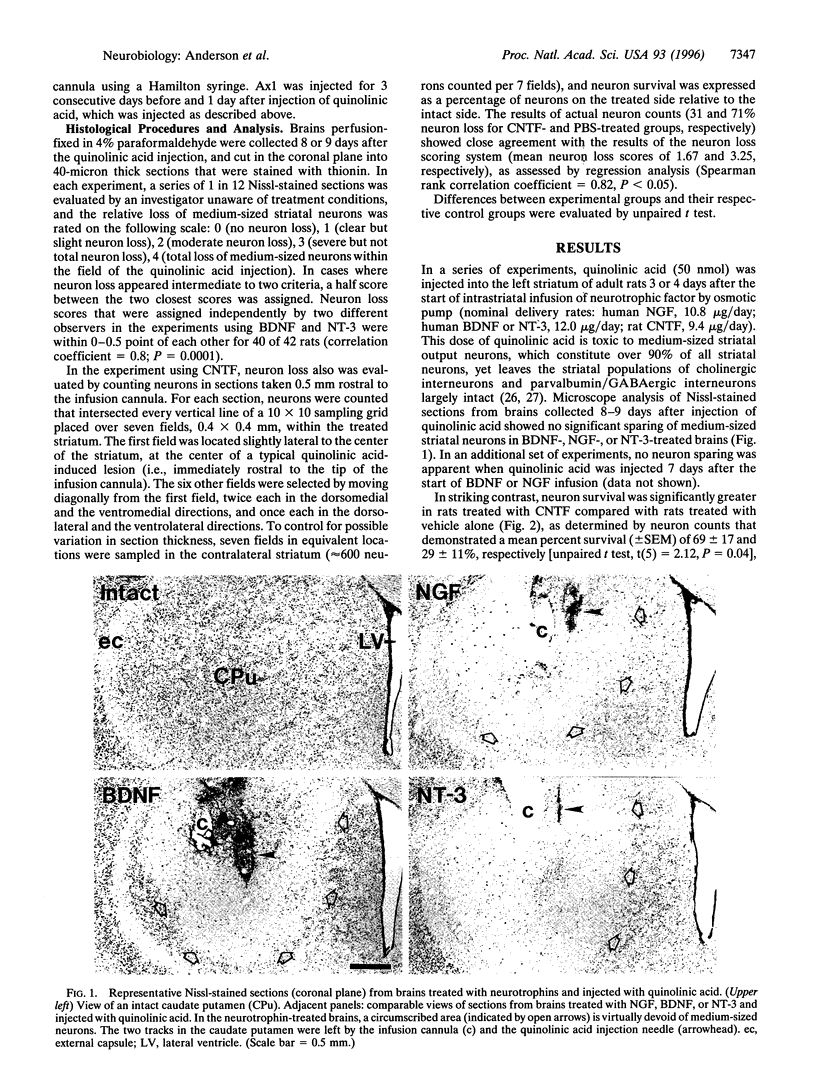
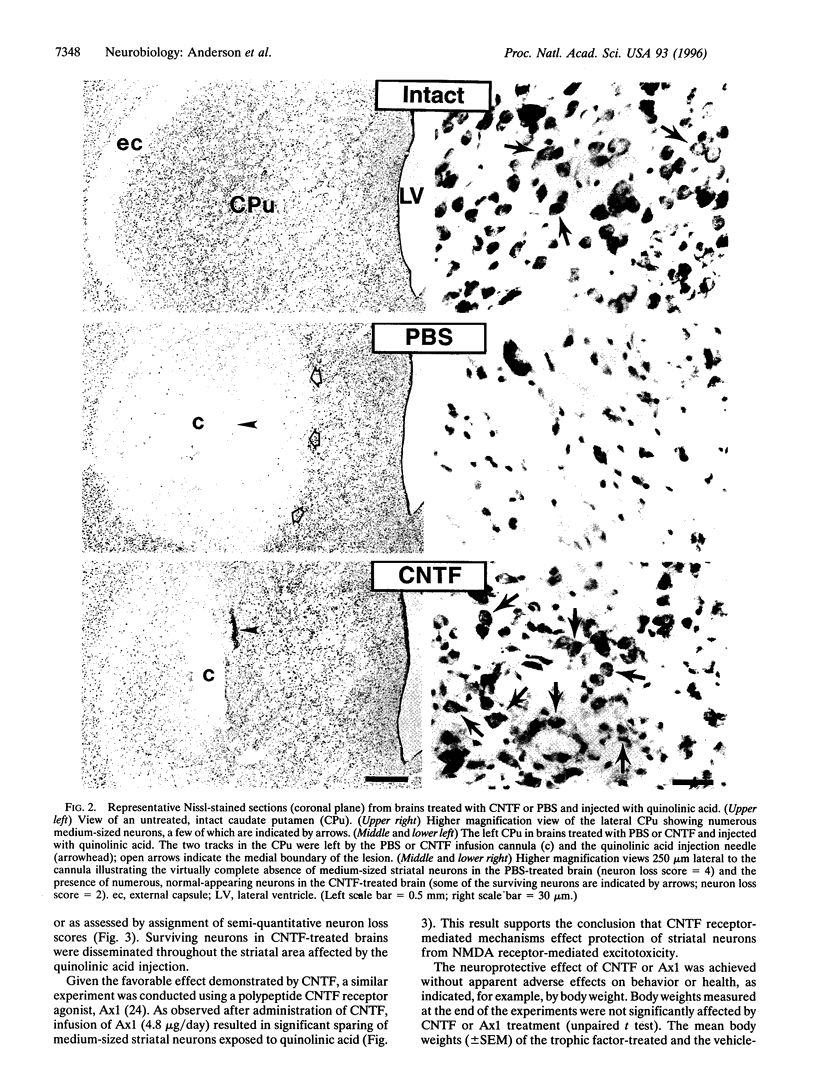
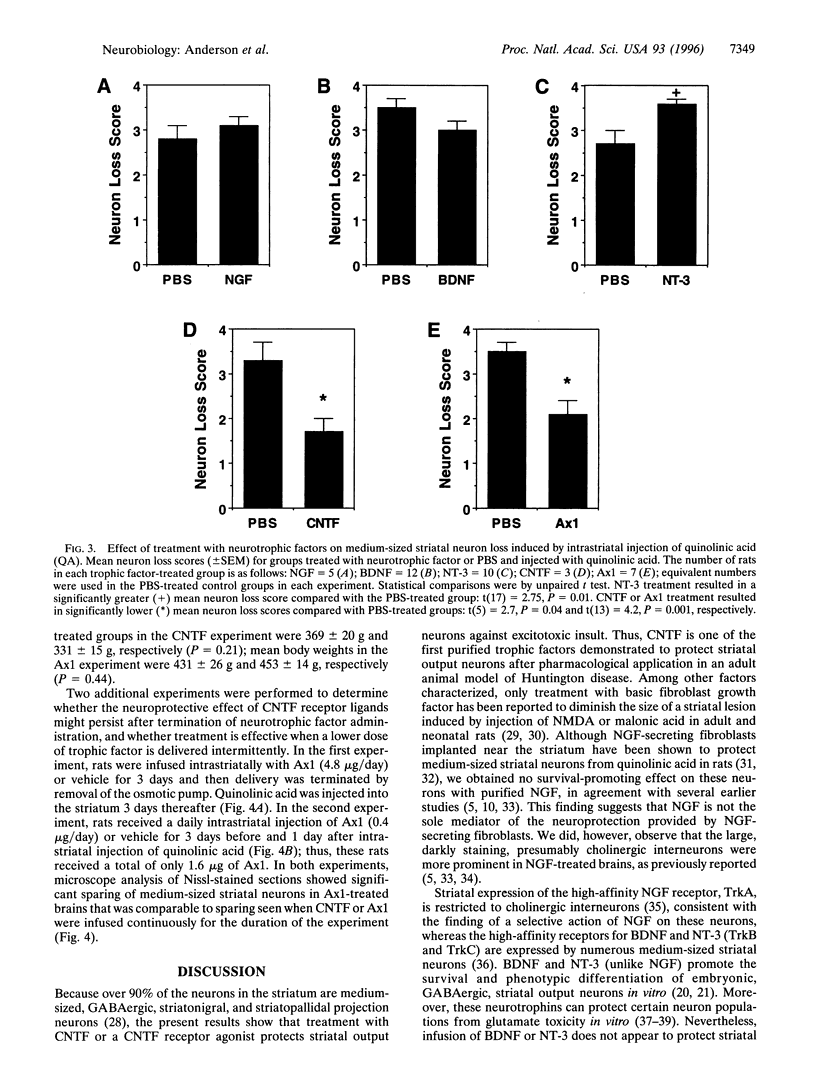
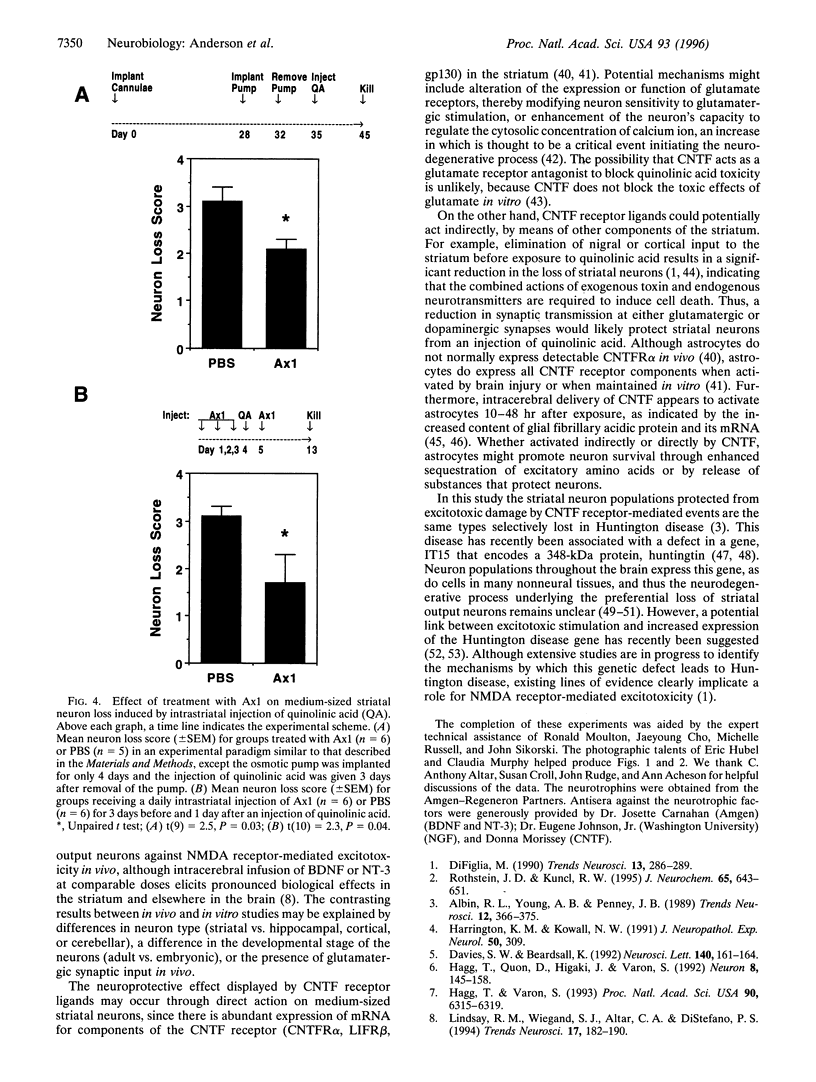
Abstract
Huntington disease is a dominantly inherited, untreatable neurological disorder featuring a progressive loss of striatal output neurons that results in dyskinesia, cognitive decline, and, ultimately, death. Neurotrophic factors have recently been shown to be protective in several animal models of neurodegenerative disease, raising the possibility that such substances might also sustain the survival of compromised striatal output neurons. We determined whether intracerebral administration of brain-derived neurotrophic factor, nerve growth factor, neurotrophin-3, or ciliary neurotrophic factor could protect striatal output neurons in a rodent model of Huntington disease. Whereas treatment with brain-derived neurotrophic factor, nerve growth factor, or neurotrophin-3 provided no protection of striatal output neurons from death induced by intrastriatal injection of quinolinic acid, an N-methyl-D-aspartate glutamate receptor agonist, treatment with ciliary neurotrophic factor afforded marked protection against this neurodegenerative insult.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albin R. L., Tagle D. A. Genetics and molecular biology of Huntington's disease. Trends Neurosci. 1995 Jan;18(1):11–14. doi: 10.1016/0166-2236(95)93943-r. [DOI] [PubMed] [Google Scholar]
- Albin R. L., Young A. B., Penney J. B. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989 Oct;12(10):366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Altar C. A., Siuciak J. A., Wright P., Ip N. Y., Lindsay R. M., Wiegand S. J. In situ hybridization of trkB and trkC receptor mRNA in rat forebrain and association with high-affinity binding of [125I]BDNF, [125I]NT-4/5 and [125I]NT-3. Eur J Neurosci. 1994 Sep 1;6(9):1389–1405. doi: 10.1111/j.1460-9568.1994.tb01001.x. [DOI] [PubMed] [Google Scholar]
- Anderson K. D., Alderson R. F., Altar C. A., DiStefano P. S., Corcoran T. L., Lindsay R. M., Wiegand S. J. Differential distribution of exogenous BDNF, NGF, and NT-3 in the brain corresponds to the relative abundance and distribution of high-affinity and low-affinity neurotrophin receptors. J Comp Neurol. 1995 Jun 26;357(2):296–317. doi: 10.1002/cne.903570209. [DOI] [PubMed] [Google Scholar]
- Buisson A., Pateau V., Plotkine M., Boulu R. G. Nigrostriatal pathway modulates striatum vulnerability to quinolinic acid. Neurosci Lett. 1991 Oct 14;131(2):257–259. doi: 10.1016/0304-3940(91)90627-6. [DOI] [PubMed] [Google Scholar]
- Carlock L., Walker P. D., Shan Y., Gutridge K. Transcription of the Huntington disease gene during the quinolinic acid excitotoxic cascade. Neuroreport. 1995 May 30;6(8):1121–1124. doi: 10.1097/00001756-199505300-00012. [DOI] [PubMed] [Google Scholar]
- Cheng B., Mattson M. P. NT-3 and BDNF protect CNS neurons against metabolic/excitotoxic insults. Brain Res. 1994 Mar 21;640(1-2):56–67. doi: 10.1016/0006-8993(94)91857-0. [DOI] [PubMed] [Google Scholar]
- Choi D. W. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988 Oct;1(8):623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Davies S. W., Beardsall K. Nerve growth factor selectively prevents excitotoxin induced degeneration of striatal cholinergic neurones. Neurosci Lett. 1992 Jun 22;140(2):161–164. doi: 10.1016/0304-3940(92)90092-l. [DOI] [PubMed] [Google Scholar]
- DiFiglia M. Excitotoxic injury of the neostriatum: a model for Huntington's disease. Trends Neurosci. 1990 Jul;13(7):286–289. doi: 10.1016/0166-2236(90)90111-m. [DOI] [PubMed] [Google Scholar]
- Emerich D. F., Hammang J. P., Baetge E. E., Winn S. R. Implantation of polymer-encapsulated human nerve growth factor-secreting fibroblasts attenuates the behavioral and neuropathological consequences of quinolinic acid injections into rodent striatum. Exp Neurol. 1994 Nov;130(1):141–150. doi: 10.1006/exnr.1994.1193. [DOI] [PubMed] [Google Scholar]
- Figueredo-Cardenas G., Anderson K. D., Chen Q., Veenman C. L., Reiner A. Relative survival of striatal projection neurons and interneurons after intrastriatal injection of quinolinic acid in rats. Exp Neurol. 1994 Sep;129(1):37–56. doi: 10.1006/exnr.1994.1145. [DOI] [PubMed] [Google Scholar]
- Frim D. M., Uhler T. A., Short M. P., Ezzedine Z. D., Klagsbrun M., Breakefield X. O., Isacson O. Effects of biologically delivered NGF, BDNF and bFGF on striatal excitotoxic lesions. Neuroreport. 1993 Apr;4(4):367–370. doi: 10.1097/00001756-199304000-00006. [DOI] [PubMed] [Google Scholar]
- Graybiel A. M. Neurotransmitters and neuromodulators in the basal ganglia. Trends Neurosci. 1990 Jul;13(7):244–254. doi: 10.1016/0166-2236(90)90104-i. [DOI] [PubMed] [Google Scholar]
- Hagg T., Quon D., Higaki J., Varon S. Ciliary neurotrophic factor prevents neuronal degeneration and promotes low affinity NGF receptor expression in the adult rat CNS. Neuron. 1992 Jan;8(1):145–158. doi: 10.1016/0896-6273(92)90116-u. [DOI] [PubMed] [Google Scholar]
- Hagg T., Varon S. Ciliary neurotrophic factor prevents degeneration of adult rat substantia nigra dopaminergic neurons in vivo. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6315–6319. doi: 10.1073/pnas.90.13.6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson C. E., Phillips H. S., Pollock R. A., Davies A. M., Lemeulle C., Armanini M., Simmons L., Moffet B., Vandlen R. A., Simpson LC corrected to Simmons L. GDNF: a potent survival factor for motoneurons present in peripheral nerve and muscle. Science. 1994 Nov 11;266(5187):1062–1064. doi: 10.1126/science.7973664. [DOI] [PubMed] [Google Scholar]
- Ip N. Y., McClain J., Barrezueta N. X., Aldrich T. H., Pan L., Li Y., Wiegand S. J., Friedman B., Davis S., Yancopoulos G. D. The alpha component of the CNTF receptor is required for signaling and defines potential CNTF targets in the adult and during development. Neuron. 1993 Jan;10(1):89–102. doi: 10.1016/0896-6273(93)90245-m. [DOI] [PubMed] [Google Scholar]
- Kirschner P. B., Henshaw R., Weise J., Trubetskoy V., Finklestein S., Schulz J. B., Beal M. F. Basic fibroblast growth factor protects against excitotoxicity and chemical hypoxia in both neonatal and adult rats. J Cereb Blood Flow Metab. 1995 Jul;15(4):619–623. doi: 10.1038/jcbfm.1995.76. [DOI] [PubMed] [Google Scholar]
- Kordower J. H., Charles V., Bayer R., Bartus R. T., Putney S., Walus L. R., Friden P. M. Intravenous administration of a transferrin receptor antibody-nerve growth factor conjugate prevents the degeneration of cholinergic striatal neurons in a model of Huntington disease. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):9077–9080. doi: 10.1073/pnas.91.19.9077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landwehrmeyer G. B., McNeil S. M., Dure L. S., 4th, Ge P., Aizawa H., Huang Q., Ambrose C. M., Duyao M. P., Bird E. D., Bonilla E. Huntington's disease gene: regional and cellular expression in brain of normal and affected individuals. Ann Neurol. 1995 Feb;37(2):218–230. doi: 10.1002/ana.410370213. [DOI] [PubMed] [Google Scholar]
- Lindholm D., Dechant G., Heisenberg C. P., Thoenen H. Brain-derived neurotrophic factor is a survival factor for cultured rat cerebellar granule neurons and protects them against glutamate-induced neurotoxicity. Eur J Neurosci. 1993 Nov 1;5(11):1455–1464. doi: 10.1111/j.1460-9568.1993.tb00213.x. [DOI] [PubMed] [Google Scholar]
- Lindsay R. M., Wiegand S. J., Altar C. A., DiStefano P. S. Neurotrophic factors: from molecule to man. Trends Neurosci. 1994 May;17(5):182–190. doi: 10.1016/0166-2236(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Masiakowski P., Liu H. X., Radziejewski C., Lottspeich F., Oberthuer W., Wong V., Lindsay R. M., Furth M. E., Panayotatos N. Recombinant human and rat ciliary neurotrophic factors. J Neurochem. 1991 Sep;57(3):1003–1012. doi: 10.1111/j.1471-4159.1991.tb08250.x. [DOI] [PubMed] [Google Scholar]
- Mattson M. P., Lovell M. A., Furukawa K., Markesbery W. R. Neurotrophic factors attenuate glutamate-induced accumulation of peroxides, elevation of intracellular Ca2+ concentration, and neurotoxicity and increase antioxidant enzyme activities in hippocampal neurons. J Neurochem. 1995 Oct;65(4):1740–1751. doi: 10.1046/j.1471-4159.1995.65041740.x. [DOI] [PubMed] [Google Scholar]
- Mitsumoto H., Ikeda K., Klinkosz B., Cedarbaum J. M., Wong V., Lindsay R. M. Arrest of motor neuron disease in wobbler mice cotreated with CNTF and BDNF. Science. 1994 Aug 19;265(5175):1107–1110. doi: 10.1126/science.8066451. [DOI] [PubMed] [Google Scholar]
- Mizuno K., Carnahan J., Nawa H. Brain-derived neurotrophic factor promotes differentiation of striatal GABAergic neurons. Dev Biol. 1994 Sep;165(1):243–256. doi: 10.1006/dbio.1994.1250. [DOI] [PubMed] [Google Scholar]
- Nozaki K., Finklestein S. P., Beal M. F. Basic fibroblast growth factor protects against hypoxia-ischemia and NMDA neurotoxicity in neonatal rats. J Cereb Blood Flow Metab. 1993 Mar;13(2):221–228. doi: 10.1038/jcbfm.1993.27. [DOI] [PubMed] [Google Scholar]
- Oppenheim R. W., Houenou L. J., Johnson J. E., Lin L. F., Li L., Lo A. C., Newsome A. L., Prevette D. M., Wang S. Developing motor neurons rescued from programmed and axotomy-induced cell death by GDNF. Nature. 1995 Jan 26;373(6512):344–346. doi: 10.1038/373344a0. [DOI] [PubMed] [Google Scholar]
- Panayotatos N., Radziejewska E., Acheson A., Pearsall D., Thadani A., Wong V. Exchange of a single amino acid interconverts the specific activity and gel mobility of human and rat ciliary neurotrophic factors. J Biol Chem. 1993 Sep 5;268(25):19000–19003. [PubMed] [Google Scholar]
- Persichetti F., Ambrose C. M., Ge P., McNeil S. M., Srinidhi J., Anderson M. A., Jenkins B., Barnes G. T., Duyao M. P., Kanaley L. Normal and expanded Huntington's disease gene alleles produce distinguishable proteins due to translation across the CAG repeat. Mol Med. 1995 May;1(4):374–383. [PMC free article] [PubMed] [Google Scholar]
- Pérez-Navarro E., Alberch J., Arenas E., Calvo N., Marsal J. Nerve growth factor and basic fibroblast growth factor protect cholinergic neurons against quinolinic acid excitotoxicity in rat neostriatum. Eur J Neurosci. 1994 May 1;6(5):706–711. doi: 10.1111/j.1460-9568.1994.tb00982.x. [DOI] [PubMed] [Google Scholar]
- Qin Y., Soghomonian J. J., Chesselet M. F. Effects of quinolinic acid on messenger RNAs encoding somatostatin and glutamic acid decarboxylases in the striatum of adult rats. Exp Neurol. 1992 Feb;115(2):200–211. doi: 10.1016/0014-4886(92)90054-t. [DOI] [PubMed] [Google Scholar]
- Radziejewski C., Robinson R. C., DiStefano P. S., Taylor J. W. Dimeric structure and conformational stability of brain-derived neurotrophic factor and neurotrophin-3. Biochemistry. 1992 May 12;31(18):4431–4436. doi: 10.1021/bi00133a007. [DOI] [PubMed] [Google Scholar]
- Rothstein J. D., Kuncl R. W. Neuroprotective strategies in a model of chronic glutamate-mediated motor neuron toxicity. J Neurochem. 1995 Aug;65(2):643–651. doi: 10.1046/j.1471-4159.1995.65020643.x. [DOI] [PubMed] [Google Scholar]
- Rudge J. S., Li Y., Pasnikowski E. M., Mattsson K., Pan L., Yancopoulos G. D., Wiegand S. J., Lindsay R. M., Ip N. Y. Neurotrophic factor receptors and their signal transduction capabilities in rat astrocytes. Eur J Neurosci. 1994 May 1;6(5):693–705. doi: 10.1111/j.1460-9568.1994.tb00981.x. [DOI] [PubMed] [Google Scholar]
- Sendtner M., Arakawa Y., Stöckli K. A., Kreutzberg G. W., Thoenen H. Effect of ciliary neurotrophic factor (CNTF) on motoneuron survival. J Cell Sci Suppl. 1991;15:103–109. doi: 10.1242/jcs.1991.supplement_15.14. [DOI] [PubMed] [Google Scholar]
- Sendtner M., Schmalbruch H., Stöckli K. A., Carroll P., Kreutzberg G. W., Thoenen H. Ciliary neurotrophic factor prevents degeneration of motor neurons in mouse mutant progressive motor neuronopathy. Nature. 1992 Aug 6;358(6386):502–504. doi: 10.1038/358502a0. [DOI] [PubMed] [Google Scholar]
- Sharp A. H., Loev S. J., Schilling G., Li S. H., Li X. J., Bao J., Wagster M. V., Kotzuk J. A., Steiner J. P., Lo A. Widespread expression of Huntington's disease gene (IT15) protein product. Neuron. 1995 May;14(5):1065–1074. doi: 10.1016/0896-6273(95)90345-3. [DOI] [PubMed] [Google Scholar]
- Shimohama S., Tamura Y., Akaike A., Tsukahara T., Ohara O., Watanabe S., Kimura J. Brain-derived neurotrophic factor pretreatment exerts a partially protective effect against glutamate-induced neurotoxicity in cultured rat cortical neurons. Neurosci Lett. 1993 Dec 24;164(1-2):55–58. doi: 10.1016/0304-3940(93)90856-g. [DOI] [PubMed] [Google Scholar]
- Steininger T. L., Wainer B. H., Klein R., Barbacid M., Palfrey H. C. High-affinity nerve growth factor receptor (Trk) immunoreactivity is localized in cholinergic neurons of the basal forebrain and striatum in the adult rat brain. Brain Res. 1993 May 28;612(1-2):330–335. doi: 10.1016/0006-8993(93)91681-h. [DOI] [PubMed] [Google Scholar]
- Tatter S. B., Galpern W. R., Hoogeveen A. T., Isacson O. Effects of striatal excitotoxicity on huntingtin-like immunoreactivity. Neuroreport. 1995 May 30;6(8):1125–1129. doi: 10.1097/00001756-199505300-00013. [DOI] [PubMed] [Google Scholar]
- Unoki K., LaVail M. M. Protection of the rat retina from ischemic injury by brain-derived neurotrophic factor, ciliary neurotrophic factor, and basic fibroblast growth factor. Invest Ophthalmol Vis Sci. 1994 Mar;35(3):907–915. [PubMed] [Google Scholar]
- Vejsada R., Sagot Y., Kato A. C. Quantitative comparison of the transient rescue effects of neurotrophic factors on axotomized motoneurons in vivo. Eur J Neurosci. 1995 Jan 1;7(1):108–115. doi: 10.1111/j.1460-9568.1995.tb01025.x. [DOI] [PubMed] [Google Scholar]
- Venero J. L., Beck K. D., Hefti F. Intrastriatal infusion of nerve growth factor after quinolinic acid prevents reduction of cellular expression of choline acetyltransferase messenger RNA and trkA messenger RNA, but not glutamate decarboxylase messenger RNA. Neuroscience. 1994 Jul;61(2):257–268. doi: 10.1016/0306-4522(94)90229-1. [DOI] [PubMed] [Google Scholar]
- Ventimiglia R., Mather P. E., Jones B. E., Lindsay R. M. The neurotrophins BDNF, NT-3 and NT-4/5 promote survival and morphological and biochemical differentiation of striatal neurons in vitro. Eur J Neurosci. 1995 Feb 1;7(2):213–222. doi: 10.1111/j.1460-9568.1995.tb01057.x. [DOI] [PubMed] [Google Scholar]
- Wen T. C., Matsuda S., Yoshimura H., Kawabe T., Sakanaka M. Ciliary neurotrophic factor prevents ischemia-induced learning disability and neuronal loss in gerbils. Neurosci Lett. 1995 May 19;191(1-2):55–58. doi: 10.1016/0304-3940(95)11574-8. [DOI] [PubMed] [Google Scholar]
- Winter C. G., Saotome Y., Levison S. W., Hirsh D. A role for ciliary neurotrophic factor as an inducer of reactive gliosis, the glial response to central nervous system injury. Proc Natl Acad Sci U S A. 1995 Jun 20;92(13):5865–5869. doi: 10.1073/pnas.92.13.5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q., Matheson C., Lopez O. T. In vivo neurotrophic effects of GDNF on neonatal and adult facial motor neurons. Nature. 1995 Jan 26;373(6512):341–344. doi: 10.1038/373341a0. [DOI] [PubMed] [Google Scholar]




