Abstract
Purpose of review
Reproduction is a tightly regulated function in which many mechanisms contribute to ensure the survival of the species. Among those, due to the elevated energy requirements of reproduction, metabolic factors exert a pivotal role in the control of hypothalamic-pituitary-gonadal axis. Although this control may occur at multiple levels of the axis, the majority of interactions between metabolic and reproductive systems take place in the hypothalamus. In this article, we present an overview of the state-of-the-art knowledge regarding the metabolic regulation of reproduction at the central level. We aim to identify the neuroanatomical location where both functions interconnect by discussing the likelihood of each component of the neuronal hierarchical network controlling gonadotropin-releasing hormone release to be first-order responders to metabolic cues, especially the peripheral metabolic signals leptin, insulin, and ghrelin.
Recent findings
Latest evidence suggests that the primary action of leptin, insulin, and ghrelin to regulate reproduction is located upstream of the main central elicitors of gonadotropin release, Kiss1 and gonadotropin-releasing hormone neurons, and neuroanatomically separated from their metabolic action.
Summary
The study of the neuronal interactions between the mechanisms governing metabolism and reproduction offers the platform to overcome or treat a number of prevailing metabolic and/or reproductive conditions.
Keywords: hypothalamus, kisspeptin, leptin, metabolism, reproduction
Introduction
Reproduction is one of the most energy demanding endeavors of any species and, hence, tight connections between the mechanisms controlling metabolism and reproductive integrity have developed during evolution. Metabolic cues from peripheral tissues and environmental cues translate information reflecting fuel storage and food availability to the central regulators of reproduction, thus assuring the appropriate timing of gestation and survival of the offspring. Sufficient energy stores are critical for the attainment of reproductive maturation and maintenance of fertility in adulthood [1]. Indeed, situations of energy depletion such as anorexia nervosa, excessive exercise, or diabetes –but also extreme energy surplus (e.g., severe obesity) – lead predominantly to delayed or absent pubertal onset in adolescents and hypogonadism in adults, which is usually characterized by hypothalamic amenorrhea [2,3]. Indeed, peripheral metabolic cues and reproductive hormones may act on different targets to regulate food intake and reproduction at multiple levels, for example, at the level of the pituitary [4▪,5▪,6]; however, there is a key central node that coordinates these two functions and that is common to both: the hypothalamus. In this article, we offer a comprehensive review of the latest advances in the identification and characterization of the neuroendocrine mechanisms that bridge metabolism and reproduction in the hypothalamus in mammals, focusing on the mechanism(s) of action of leptin, insulin, and ghrelin as the three major representatives of peripheral metabolic cues.
Transmission of the Metabolic State to the Brain
Energy storage occurs mainly at the level of white adipose tissue, where adipocytes secrete the anorexigenic adipokine leptin [7]. Circulating levels of leptin reflect body fat mass, serving as a messenger for the metabolic state to the central neuroendocrine regulatory centers of appetite, energy homeostasis, and metabolism [8]. Additionally, a number of peripheral organs secrete factors that provide further information about nutritional status and hence may impinge upon metabolic function, such as the pancreatic hormone insulin – released as a consequence of increasing glucose and, in the long term, body fat levels, and serving to increase anorexigenic inputs to the hypothalamus [9▪] – and gut hormones, among which ghrelin has gathered particular attention due to its orexigenic effects [10,11].
Disruption of proper metabolic signaling inflicts deleterious effects upon reproductive function [1]. For instance, humans and laboratory animals with leptin or insulin deficiency or resistance and/or increased ghrelin levels exhibit delayed or absent puberty and frequently display hypogonadotropic hypogonadism, which prevents fertility [1,9▪,12,13]. Ghrelin suppresses pulsatile gonadotropin-releasing hormone (GnRH) release [14,15], thus serving as a signal to suppress reproduction in times of famine [16,17▪]. Exactly how the energy state impinges on GnRH release remains unresolved.
Central Action of Metabolic Cues to Regulate Gonadotropin-Releasing Hormone Release
The hypothalamus is the nodal regulatory center where the mechanisms controlling metabolic state and reproductive function collide [18]. On the one hand, GnRH, the ultimate elicitor of gonadotropin function, is synthesized predominantly at the level of the hypothalamic preoptic area (POA) and is responsible for the awakening of the gonadotropic axis at puberty as well as its maintenance thereafter [19]. On the other hand, metabolic control is centered particularly in the ventromedial nucleus and the arcuate nucleus (ARC) [20▪]. In the ARC, two neuropeptides play crucial roles in the control of metabolic function through the melanocortin system: α-melanocyte-stimulating hormone-a [MSH; agonist of the melanocortin-3 and melanocortin-4 receptors (MC3R and MC4R)] and the agouti-related protein (AgRP; inverse agonist of MC3R and MC4R) [21,22], produced by pro-opiomelanocortin and cocaine and amphetamine-regulated transcript (POMC/CART) and neuropeptide Y (NPY)/AgRP neurons, respectively [9▪,20▪]. Neuropeptides derived from POMC/CART neurons exert a potent anorectic action, thus decreasing food intake and body weight, whereas AgRP and NPY have the opposite (orexigenic) effect, inducing food intake. A body of evidence also supports the role of these neurons as potent regulators of gonadotrope function, although, admittedly, conflicting effects have been reported regarding their action upon gonadotropin release, depending on the species and/or neuroanatomical site of action [23–26]. In the following section, we address the role of these and other neurons as potential first-order responders to energy status in the hierarchy of neurocircuits that control gonadotropin release.
Gonadotropin-releasing hormone neurons
One possibility is that GnRH neurons themselves receive direct input from metabolic cues. In this vein, GnRH neurons have been shown to express insulin receptor mRNA and protein [27] and are activated by insulin [28]. Indeed, insulin action in the brain is essential for proper gonadotropin secretion, as observed in mice with neuronal deletion of insulin receptors (NIRKO mice) [29]. Nonetheless, recent evidence has challenged the notion of an important direct action of insulin on GnRH neurons, as mice with selective deletion of insulin receptors in GnRH neurons have normal puberty and fertility [30]. Moreover, the possibility that GnRH neurons are first-order responders to leptin or ghrelin action has been disproven due to the absence of receptors for leptin (LepR) [18] or ghrelin (growth hormone secretagogue receptor, GHSR) [31▪▪] in GnRH neurons (Fig. 1). Therefore, although a direct role of insulin on GnRH neurons cannot be fully excluded, the putative site for the metabolic control of reproduction must necessarily rest upstream of GnRH neurons, at least for these metabolic factors (although additional fine-tuning of gonadotropin release may occur directly at the level of the pituitary, as mentioned previously).
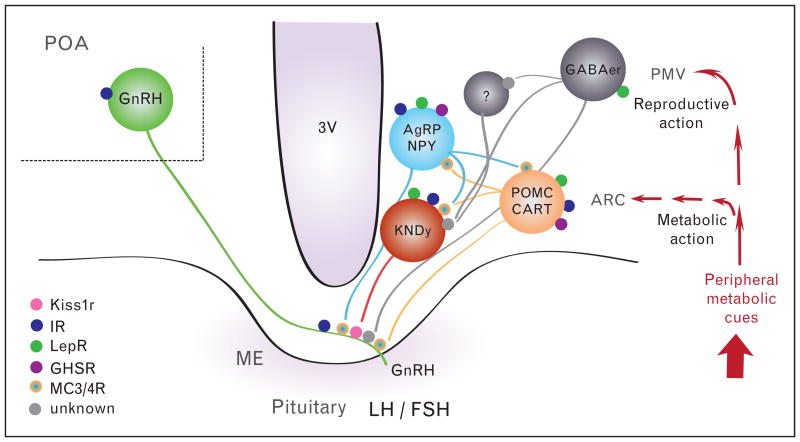
Figure 1.
Schematic representation of the neural interactions between metabolic and reproductive functions depicting the likely sites of action of leptin, insulin, and ghrelin to control gonadotropin-releasing hormone (GnRH) release. 3V, third ventricle; ARC, arcuate nucleus; ME, median eminence; PMV, ventral premammillary nucleus; POA, preoptic area.
Kiss1 neurons
Kisspeptins (encoded by KISS1) have been identified in the last decade as the most potent secretagogues of GnRH release. Kiss1 neurons, located mainly in the ARC and the anteroventral-periventricular nucleus (AVPV) in rodents, contact GnRH neurons directly and are important for puberty onset and maintenance of reproductive function [32▪▪]. Importantly, the subpopulation of Kiss1 neurons in the ARC, referred to as KNDy neurons, coexpress the neuropeptides, dynorphin, and neurokinin B (NKB), and have been suggested to play a crucial role in the shaping of GnRH pulses [33▪]. This pivotal role of Kiss1 neurons in the neuroendocrine control of reproduction places them as likely conveyors of metabolic influences on GnRH neurons. However, mounting evidence suggests that although Kiss1 neurons may actively participate in this integrative process (see below), they are not direct targets of these metabolic cues (Fig. 1). First, although initial studies suggested the existence of LepR in a subset of Kiss1 neurons [34], recent studies using genetic models of selective ablation of LepR in Kiss1 neurons have documented that direct leptin signaling to Kiss1 neurons is not required for pubertal onset [35]. Similarly, removal of insulin receptors from Kiss1 neurons using Kiss1-specific insulin receptor knockout (KIRKO) mice [36▪▪] disclosed parallel findings, and further, Kiss1 neurons do not express ghrelin receptor GHSR [31▪▪]. Altogether, these data strongly suggest that Kiss1 neurons are not the primary conduit of the metabolic state to the reproductive axis.
Nonetheless, kisspeptin and NKB, as cotransmitters of KNDy neurons, have been suggested to participate in the transmission of information regarding energy balance to GnRH neurons. Caloric restriction significantly blunts the expression of Kiss1 and Tac2 (encoding NKB), leading to decreased gonadotropin release that can be reversed by administration of exogenous kisspeptin or senktide, an agonist of the NKB receptor (NK3R) [37▪▪,38,39]. Interestingly, luteinizing hormone (LH) responses to kisspeptin and senktide administration in pubertal rats are greater in conditions of energy deficit [38,40]. Furthermore, chronic administration of kisspeptin or senktide to prepubertal female rats subjected to caloric restriction induced a partial recovery of puberty onset (i.e., vaginal opening and LH secretion) [37▪▪,40]. On the contrary, excessive energy storage may also critically impinge on the activity of KNDy neurons, depending on the timing and developmental stage. Thus, prepubertal rats fed a high-fat diet displayed precocious puberty, characterized by early increases of Kiss1 and Tac2 expression as well as LH pulsatility [41]. In contrast, diet-induced obesity in adult mice caused a significant inhibition of Kiss1 mRNA in the ARC and AVPV [42]. Overall, KNDy neurons – and potentially also AVPV Kiss1 neurons – are susceptible to regulation by metabolic factors that may modify the pattern of kisspeptin/GnRH release. An additional study supports this contention by documenting the role of KNDy neurons in the negative action exerted by estrogens on body weight [43▪].
Pro-opiomelanocortin and agouti-related protein neurons in the arcuate nucleus
The exclusion of GnRH and Kiss1 neurons as first-order responders in the metabolic/reproductive regulatory circuit turns attention to other neuronal populations in the ARC as potential immediate upstream regulators of Kiss1 and/or GnRH neurons. A plethora of neuropeptides potentially involved in the control of metabolism and reproduction are located within this nucleus. As described earlier, the melanocortin system and its two distinct populations of neurons (POMC/CART and NPY/AgRP) have been extensively documented to play regulatory roles in both metabolic and reproductive pathways [1,20▪]. Indeed, both groups of neurons express LepR, insulin receptor, and GHSR [44–48] (Fig. 1) and both leptin and insulin stimulate Pomc and inhibit Agrp expression [49,50], whereas ghrelin has been shown to inhibit POMC neurons and activate AgRP neurons [51,52▪]. Interestingly, GnRH neurons express MC3R and MC4R [53▪] and melanocortins and NPY can directly modify the activity of GnRH neurons [54▪], suggesting this as a possible route of regulation of the reproductive axis. Additionally, POMC/CART and NPY/AgRP neurons also display reciprocal connections with Kiss1 neurons [26]; however, in this case, as leptin inhibits Npy expression [55] and both leptin and NPY stimulate Kiss1 expression [25,56▪], it is unlikely that any stimulatory effect of leptin on Kiss1 neurons happens through NPY action. AgRP, in turn, may play a role conveying leptin action to Kiss1 and/or GnRH neurons. Even though AgRP neurons are not essential for reproduction [57], recent studies by Wu et al. [58▪▪,59▪▪] suggested that hyperstimulation of AgRP neurons – but not POMC neurons – in leptin-deficient (ob/ob) mice might account for the suppression of the reproductive axis in these mice, as ablation of AgRP neurons resulted in decreased body weight and recovery of fertility. As NPY/AgRP neurons have been reported to project to GnRH neurons [60] and also to Kiss1 neurons [26], further examination of this model is required to identify their primary target(s).
In support of the ARC as a conveyor of metabolic/reproductive interactions, this nucleus hosts a dense network of neuronal fibers [61] and has access to circulating molecules outside the blood–brain barrier [62]. However, recent publications have disputed this role, at least for melanocortin neurons, as mouse models of selective deletion of LepR from POMC neurons, AgRP neurons, or both, results in minimal increases in body weight [44,63,64], suggesting that these neurons might be downstream of leptin-responsive neurons situated at a higher level.
The arcuate nucleus and beyond
Recent investigations have tried to narrow down the candidates in the search for the putative targets of leptin action in the brain to control body weight by parsing out excitatory (glutamatergic) vs. inhibitory (GABAergic) first-order leptin-responsive neurons. Deletion of LepR from GABAergic (VGAT+) but not glutamatergic (VGLUT2+) neurons resulted in loss of the antiobesity action of leptin by reducing the inhibitory tone on POMC neurons [65]. More interestingly, these mice also indicated that the facilitatory role that leptin exerts on reproduction is also mediated by the GABAergic subpopulation of LepR-expressing neurons, as they recapitulated the reproductive phenotype of ob/ob mice (delayed or absent puberty onset and infertility) [66▪▪]. Identifying the nature of this GABAergic population of neurons is essential for understanding the central mechanisms governing leptin action on reproductive function. It is worth mentioning that, whereas AgRP neurons are GABAergic [65,67,68], most GABAergic neurons do not express AgRP [65] and, given the minimal metabolic phenotype of mice lacking LepR in AgRP neurons, this neuronal population is unlikely to hold this integrative role. In this regard, a recent study by Kong et al. [69▪▪] uncovered a role for a new population of GABAergic neurons in the ARC that express rat insulin-2 promoter in the control of energy expenditure, which may contribute to the action of leptin; however, whether these neurons are first-order leptin-responsive neurons and whether they participate in the control of reproductive function remains to be determined.
Additionally, a number of other players within the ARC may also serve as potential conveyors of metabolic control of reproduction, such as the newly identified anorexigenic peptide nesfatin-1 [70], which is coexpressed with POMC [71]. Nesfatin-1 blockade increases body weight and food intake in rats in a leptin-independent (and melanocortin-dependent) manner [70,72]. Interestingly, nesfatin-1 blockade delays puberty onset in rats [73] and reduces Kiss1 mRNA in the ARC and AVPV, and central injection of nesfatin-1 induces LH release [74▪▪], suggesting a stimulatory role in kisspeptin release. This action however, appears to be indirect, as nesfatin-1 does not modify Kiss1 neuronal activity directly as measured by whole-cell clamp recordings [74▪▪], but rather may be mediated by inhibition of NPY neurons in the ARC [75]. Nevertheless, these studies suggest a role for nesfatin-1 neurons in the metabolic control of reproductive function. Given the wide distribution of nesfatin-1 in the brain [70,73], precisely which subpopulation of these neurons exerts the metabolic vs. reproductive role and whether they are first-order responsive neurons remains unknown.
Additional populations of leptin-responsive neurons have been emerging in recent years, indicating that the overall action of leptin may result from the contributions of a number of neuronal circuits. In this regard, particularly relevant is the finding that nitric oxide, produced in LepR-expressing neurons of the ventral premammillary nucleus (PMV), dorsomedial nucleus (DMH), and the ARC greatly influences leptin's action. Ablation of LepR in these neurons – of which 20–30% are GABAergic – leads to hyperphagic obesity, decreased energy expenditure, and hyperglycemia [76▪▪] comparable to that of constitutive LepR-null mice. Strikingly, these mice show only a slight delay in reproductive maturation and maintain normal fertility. These findings suggest that, indeed, the metabolic vs. reproductive role of leptin may lie in distinct neuronal populations, yet all seem to be predominantly GABAergic. Then, where in the brain is the reproductive role of leptin relayed? A number of recent studies have posited the PMV as the holder of this action. Animals with a lesioned PMV have impaired reproductive function and leptin is no longer able to induce LH release [35,77]. Interestingly, LepR-expressing neurons from the PMV may exert a direct action on GnRH neurons as contacts between the two populations of neurons have been identified [78] (Fig. 1). However, the documented inhibition of Kiss1 mRNA in congenitally leptin-deficient mice and the increased Kiss1 mRNA in the ARC and improved gonadotropin levels following leptin administration suggest effects at the level of Kiss1 neurons as well [34,79]. Furthermore, we have recently observed that LepR ablation from GABAergic neurons is accompanied by decreased kisspeptin input, and the hypogonadotropic state is rescued by exogenous kisspeptin [66▪▪]. These data argue against GnRH neurons as the exclusive target of the PMV neurons and strongly suggest that Kiss1 neurons, at least the population in the ARC (i.e., KNDy neurons), convey metabolic information [78].
Conclusion
The development of new and powerful genetic tools in recent years is allowing an unprecedented increase in our knowledge of the neuronal interactions that govern energy homeostasis and its influences on reproductive maturation and function. Interestingly, although both functions are critical for the survival of an individual and appear very tightly interconnected, mounting data suggest the existence of different neuronal circuits behind each function though, eventually, the metabolic influences on reproduction converge at the level of KNDy neurons. Admittedly, branches from both axes exist and may interact upstream of KNDy neurons to, perhaps, favor a tuned and coherent response of kisspeptins and, hence, GnRH release (Fig. 1). As new neuropeptides and neuronal interactions are being discovered, the hierarchical characterization of these routes will offer a more mechanistic view of these interactions that will, perhaps, allow us to target specific neurons to facilitate, overcome, or treat a number of prevailing metabolic and/or reproductive conditions. For instance, studies have shown that women with hypothalamic amenorrhea who are treated with leptin can have restoration of pulsatile LH secretion and menstrual cyclicity; however, this treatment may also result in further weight loss in these women [80]. In these situations, separation of the effects of metabolic pathways on energy homeostasis from those on reproductive function might allow restoration of reproductive function without causing worsening of the metabolic homeostasis. Similarly, conditions of severe obesity or insulin resistance may present with associated hypogonadotropic hypogonadism. Currently, the use of kisspeptin administration to reverse these reproductive impairments is being explored [81,82▪] and kisspeptin analogues are being developed to facilitate their therapeutic administration.
Key Points.
Sufficient energy stores are necessary for reproductive success.
Hypothalamic Kiss1 neurons are essential conduits of the energy status to GnRH neurons, although they do not appear to be direct targets of metabolic factors.
Compelling evidence suggests that metabolic factors act on distinct hypothalamic centers to exert their metabolic vs. reproductive actions.
Acknowledgments
The authors are supported by The Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (NIH), through cooperative agreement U54 HD28138 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research, NIH/NICHD R01 HD061577, R01 HD019938, and R21 HD066495 (U.B.K.) and the NIH/NICHD K99 HD071970 (V.M.N.).
Footnotes
Conflicts of interest: There are no conflicts of interest.
References and Recommended Reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 363–364).
- 1.Roa J, Garcia-Galiano D, Castellano JM, et al. Metabolic control of puberty onset: new players, new mechanisms. Mol Cell Endocrinol. 2010;324:87–94. doi: 10.1016/j.mce.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 2.Bruni V, Dei M, Morelli C, et al. Body composition variables and leptin levels in functional hypothalamic amenorrhea and amenorrhea related to eating disorders. J Pediatr Adolesc Gynecol. 2011;24:347–352. doi: 10.1016/j.jpag.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Genazzani AD, Chierchia E, Santagni S, et al. Hypothalamic amenorrhea: from diagnosis to therapeutical approach. Ann Endocrinol (Paris) 2010;71:163–169. doi: 10.1016/j.ando.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 4▪.Akhter N, Odle AK, Allensworth-James ML, et al. Ablation of leptin signaling to somatotropes: changes in metabolic factors that cause obesity. Endocrinology. 2012;153:4705–4715. doi: 10.1210/en.2012-1331. This study shows that leptin may act at the level of the pituitary to exert its metabolic action. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5▪.Syed M, Cozart M, Haney AC, et al. Ghrelin restoration of function in vitro in somatotropes from male mice lacking the Janus kinase (JAK)-binding site of the leptin receptor. Endocrinology. 2013;154:1565–1576. doi: 10.1210/en.2012-2254. This study shows that ghrelin may act at the level of the pituitary to exert its metabolic action. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brothers KJ, Wu S, DiVall SA, et al. Rescue of obesity-induced infertility in female mice due to a pituitary-specific knockout of the insulin receptor. Cell metabolism. 2010;12:295–305. doi: 10.1016/j.cmet.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Proenca R, Maffei M, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 8.Gautron L, Elmquist JK. Sixteen years and counting: an update on leptin in energy balance. J Clin Invest. 2011;121:2087–2093. doi: 10.1172/JCI45888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9▪.Varela L, Horvath TL. Leptin and insulin pathways in POMC and AgRP neurons that modulate energy balance and glucose homeostasis. EMBO Rep. 2012;13:1079–1086. doi: 10.1038/embor.2012.174. This review article highlights the action of leptin and insulin on POMC and AgRP neurons to control energy balance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Briggs DI, Andrews ZB. Metabolic status regulates ghrelin function on energy homeostasis. Neuroendocrinology. 2011;93:48–57. doi: 10.1159/000322589. [DOI] [PubMed] [Google Scholar]
- 11.Andrews ZB. Central mechanisms involved in the orexigenic actions of ghrelin. Peptides. 2011;32:2248–2255. doi: 10.1016/j.peptides.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 12.Chan JL, Mantzoros CS. Role of leptin in energy-deprivation states: normal human physiology and clinical implications for hypothalamic amenorrhoea and anorexia nervosa. Lancet. 2005;366:74–85. doi: 10.1016/S0140-6736(05)66830-4. [DOI] [PubMed] [Google Scholar]
- 13.Small CJ, Stanley SA, Bloom SR. Appetite control and reproduction: leptin and beyond. Semin Reprod Med. 2002;20:389–398. doi: 10.1055/s-2002-36712. [DOI] [PubMed] [Google Scholar]
- 14.Lebrethon MC, Aganina A, Fournier M, et al. Effects of in vivo and in vitro administration of ghrelin, leptin and neuropeptide mediators on pulsatile gonadotrophin-releasing hormone secretion from male rat hypothalamus before and after puberty. J Neuroendocrinol. 2007;19:181–188. doi: 10.1111/j.1365-2826.2006.01518.x. [DOI] [PubMed] [Google Scholar]
- 15.Ogata R, Matsuzaki T, Iwasa T, et al. Hypothalamic Ghrelin suppresses pulsatile secretion of luteinizing hormone via beta-endorphin in ovariectomized rats. Neuroendocrinology. 2009;90:364–370. doi: 10.1159/000257421. [DOI] [PubMed] [Google Scholar]
- 16.El-Eshmawy MM, Abdel Aal IA, El Hawary AK. Association of ghrelin and leptin with reproductive hormones in constitutional delay of growth and puberty. Reprod Biol Endocrinol. 2010;8:153. doi: 10.1186/1477-7827-8-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17▪.Kluge M, Schussler P, Schmidt D, et al. Ghrelin suppresses secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) in women. J Clin Endocrinol Metab. 2012;97:E448–E451. doi: 10.1210/jc.2011-2607. This study describes the reproductive effect of ghrelin in humans. [DOI] [PubMed] [Google Scholar]
- 18.Donato J, Jr, Cravo RM, Frazao R, Elias CF. Hypothalamic sites of leptin action linking metabolism and reproduction. Neuroendocrinology. 2011;93:9–18. doi: 10.1159/000322472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herbison A. Physiology of the GnRH neuronal network. In: Neil J, Knobil E, editors. Physiology of reproduction. San Diego, CA: Academic Press; 2006. pp. 1415–1482. [Google Scholar]
- 20▪.Myers MG, Jr, Olson DP. Central nervous system control of metabolism. Nature. 2012;491:357–363. doi: 10.1038/nature11705. Elegant review of the state-of-the-art in the neural circuits involved in the control of food intake. [DOI] [PubMed] [Google Scholar]
- 21.Mountjoy KG, Robbins LS, Mortrud MT, Cone RD. The cloning of a family of genes that encode the melanocortin receptors. Science. 1992;257:1248–1251. doi: 10.1126/science.1325670. [DOI] [PubMed] [Google Scholar]
- 22.Ollmann MM, Wilson BD, Yang YK, et al. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 23.Kalra SP, Crowley WR. Neuropeptide Y: a novel neuroendocrine peptide in the control of pituitary hormone secretion, and its relation to luteinizing hormone. Front Neuroendocrinol. 1992;13:1–46. [PubMed] [Google Scholar]
- 24.Pralong FP. Insulin and NPY pathways and the control of GnRH function and puberty onset. Mol Cell Endocrinol. 2010;324:82–86. doi: 10.1016/j.mce.2010.01.037. [DOI] [PubMed] [Google Scholar]
- 25.Luque RM, Kineman RD, Tena-Sempere M. Regulation of hypothalamic expression of KiSS-1 and GPR54 genes by metabolic factors: analyses using mouse models and a cell line. Endocrinology. 2007;148:4601–4611. doi: 10.1210/en.2007-0500. [DOI] [PubMed] [Google Scholar]
- 26.Backholer K, Smith J, Clarke IJ. Melanocortins may stimulate reproduction by activating orexin neurons in the dorsomedial hypothalamus and kisspeptin neurons in the preoptic area of the ewe. Endocrinology. 2009;150:5488–5497. doi: 10.1210/en.2009-0604. [DOI] [PubMed] [Google Scholar]
- 27.Kim HH, DiVall SA, Deneau RM, Wolfe A. Insulin regulation of GnRH gene expression through MAP kinase signaling pathways. Mol Cell Endocrinol. 2005;242:42–49. doi: 10.1016/j.mce.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Salvi R, Castillo E, Voirol MJ, et al. Gonadotropin-releasing hormone-expressing neurons immortalized conditionally are activated by insul implication of the mitogen-activated protein kinase pathway. Endocrinology. 2006;147:816–826. doi: 10.1210/en.2005-0728. [DOI] [PubMed] [Google Scholar]
- 29.Bruning JC, GautamF JC, Burks DJ, et al. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- 30.DiVall SA, Williams TR, Carver SE, et al. Divergent roles of growth factors in the GnRH regulation of puberty in mice. J Clin Invest. 2010;120:2900–2909. doi: 10.1172/JCI41069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31▪▪.Smith J, Reichenbach A, Lemus M, Andrews Z, editors. Ghrelin does not act directly on kisspeptin neurons to suppress the reproductive system. 2nd world Conference of Kisspeptin Signaling in the Brain; Tokyo, Japan. 2012. These results offer important information to understand the reproductive role of ghrelin. [Google Scholar]
- 32▪▪.Pinilla L, Aguilar E, Dieguez C, et al. Kisspeptins and reproduction: physiological roles and regulatory mechanisms. Physiol Rev. 2012;92:1235–1316. doi: 10.1152/physrev.00037.2010. This is a very elegant and complete review of the current knowledge on the reproductive facet of kisspeptins. [DOI] [PubMed] [Google Scholar]
- 33▪.Navarro VM. New insights into the control of pulsatile GnRH release: the role of Kiss1/neurokinin B neurons. Front Endocrinol (Lausanne) 2012;3:48. doi: 10.3389/fendo.2012.00048. Discussion of the proposed role of KNDy neurons as pacemakers of GnRH secretion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith JT, Acohido BV, Clifton DK, Steiner RA. KiSS-1 neurones are direct targets for leptin in the ob/ob mouse. J Neuroendocrinol. 2006;18:298–303. doi: 10.1111/j.1365-2826.2006.01417.x. [DOI] [PubMed] [Google Scholar]
- 35.Donato J, Jr, Cravo RM, Frazao R, et al. Leptin's effect on puberty in mice is relayed by the ventral premammillary nucleus and does not require signaling in Kiss1 neurons. J Clin Invest. 2011;121:355–368. doi: 10.1172/JCI45106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36▪▪.Qiu X, Dowling AR, Marino JS, et al. Delayed puberty but normal fertility in mice with selective deletion of insulin receptors from Kiss1 cells. Endocrinology. 2013;154:1337–1348. doi: 10.1210/en.2012-2056. Important study showing that Kiss1 neurons are not first-order responders of insulin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37▪▪.Navarro VM, Ruiz-Pino F, Sanchez-Garrido MA, et al. Role of neurokinin B in the control of female puberty and its modulation by metabolic status. J Neurosci. 2012;32:2388–2397. doi: 10.1523/JNEUROSCI.4288-11.2012. Description of the metabolic role of NKB during puberty onset. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tovar S, Vazquez MJ, Navarro VM, et al. Effects of single or repeated intravenous administration of kisspeptin upon dynamic LH secretion in conscious male rats. Endocrinology. 2006;147:2696–2704. doi: 10.1210/en.2005-1397. [DOI] [PubMed] [Google Scholar]
- 39.True C, Kirigiti MA, Kievit P, et al. Leptin is not the critical signal for kisspeptin or luteinising hormone restoration during exit from negative energy balance. J Neuroendocrinol. 2011;23:1099–1112. doi: 10.1111/j.1365-2826.2011.02144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Castellano JM, Navarro VM, Fernandez-Fernandez R, et al. Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology. 2005;146:3917–3925. doi: 10.1210/en.2005-0337. [DOI] [PubMed] [Google Scholar]
- 41.Li XF, Lin YS, Kinsey-Jones JS, O'Byrne KT. High-fat diet increases LH pulse frequency and kisspeptin-neurokinin B expression in puberty-advanced female rats. Endocrinology. 2012;153:4422–4431. doi: 10.1210/en.2012-1223. [DOI] [PubMed] [Google Scholar]
- 42.Quennell JH, Howell CS, Roa J, et al. Leptin deficiency and diet-induced obesity reduce hypothalamic kisspeptin expression in mice. Endocrinology. 2011;152:1541–1550. doi: 10.1210/en.2010-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43▪.Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, et al. Arcuate kisspeptin/neurokinin B/dynorphin (KNDy) neurons mediate the estrogen suppression of gonadotropin secretion and body weight. Endocrinology. 2012;153:2800–2812. doi: 10.1210/en.2012-1045. First evidence of the role of KNDy neurons in the estrogen-mediated regulation of body weight. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hill JW, Elias CF, Fukuda M, et al. Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell metabolism. 2010;11:286–297. doi: 10.1016/j.cmet.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kamegai J, Tamura H, Shimizu T, et al. Chronic central infusion of ghrelin increases hypothalamic neuropeptide Y and Agouti-related protein mRNA levels and body weight in rats. Diabetes. 2001;50:2438–2443. doi: 10.2337/diabetes.50.11.2438. [DOI] [PubMed] [Google Scholar]
- 46.Osterstock G, Escobar P, Mitutsova V, et al. Ghrelin stimulation of growth hormone-releasing hormone neurons is direct in the arcuate nucleus. PLoS One. 2010;5:e9159. doi: 10.1371/journal.pone.0009159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baskin DG, Breininger JF, Schwartz MW. Leptin receptor mRNA identifies a subpopulation of neuropeptide Y neurons activated by fasting in rat hypothalamus. Diabetes. 1999;48:828–833. doi: 10.2337/diabetes.48.4.828. [DOI] [PubMed] [Google Scholar]
- 48.Williams KW, Margatho LO, Lee CE, et al. Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. J Neurosci. 2010;30:2472–2479. doi: 10.1523/JNEUROSCI.3118-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mesaros A, Koralov SB, Rother E, et al. Activation of Stat3 signaling in AgRP neurons promotes locomotor activity. Cell Metab. 2008;7:236–248. doi: 10.1016/j.cmet.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 50.Ernst MB, Wunderlich CM, Hess S, et al. Enhanced Stat3 activation in POMC neurons provokes negative feedback inhibition of leptin and insulin signaling in obesity. J Neurosci. 2009;29:11582–11593. doi: 10.1523/JNEUROSCI.5712-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cowley MA, Smith RG, Diano S, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 52▪.Schaeffer M, Langlet F, Lafont C, et al. Rapid sensing of circulating ghrelin by hypothalamic appetite-modifying neurons. Proc Natl Acad Sci U S A. 2013;110:1512–1517. doi: 10.1073/pnas.1212137110. This study addresses the action of ghrelin on POMC and NPY neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53▪.Israel DD, Sheffer-Babila S, de Luca C, et al. Effects of leptin and melanocortin signaling interactions on pubertal development and reproduction. Endocrinology. 2012;153:2408–2419. doi: 10.1210/en.2011-1822. The authors document the existence of melanocortin receptors in GnRH neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54▪.Roa J, Herbison AE. Direct regulation of GnRH neuron excitability by arcuate nucleus POMC and NPY neuron neuropeptides in female mice. Endocrinology. 2012;153:5587–5599. doi: 10.1210/en.2012-1470. Important evidence of direct actions of POMC and NPY neurons on GnRH neurons. [DOI] [PubMed] [Google Scholar]
- 55.Schwartz MW, Seeley RJ, Campfield LA, et al. Identification of targets of leptin action in rat hypothalamus. J Clin Invest. 1996;98:1101–1106. doi: 10.1172/JCI118891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56▪.Navarro VM, Tena-Sempere M. Neuroendocrine control by kisspeptins: role in metabolic regulation of fertility. Nat Rev Endocrinol. 2012;8:40–53. doi: 10.1038/nrendo.2011.147. Description of the role of kisspeptins in the metabolic control of reproduction, including the role of NKB in ARC Kiss1 neurons. [DOI] [PubMed] [Google Scholar]
- 57.Phillips CT, Palmiter RD. Role of agouti-related protein-expressing neurons in lactation. Endocrinology. 2008;149:544–550. doi: 10.1210/en.2007-1153. [DOI] [PubMed] [Google Scholar]
- 58▪▪.Wu Q, Clark MS, Palmiter RD. Deciphering a neuronal circuit that mediates appetite. Nature. 2012;483:594–597. doi: 10.1038/nature10899. Evidence of the role of AgRP neurons in the transmission of the metabolic action of leptin in the brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59▪▪.Wu Q, Whiddon BB, Palmiter RD. Ablation of neurons expressing agouti-related protein, but not melanin concentrating hormone, in leptin-deficient mice restores metabolic functions and fertility. Proc Natl Acad Sci U S A. 2012;109:3155–3160. doi: 10.1073/pnas.1120501109. Evidence of the role of AgRP neurons in the transmission of the metabolic action of leptin in the brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Turi GF, Liposits Z, Moenter SM, et al. Origin of neuropeptide Y-containing afferents to gonadotropin-releasing hormone neurons in male mice. Endocrinology. 2003;144:4967–4974. doi: 10.1210/en.2003-0470. [DOI] [PubMed] [Google Scholar]
- 61.Krajewski SJ, Burke MC, Anderson MJ, et al. Forebrain projections of arcuate neurokinin B neurons demonstrated by anterograde tract-tracing and mono-sodium glutamate lesions in the rat. Neuroscience. 2010;166:680–697. doi: 10.1016/j.neuroscience.2009.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cone RD, Cowley MA, Butler AA, et al. The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. Int J Obes Relat Metab Disord. 2001;25(Suppl 5):S63–Sv67. doi: 10.1038/sj.ijo.0801913. [DOI] [PubMed] [Google Scholar]
- 63.Balthasar N, Coppari R, McMinn J, et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 64.van de Wall E, Leshan R, Xu AWv, et al. Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology. 2008;149:1773–1785. doi: 10.1210/en.2007-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vong L, Ye C, Yang Z, et al. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron. 2011;71:142–154. doi: 10.1016/j.neuron.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66▪▪.Martin CM, Navarro VM, Vong L, et al. Leptin-responsive GABAergic neurons are necessary for onset of puberty in females. The Endocrine Society Conference; San Francisco, CA. 2013. This work documents the role of GABAergic neurons in the reproductive action of leptin. [Google Scholar]
- 67.Cowley MA, Smart JL, Rubinstein M, et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 68.Tong Q, Ye CP, Jones JE, et al. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat Neurosci. 2008;11:998–1000. doi: 10.1038/nn.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69▪▪.Kong D, Tong Q, Ye C, et al. GABAergic RIP-Cre neurons in the arcuate nucleus selectively regulate energy expenditure. Cell. 2012;151:645–657. doi: 10.1016/j.cell.2012.09.020. Description of a new set of arcuate neurons involved in the control of energy balance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oh IS, Shimizu H, Satoh T, et al. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature. 2006;443:709–712. doi: 10.1038/nature05162. [DOI] [PubMed] [Google Scholar]
- 71.Foo KS, Brismar H, Broberger C. Distribution and neuropeptide coexistence of nucleobindin-2 mRNA/nesfatin-like immunoreactivity in the rat CNS. Neuroscience. 2008;156:563–579. doi: 10.1016/j.neuroscience.2008.07.054. [DOI] [PubMed] [Google Scholar]
- 72.Garcia-Galiano D, Navarro VM, Gaytan F, Tena-Sempere M. Expanding roles of NUCB2/nesfatin-1 in neuroendocrine regulation. J Mol Endocrinol. 2010;45:281–290. doi: 10.1677/JME-10-0059. [DOI] [PubMed] [Google Scholar]
- 73.Garcia-Galiano D, Navarro VM, Roa J, et al. The anorexigenic neuropeptide, nesfatin-1, is indispensable for normal puberty onset in the female rat. J Neurosci. 2010;30:7783–7792. doi: 10.1523/JNEUROSCI.5828-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74▪▪.Garcia-Galiano D, Wu M, Ruiz-Pino F, et al., editors. Evidence for indirect regulatory actions of the puberty-modulating peptide, nesfatin-1, on the Kiss1 system: role in the gonadotropic effect of nesfatin-1. 2nd World Conference of Kisspepin Signaling in the Brain; Tokyo, Japan. 2012. This works aims to identify the mechanism/s of action of nesfatin-1 in the central control of reproduction. [Google Scholar]
- 75.Price CJ, Samson WK, Ferguson AV. Nesfatin-1 inhibits NPY neurons in the arcuate nucleus. Brain Res. 2008;1230:99–106. doi: 10.1016/j.brainres.2008.06.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76▪▪.Leshan RL, Greenwald-Yarnell M, Patterson CM, et al. Leptin action through hypothalamic nitric oxide synthase-1-expressing neurons controls energy balance. Nat Med. 2012;18:820–823. doi: 10.1038/nm.2724. Description of a new set of hypothalamic neurons involved in the control of energy balance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Donato J, Jr, Elias CF. The ventral premammillary nucleus links metabolic cues and reproduction. Front Endocrinol (Lausanne) 2011;2:57. doi: 10.3389/fendo.2011.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Louis GW, Greenwald-Yarnell M, Phillips R, et al. Molecular mapping of the neural pathways linking leptin to the neuroendocrine reproductive axis. Endocrinology. 2011;152:2302–2310. doi: 10.1210/en.2011-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Castellano JM, Navarro VM, Fernandez-Fernandez R, et al. Expression of hypothalamic KiSS-1 system and rescue of defective gonadotropic responses by kisspeptin in streptozotocin-induced diabetic male rats. Diabetes. 2006;55:2602–2610. doi: 10.2337/db05-1584. [DOI] [PubMed] [Google Scholar]
- 80.Welt CK, Chan JL, Bullen J, et al. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med. 2004;351:987–997. doi: 10.1056/NEJMoa040388. [DOI] [PubMed] [Google Scholar]
- 81.George JT, Millar RP, Anderson RA. Hypothesis: kisspeptin mediates male hypogonadism in obesity and type 2 diabetes. Neuroendocrinology. 2010;91:302–307. doi: 10.1159/000299767. [DOI] [PubMed] [Google Scholar]
- 82▪.Ratnasabapathy R, Dhillo WS. The effects of kisspeptin in human reproductive function: therapeutic implications. Curr Drug Targets. 2013;14:365–371. Summary of human studies using kisspeptin treatment. [PubMed] [Google Scholar]