Abstract
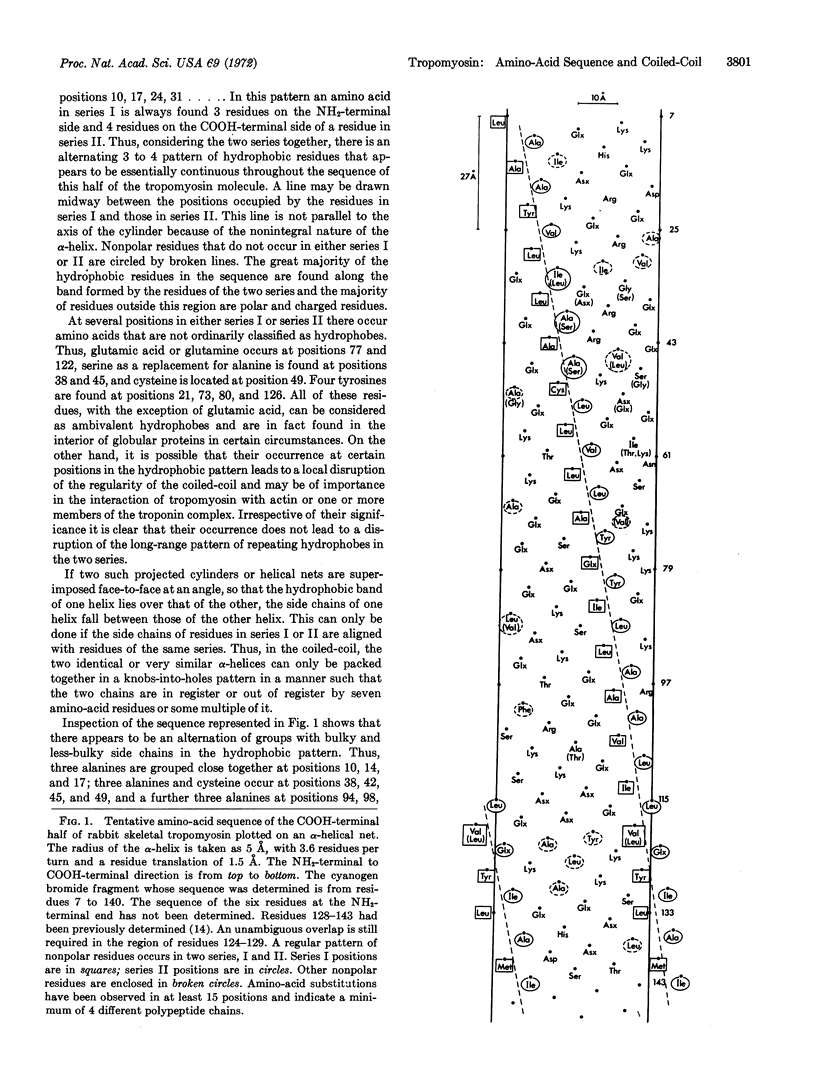
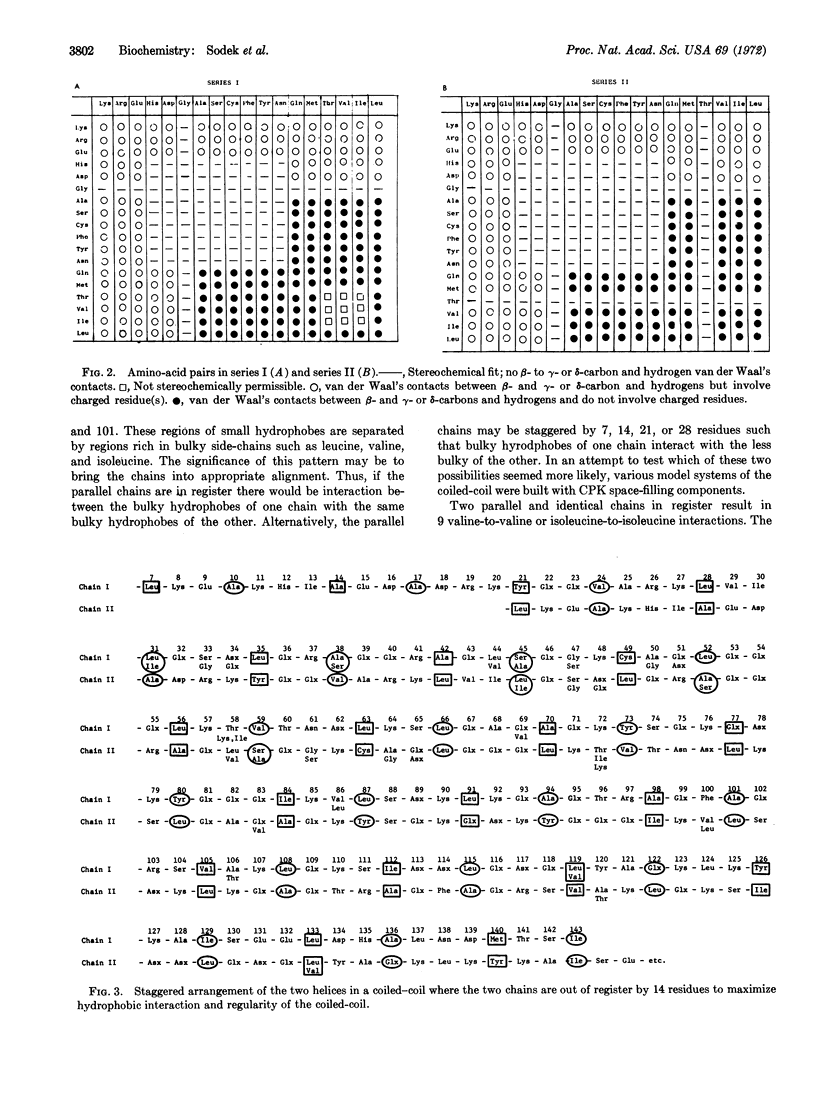
A tentative amino-acid sequence for the COOH-terminal half of rabbit skeletal tropomyosin is reported. These studies confirm our previous conclusions that this tropomyosin consists of several different but similar polypeptide chains. In the sequence, nonpolar residues occur in two series at intervals of seven residues. Amino-acid residues in series I are three residues on the NH2-terminal side of, and four residues on the COOH-terminal side of, residues in series II. The presence of occasional charged or ambivalent residues in the positions of series I or II does not lead to a disruption of this long-range pattern. The majority of residues located between the nonpolar residues are charged or polar amino acids. Two highly similar or identical α-helices with the reported sequence can be packed together in parallel in a coiled-coil structure. These may be in register or staggered by seven residues or some multiple of it. The observation that groups of small hydrophobic side chains appear to alternate with groups of bulky side chains suggests that a staggered arrangement of the two α-helices would maximize the regularity and hydrophobic interactions of the coiled-coil. Model building considerations show that this would occur with a stagger of 14 residues. Such an arrangement could account for the end-to-end aggregation of tropomyosin in solution, and in crystal and tactoid filaments. However, a structure in which the two polypeptides are in register cannot be ruled out.
Keywords: COOH-terminal, α-helix, three-dimensional structure
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COHEN C., HOLMES K. C. X-ray diffraction evidence for alpha-helical coiled-coils in native muscle. J Mol Biol. 1963 May;6:423–432. doi: 10.1016/s0022-2836(63)80053-4. [DOI] [PubMed] [Google Scholar]
- Caspar D. L., Cohen C., Longley W. Tropomyosin: crystal structure, polymorphism and molecular interactions. J Mol Biol. 1969 Apr 14;41(1):87–107. doi: 10.1016/0022-2836(69)90128-4. [DOI] [PubMed] [Google Scholar]
- Cohen C., Caspar D. L., Parry D. A., Lucas R. M. Tropomyosin crystal dynamics. Cold Spring Harb Symp Quant Biol. 1972;36:205–216. doi: 10.1101/sqb.1972.036.01.028. [DOI] [PubMed] [Google Scholar]
- Cummins P., Perry S. V. Subunit structure and biological activity of tropomyosin B from different muscle types. Biochem J. 1972 Jul;128(3):106P–107P. doi: 10.1042/bj1280106pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges R. S., Smillie L. B. Chemical evidence for chain heterogeneity in rabbit muscle tropomyosin. Biochem Biophys Res Commun. 1970 Nov 25;41(4):987–994. doi: 10.1016/0006-291x(70)90182-8. [DOI] [PubMed] [Google Scholar]
- Hodges R. S., Smillie L. B. Cysteine sequences of rabbit skeletal tropomyosin. Can J Biochem. 1972 Mar;50(3):330–343. doi: 10.1139/o72-045. [DOI] [PubMed] [Google Scholar]
- Hodges R. S., Smillie L. B. The histidine and methionine sequences of rabbit skeletal tropomyosin. Can J Biochem. 1972 Mar;50(3):312–329. doi: 10.1139/o72-044. [DOI] [PubMed] [Google Scholar]
- Hodges R. S., Sodek J., Smillie L. B. The cyanogen bromide fragments of rabbit skeletal-muscle tropomyosin. Biochem J. 1972 Jul;128(3):102P–102P. doi: 10.1042/bj1280102p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWEY S. COMPARATIVE STUDY OF THE ALPHA-HELICAL MUSCLE PROTEINS. TYROSYL TITRATION AND EFFECT OF PH ON CONFORMATION. J Biol Chem. 1965 Jun;240:2421–2427. [PubMed] [Google Scholar]
- Mccubbin W. D., Kouba R., Kay C. M. Physicochemical studies on bovine cardiac tropomyosin. Biochemistry. 1967 Aug;6(8):2417–2425. doi: 10.1021/bi00860a018. [DOI] [PubMed] [Google Scholar]
- Olander J., Emerson M. F., Holtzer A. On the dissociation and reassociation of the polypeptide chains of tropomyosin and paramyosin. J Am Chem Soc. 1967 Jun 7;89(12):3058–3059. doi: 10.1021/ja00988a051. [DOI] [PubMed] [Google Scholar]
- Parry D. A. A proposed conformation for alpha-fibrous proteins. J Theor Biol. 1970 Mar;26(3):429–435. doi: 10.1016/0022-5193(70)90094-9. [DOI] [PubMed] [Google Scholar]
- Sender P. M. Muscle fibrils: Solubilization and gel electrophoresis. FEBS Lett. 1971 Sep 15;17(1):106–110. doi: 10.1016/0014-5793(71)80575-6. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Woods E. F. Comparative physicochemical studies on vertebrate tropomyosins. Biochemistry. 1969 Nov;8(11):4336–4344. doi: 10.1021/bi00839a017. [DOI] [PubMed] [Google Scholar]
- Woods E. F. Molecular weight and subunit structure of tropomyosin B. J Biol Chem. 1967 Jun 25;242(12):2859–2871. [PubMed] [Google Scholar]