neuroimage.2011.03.048. [DOI] [PubMed] [Google Scholar]
- 14.Weissman DH., Roberts KC., Visscher KM., Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- 15.Gross JJ. Emotion regulation: conceptual foundations. In: Gross JJ, ed. Handbook of Emotion Regulation. New York, NY: Guilford Press. 2007 [Google Scholar]
- 16.Gyurak A., Gross JJ., Etkin A. Explicit and implicit emotion regulation: a dual-process framework. Cognition Emotion. 2011;25:400–412. doi: 10.1080/02699931.2010.544160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buhle JT., Silvers JA., Wager TD., et al Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb Cortex. 2013. [Epub ahead of print] doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalisch R. The functional neuroanatomy of reappraisal: time matters. Neurosci Biobehav Rev. 2009,33:1215-1226;33:1215–1226. doi: 10.1016/j.neubiorev.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Egner T., Etkin A., Gale S., Hirsch J. Dissociable neural systems resolve conflict from emotional versus nonemotional distracters. Cereb Cortex. 2008;18:1475–1484. doi: 10.1093/cercor/bhm179. [DOI] [PubMed] [Google Scholar]
- 20.Etkin A., Egner T., Peraza DM., Kandel ER., Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 21.Maier ME., di Pellegrino G. Impaired conflict adaptation in an emotional task context following rostral anterior cingulate cortex lesions in humans. J Cogn Neurosci. 2012;24:2070–2079. doi: 10.1162/jocn_a_00266. [DOI] [PubMed] [Google Scholar]
- 22.Harvey PD., Strassnig M. Predicting the severity of everyday functional disability in people with schizophrenia: cognitive deficits, functional capacity, symptoms, and health status. World Psychiatry . 2012;11:73–79. doi: 10.1016/j.wpsyc.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fioravanti M., Bianchi V., Cinti ME. Cognitive deficits in schizophrenia: an updated metanalysis of the scientific evidence. BMC Psychiatry. 2012;12:64. doi: 10.1186/1471-244X-12-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fett AK., Viechtbauer W., Dominguez MD., Penn DL., van Os J., Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci Biobehav Rev. 2011;35:573–588. doi: 10.1016/j.neubiorev.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Harvey PD., Green MF., Keefe RS., Velligan DI. Cognitive functioning in schizophrenia: a consensus statement on its role in the definition and evaluation of effective treatments for the illness. J Clin Psychiatry. 2004;65:361–372. [PubMed] [Google Scholar]
- 26.Fusar-Poli P., Deste G., Smieskova R., et al Cognitive functioning in prodromal psychosis: a meta-analysis. Arch Gen Psychiatry. 2012;69:562–571. doi: 10.1001/archgenpsychiatry.2011.1592. [DOI] [PubMed] [Google Scholar]
- 27.Snitz BE., Macdonald AW 3rd., Carter CS. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schizophr Bull. 2006;32:179–194. doi: 10.1093/schbul/sbi048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Owens SF., Rijsdijk F., Picchioni MM., et al Genetic overlap between schizophrenia and selective components of executive function. Schizophr Res. 2011;127:181–187. doi: 10.1016/j.schres.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Bora E., Yucel M., Pantelis C. Cognitive endophenotypes of bipolar disorder: a meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. J Affect Disord. 2009;113:1–20. doi: 10.1016/j.jad.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 30.Depp CA., Mausbach BT., Harmell AL., et al Meta-analysis of the association between cognitive abilities and everyday functioning in bipolar disorder. Bipolar Disord. 2012;14:217–226. doi: 10.1111/j.1399-5618.2012.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bora E., Yucel M., Pantelis C. Cognitive impairment in affective psychoses: a meta-analysis. Schizophr Bull. 2010;36:112–125. doi: 10.1093/schbul/sbp093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minzenberg MJ., Laird AR., Thelen S., Carter CS., Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66:811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Callicott JH., Mattay VS., Bertolino A., et al Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb Cortex. 1999;9:20–26. doi: 10.1093/cercor/9.1.20. [DOI] [PubMed] [Google Scholar]
- 34.Callicott JH., Mattay VS., Verchinski BA., Marenco S., Egan MF., Weinberger DR. Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. Ami J Psychiatry. 2003;160:2209–2215. doi: 10.1176/appi.ajp.160.12.2209. [DOI] [PubMed] [Google Scholar]
- 35.Whitfield-Gabrieli S., Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- 36.Whitfield-Gabrieli S., Thermenos HW., Milanovic S., et al Hyperactivity and hyperconnectivity of the default network in schizophrenia and in firstdegree relatives of persons with schizophrenia. Proc Natl Acad Sci USA. 2009;106:1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chai XJ., Whitfield-Gabrieli S., Shinn AK., et al Abnormal medial prefrontal cortex resting-state connectivity in bipolar disorder and schizophrenia. Neuropsychopharmacology. 2011;36:2009–2017. doi: 10.1038/npp.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Callicott JH., Egan MF., Mattay VS., et al Abnormal fMRI response of the dorsolateral prefrontal cortex in cognitively intact siblings of patients with schizophrenia. Am J Psychiatry. 2003;160:709–719. doi: 10.1176/appi.ajp.160.4.709. [DOI] [PubMed] [Google Scholar]
- 39.Shim G., Oh JS., Jung WH., et al Altered resting-state connectivity in subjects at ultra-high risk for psychosis: an fMRI study. Behav Brain Fund. 2010;6 doi: 10.1186/1744-9081-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anticevic A., Gancsos M., Murray JD., et al NMDA receptor function in large-scale anticorrelated neural systems with implications for cognition and schizophrenia. Proc Natl Acad Sci U S A. 2012;109:16720–16725. doi: 10.1073/pnas.1208494109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fernandez-Corcuera P., Salvador R., Monte GC., et al Bipolar depressed patients show both failure to activate and failure to de-activate during performance of a working memory task. J Affect Disord. 2013;148:170–178. doi: 10.1016/j.jad.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 42.Townsend J., Bookheimer SY., Foland-Ross LC., Sugar CA., Altshuler LL. fMRI abnormalities in dorsolateral prefrontal cortex during a working memory task in manic, euthymic and depressed bipolar subjects. Psychiatry Res. 2010;182:22–29. doi: 10.1016/j.pscychresns.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thermenos HW., Goldstein JM., Milanovic SM., et al An fMRI study of working memory in persons with bipolar disorder or at genetic risk for bipolar disorder. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:120–131. doi: 10.1002/ajmg.b.30964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drapier D., Surguladze S., Marshall N., et al Genetic liability for bipolar disorder is characterized by excess frontal activation in response to a working memory task. Biol Psychiatry. 2008;64:513–520. doi: 10.1016/j.biopsych.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 45.Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychol Bull. 2013;139:81–132. doi: 10.1037/a0028727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pitman RK., Rasmusson AM., Koenen KC., et al Biological studies of posttraumatic stress disorder. Nat Rev Neurosci. 2012;13:769–787. doi: 10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aupperle RL., Melrose AJ., Stein MB., Paulus MP. Executive function and PTSD: disengaging from trauma. Neuropharmacology. 2012;62:686–694. doi: 10.1016/j.neuropharm.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Polak AR., Witteveen AB., Reitsma JB., Olff M. The role of executive function in posttraumatic stress disorder: a systematic review. J Affect Disord. 2012;141:11–21. doi: 10.1016/j.jad.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 49.O'Toole MS., Pedersen AD. A systematic review of neuropsychological performance in social anxiety disorder. Nord J Psychiatry. 2011;65:147–161. doi: 10.3109/08039488.2011.565801. [DOI] [PubMed] [Google Scholar]
- 50.Menzies L., Chamberlain SR., Laird AR., Thelen SM., Sahakian BJ., Bullmore ET. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: the orbitofronto-striatal model revisited. Neurosci Biobehav Rev. 2008;32:525–549. doi: 10.1016/j.neubiorev.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Olley A., Malhi G., Sachdev P. Memory and executive functioning in obsessive-compulsive disorder: a selective review. J Affect Disord. 2007;104:15–23. doi: 10.1016/j.jad.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 52.Chang CT., Chang YH., Yung-Wei Wu J., et al Neuropsychological functions impairment in different subtypes of bipolar disorder with or without comorbid anxiety disorders. Psychiatry Res. 2012;200:246–251. doi: 10.1016/j.psychres.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 53.Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2011;36:183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barch DM., Sheline Yl., Csernansky JG., Snyder AZ. Working memory and prefrontal cortex dysfunction: specificity to schizophrenia compared with major depression. Biol Psychiatry. 2003;53:376–384. doi: 10.1016/s0006-3223(02)01674-8. [DOI] [PubMed] [Google Scholar]
- 55.Holmes AJ., MacDonald AW 3rd., Carter CS., Barch DM., Andrew Stenger V., Cohen JD. Prefrontal functioning during context processing in schizophrenia and major depression: an event-related fMRI study. Schizophr Res. 2005;76:199–206. doi: 10.1016/j.schres.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 56.Sheline Yl., Barch DM., Price JL., et al The default mode network and self-referential processes in depression. Proc Na tl Acad Sci US A. 2009;106:1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walter M., Henning A., Grimm S., et al The relationship between aberrant neuronal activation in the pregenual anterior cingulate, altered glutamatergic metabolism, and anhedonia in major depression. Arch Gen Psychiatry. 2009;66:478–486. doi: 10.1001/archgenpsychiatry.2009.39. [DOI] [PubMed] [Google Scholar]
- 58.Hamilton JP., Furman DJ., Chang C., Thomason ME., Dennis E., Gotlib IH. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol Psychiatry. 2011;70:327–333. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Etkin A., Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moores KA., Clark CR., McFarlane AC., Brown GC., Puce A., Taylor DJ. Abnormal recruitment of working memory updating networks during maintenance of trauma-neutral information in post-traumatic stress disorder. Psychiatry Res. 2008;163:156–170. doi: 10.1016/j.pscychresns.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 61.Chen AC., Etkin A. Hippocampal network connectivity and activation differentiates post-traumatic stress disorder from generalized anxiety disorder. Neuropsychopharmacology. 2013;38:1889–1898. doi: 10.1038/npp.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gotlib IH., Joormann J. Cognition and depression: current status and future directions. Annu Rev Clin Psychol. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anticevic A., Cole MW., Murray JD., Corlett PR., Wang XJ., Krystal JH. The role of default network deactivation in cognition and disease. Trends Cogn Sci. 2012;16:584–592. doi: 10.1016/j.tics.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morris RW., Sparks A., Mitchell PB., Weickert CS., Green MJ. Lack of cortico-limbic coupling in bipolar disorder and schizophrenia during emotion regulation. Transl Psychiatry. 2012;2:e90. doi: 10.1038/tp.2012.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Delvecchio G., Sugranyes G., Frangou S. Evidence of diagnostic specificity in the neural correlates of facial affect processing in bipolar disorder and schizophrenia: a meta-analysis of functional imaging studies. Psychol Med. 2013;43:553–569. doi: 10.1017/S0033291712001432. [DOI] [PubMed] [Google Scholar]
- 66.Townsend JD., Torrisi SJ., Lieberman MD., Sugar CA., Bookheimer SY., Altshuler LL. Frontal-amygdala connectivity alterations during emotion downregulation in bipolar I disorder. Biol Psychiatry. 2013;73:127–135. doi: 10.1016/j.biopsych.2012.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Greening SG., Osuch EA., Williamson PC., Mitchell DG. The neural correlates of regulating positive and negative emotions in medication-free major depression. Soc Cogn Affect Neurosci. 2013. [Epub ahead of print] doi: 10.1093/scan/nst027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kanske P., Heissler J., Schonfelder S., Wessa M. Neural correlates of emotion regulation deficits in remitted depression: the influence of regulation strategy, habitual regulation use, and emotional valence. Neuroimage. 2012;61:686–693. doi: 10.1016/j.neuroimage.2012.03.089. [DOI] [PubMed] [Google Scholar]
- 69.Johnstone T., van Reekum CM., Urry HL., Kalin NH., Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Erk S., Mikschl A., Stier S., et al Acute and sustained effects of cognitive emotion regulation in major depression. J Neurosci. 2010;30:15726–15734. doi: 10.1523/JNEUROSCI.1856-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smoski MJ., Keng SL., Schiller CE., Minkel J., Dichter GS. Neural mechanisms of cognitive reappraisal in remitted major depressive disorder. J Affect Disord. 2013;151:171–177. doi: 10.1016/j.jad.2013.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.New AS., Fan J., Murrough JW., et al A functional magnetic resonance imaging study of deliberate emotion regulation in resilience and posttraumatic stress disorder. Biol Psychiatry. 2009;66:656–664. doi: 10.1016/j.biopsych.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 73.Ball TM., Ramsawh HJ., Campbell-Sills L., Paulus MP., Stein MB. Prefrontal dysfunction during emotion regulation in generalized anxiety and panic disorders. Psychol Med. 2013;43:1475–1486. doi: 10.1017/S0033291712002383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goldin PR., Manber T., Hakimi S., Canli T., Gross JJ. Neural bases of social anxiety disorder: emotional reactivity and cognitive regulation during social and physical threat. Arch Gen Psychiatry. 2009;66:170–180. doi: 10.1001/archgenpsychiatry.2008.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Etkin A., Schatzberg AF. Common abnormalities and disorder-specific compensation during implicit regulation of emotional processing in generalized anxiety and major depressive disorders. Am J Psychiatry. 2011;168:968–978. doi: 10.1176/appi.ajp.2011.10091290. [DOI] [PubMed] [Google Scholar]
- 76.Milad MR., Pitman RK., Ellis CB., et al Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Holt DJ., Coombs G., Zeidan MA., Goff DC., Milad MR. Failure of neural responses to safety cues in schizophrenia. Arch Gen Psychiatry. 2012;69:893–903. doi: 10.1001/archgenpsychiatry.2011.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Milad MR., Furtak SC., Greenberg JL., et al Deficits in conditioned fear extinction in obsessive-compulsive disorder and neurobiological changes in the fear circuit. JAMA Psychiatry. 2013;70:608–618. doi: 10.1001/jamapsychiatry.2013.914. [DOI] [PubMed] [Google Scholar]
- 79.Buonomano DV., Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- 80.Jaeggi SM., Buschkuehl M., Jonides J., Perrig WJ. Improving fluid intelligence with training on working memory. Proc Natl Acad Sci USA. 2008;105:6829–6833. doi: 10.1073/pnas.0801268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Subramaniam K., Luks TL., Fisher M., Simpson GV., Nagarajan S., Vinogradov S. Computerized cognitive training restores neural activity within the reality monitoring network in schizophrenia. Neuron. 2012;73:842–853. doi: 10.1016/j.neuron.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vinogradov S., Fisher M., de Villers-Sidani E. Cognitive training for impaired neural systems in neuropsychiatric illness. Neuropsychopharmacology. 2012;37:43–76. doi: 10.1038/npp.2011.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Siegle GJ., Ghinassi F., Thase ME. Neurobehavioral therapies in the 21st century: summary of an emerging field and an extended example of cognitive control training for depression. Cogn Ther Res. 2007;31:235–262. [Google Scholar]
- 84.Hakamata Y., Lissek S., Bar-Haim Y., et al Attention bias modification treatment: a meta-analysis toward the establishment of novel treatment for anxiety. Biol Psychiatry. 2010;68:982–990. doi: 10.1016/j.biopsych.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hallion LS., Ruscio AM. A meta-analysis of the effect of cognitive bias modification on anxiety and depression. Psychol Bull. 2011;137:940–958. doi: 10.1037/a0024355. [DOI] [PubMed] [Google Scholar]
- 86.Guse B., Falkai P., Wobrock T. Cognitive effects of high-frequency repetitive transcranial magnetic stimulation: a systematic review. J Neural Transm. 2010;117:105–122. doi: 10.1007/s00702-009-0333-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Barr MS., Farzan F., Rajji TK., et al Can repetitive magnetic stimulation improve cognition in schizophrenia? Pilot data from a randomized controlled trial. Biol Psychiatry. 2013;73:510–517. doi: 10.1016/j.biopsych.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 88.Fox MD., Buckner RL., White MP., Greicius MD., Pascual-Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry. 2012;72:595–603. doi: 10.1016/j.biopsych.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bestmann S., Ruff CC., Blankenburg F., Weiskopf N., Driver J., Rothwell JC. Mapping causal interregional influences with concurrent TMS-fMRI. Exp Brain Res. 2008;191:383–402. doi: 10.1007/s00221-008-1601-8. [DOI] [PubMed] [Google Scholar]
- 90.Chen AC., Chang C., Oathes DJ., et al Causal interactions between frontoparietal central executive and default-mode networks in humans. Proc. Natl Acad Sci US A. November 18, 2013 [Epub ahead of print] doi: 10.1073/pnas.1311772110. [DOI] [PMC free article] [PubMed] [Google Scholar]