Abstract
In the present study we employ FIB/SEM tomography for analyzing 3-D architecture of dictyosomes and formation of multivesicular bodies (MVB) in high pressure frozen and cryo-substituted interphase cells of the green algal model system Micrasterias denticulata. The ability of FIB/SEM of milling very thin ‘slices’ (5–10 nm), viewing the block face and of capturing cytoplasmic volumes of several hundred μm3 provides new insight into the close spatial connection of the ER–Golgi machinery in an algal cell particularly in z-direction, complementary to informations obtained by TEM serial sectioning or electron tomography.
Our FIB/SEM series and 3-D reconstructions show that interphase dictyosomes of Micrasterias are not only closely associated to an ER system at their cis-side which is common in various plant cells, but are surrounded by a huge “trans-ER” sheath leading to an almost complete enwrapping of dictyosomes by the ER. This is particularly interesting as the presence of a trans-dictyosomal ER system is well known from mammalian secretory cells but not from cells of higher plants to which the alga Micrasterias is closely related. In contrast to findings in plant storage tissue indicating that MVBs originate from the trans-Golgi network or its derivatives our investigations show that MVBs in Micrasterias are in direct spatial contact with both, trans-Golgi cisternae and the trans-ER sheath which provides evidence that both endomembrane compartments are involved in their formation.
Keywords: FIB/SEM tomography, Dictyosomes, Multivesicular bodies, ER, Micrasterias denticulata, TEM
1. Introduction
The plant Golgi apparatus positioned at the cross-road between the secretory-biosynthetic and the endocytotic-vacuolar pathway fulfills a great number of different functions ranging from polysaccharide synthesis, protein glycosylations, and transfer, sorting of products, regulation of vesicle trafficking up to vacuole formation and participation in degradation processes (among numerous others, see reviews by Faso et al. (2009), Hawes and Satiat-Jeunemaitre (2005)). As synthesis site for most constituents of the cell wall, it is central in plant development and plays an important role in response to environmental impact. The diverse functions of the Golgi apparatus are reflected in the unique morphology and structural integrity of the numerous motile dictyosomes of a plant cell (Hawes, 2005). While spatially and functionally tightly associated to the ER from where they are supplied with proteins and lipids, the flattened closely attached cisternae of a dictyosome usually display clear cis–trans-polarity. Cis-, median- and trans-cisternae of a Golgi stack are involved in different steps of product processing and the tubular-reticular trans-Golgi network (TGN) acts as a sorting station that marks outgoing cargo for its destination and also holds the role of an early endosome (Hwang and Robinson, 2009; Staehelin and Moore, 1995; Viotti et al., 2010). The early endosome is the first compartment that receives cargo endocytosed from the plasma membrane (Otegui and Spitzer, 2008). Via different intermediate stages it may then mature to the late endosome which is frequently named multivesicular body (MVB). MVBs are essential for membrane recycling back to the TGN, may act as constituents of the degradation pathway ending up in the vacuole (Lam et al., 2007; Otegui and Spitzer, 2008; Robinson et al., 2008; Tanchak and Fowke, 1987) or may even be involved in the formation of autophagosomes (for references see below).
By means of three-dimensional high-voltage electron microscopy early studies by Marty (1978, 1999) provided evidence that the TGN acts as starting point for generation of vesicle like provacuoles which develop to tubular prevacuoles by microinvagination of their membranes (see also Bassham et al. (2006)). Provacuoles, prevacuoles or prevacuolar compartments are regarded as convergence point for either the endocytotic pathway or autophagy. Provacuoles may sequester portions of cytoplasm by forming digitate extensions thus developing to early double-membrane autophagosomes lateron (Marty, 1978, 1999). Although the observations of Marty were based on chemically fixed tissue in which artificial membrane alterations cannot be excluded, the results are intriguing and deserve verification by means of a more reliable structure preservation technique. More recent publications on high pressure frozen Nicotiana BY-2 cells (Tse et al., 2004) and Arabidopsis root tips (Scheuring et al., 2011) identified MVBs as prevacuolar compartments or provacuoles arising from one particular domain of the TGN and able to fuse with the vacuole in a non-vesicular way. This process requires both TGN integrity and V-ATPase activity (Scheuring et al., 2011). In protein storage tissue of Arabidopsis embryos it has been found that two different populations of TGN derived vesicles containing either the storage protein or the processing enzymes, fuse into MVBs functioning as pre-vacuolar compartments (Otegui et al., 2006). Whereas numerous participants in these different degradation pathways have been identified, the structural transformation from the TGN into the MVB is still obscure. This process however is crucial for both understanding the endocytotic and the degenerative pathway.
High pressure freeze fixation (HPF) for best structural preservation (Staehelin et al., 1990) combined with 3-D analysis such as electron tomography has been proved to be an excellent tool for getting insight into the development of dictyosomal or ER derived structures and their functions at high resolution (Donohoe et al., 2006; Hayashi-Nishino et al., 2009; Kang and Staehelin, 2008; Kang et al., 2011; Knott et al., 2008; Mogelsvang et al., 2004; Ylä-Anttila et al., 2009). Although the benefit of this technique for detailed structural analysis is undoubted, its limitations arise from the maximum thickness of the sections (400 nm; see Donohoe et al. (2006)), from the maximum tilt angle of about 70° causing a “missing wedge” and from the relatively small volume (max. ∼25 μm3) that can be calculated for the 3-D reconstructions. The new technique of focused ion beam milling and viewing by field emission scanning electron microscopy (FIB/SEM) overcomes these problems and provides additional structural information by its ability of sectioning very thin slices (5–10 nm) parallel to the block face and by covering volumes of several hundreds of μm3 (Knott et al., 2008; Schroeder-Reiter and Wanner, 2009; Schroeder-Reiter et al., 2009, 2012). The resolution of FIB/SEM tomography does not yet reach the resolution of TEM in x/y but is close to it as clearly demonstrated in a recent publication (Villinger et al., 2012). In respect to analysis of dictyosomal derived membranes an additional advantage of this method is provided by the fact that several dictyosomes of a cell can be captured at the same time.
In the present study we employed this technique for analyzing the 3-D architecture of interphase dictyosomes of the algal model system Micrasterias denticulata (Meindl, 1993) and to obtain insight into structural connections between MVBs and endomembrane systems. This alga is very well suited for such investigations as it possesses large dictyosomes with a constant average number of 11 cisternae throughout the cell cycle and their vesicular products during different developmental stages are well defined (Eder and Lütz-Meindl, 2008; Eder et al., 2008; Lütz-Meindl and Brosch-Salomon, 2000; Oertel et al., 2004). Moreover, numerous studies influencing the secretion pathway have provided information on the regulation of the secretory machinery (Lehner et al., 2009; Salomon and Meindl, 1996). Recently evidence has been obtained that Micrasterias is capable of performing autophagy and programmed cell death upon induction by abiotic stressors such as oxidative stress, high salinity or cadmium (Affenzeller et al., 2009a,b; Andosch et al., 2012). Coincidently with the occurrence of autophagy, environmental stress evokes severe structural alterations at dictyosomes (Affenzeller et al., 2009a,b; Darehshouri et al., 2008; Volland et al., 2011). Particularly cadmium induces a dose and time dependent complete disintegration of dictyosomes combined with an increase in the number of MVBs (Andosch et al., 2012). Although, like in other plant and animal systems, the ER contributes to autophagosome formation in Micrasterias there are several indications that MVBs may functions as sources for stress induced degeneration or even autophagosome formation as well (see above). Information on the structural origin of MVBs as provided by FIB/SEM tomography will therefore be important as a basis to understand stress induced degradation processes. The present study shows that MVBs are frequently found in structural contact with both trans-Golgi cisternae and a “trans-ER”. As Micrasterias is a member of Streptophyta belonging to the closest relatives of higher plants (Wodniok et al., 2011) we expect that the results obtained may be of general interest for plant cells and may also provide insight into evolutionary aspects of the first steps of degradation.
2. Materials and methods
All chemicals were purchased from Roth (Karlsruhe, Germany) or Sigma–Aldrich (Vienna, Austria) unless stated differently.
2.1. Cell cultures
The unicellular freshwater green alga M. denticulata was cultivated in a liquid Desmidiacean medium (Schlösser, 1982) in Erlenmeyer flaks at constant temperature of 20 °C and a light/dark regime of 14/10 h. Cells were subcultured every 4–5 weeks. To obtain defined interphase stages for FIB/SEM analyses, developmental stages were collected and were allowed to grow in nutrient solution for 48 h.
2.2. High pressure freeze fixation for TEM and FIB/SEM tomography
Interphase cells of M. denticulata were high-pressure frozen and cryo-substituted following the protocol of Aichinger and Lütz-Meindl (2005), Meindl et al. (1992). In brief the cells were wrapped in cotton fibers to which they stick due to their surface mucilage, and frozen in a Leica EMPACT HPF device (Leica Microsystems, Austria). Freeze substitution was performed in a Leica EM AFS in 2% OsO4 and 0.05% uranyl acetate in anhydrous acetone. Cells were embedded in epoxy resin (Agar low viscosity resin; Agar Scientific, Essex, UK) and sectioned on a Leica UC7 ultramicrotome for TEM. 60–80 nm sections were mounted on formvar coated copper grids and investigated in a LEO 912 AB Omega TEM (Zeiss, Oberkochen, Germany) at 80 kV by using zero-loss energy filtering (Lütz-Meindl and Aichinger, 2004). Sections of all samples were viewed in TEM prior to analysis by FIB/SEM tomography.
2.3. FIB/SEM tomography
High pressure frozen and freeze substituted interphase cells of M. denticulata were washed in acetone and transferred into propylenoxide and mixtures of epoxy resin/propylenoxide in ratios 1:3 and 1:2 and finally embedded in pure epoxy resin (see above). Cells were mounted on aluminum stubs covered with a thin layer of polymerized epoxy resin. Blocks were trimmed with a LKB Pyramitome with glass knives that vertical faces allowed lateral milling of the cells by the FIB. Tomographic datasets were obtained using the ‘‘slice and view” technique using a Zeiss Auriga 60 dual beam workstation (Carl Zeiss Microscopy, Oberkochen, Germany). For slicing with the focused ion beam, the conditions were as follows: 500 pA milling current of the Ga-emitter; with each step 7–10 nm of the epoxy resin was removed with the FIB. SEM images were recorded with an aperture of 60 μm in the high current mode at 1.5 kV of the In-lens EsB detector with the EsB grid set to −1400 V. Line averaging 4 was performed. The image pixel size was 5.3 × 5.3 nm in x/y. The dimensions of the recorded images were 2048 × 1536 pixel. According to the Nyquist criterion the resolution lies thus in the range of 12 nm. The time to acquire one frame was 90s. The slice and view process was repeated 500–1500 times to obtain the different datasets. The contrast of the images was inversed, so that they appeared like a conventional bright field TEM image.
2.4. Data processing and 3-D reconstruction
Image stacking and alignment was performed with the open source software ImageJ (http://rsbweb.nih.gov/ij/index.html). Segmentation was performed using Amira Software (VSG; Visualization Sciences Group).
3. Results
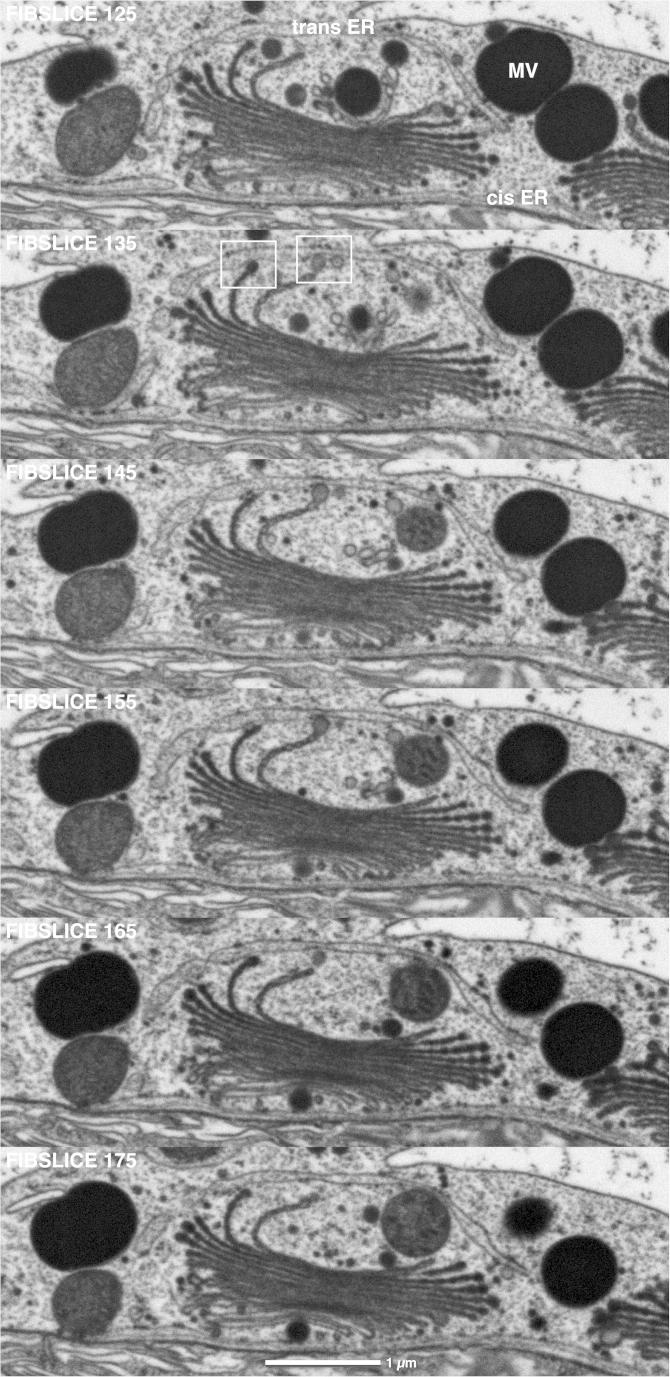
Dictyosomes of Micrasterias interphase cells are mostly accumulated in the cell center around the nucleus or along the edges of the large chloroplast which covers each of the two semicells. FIB/SEM slicing and 3-D analysis of the block-face images shows that the Golgi stacks consist of flat unfenestrated cisternae with lacerated rims (Fig. 1a,b). Interconnections between the cisternae are frequently visible at the images series. The outermost trans-cisterna is frequently shorter and more involute and differs in its structure from the others. On FIB/SEM series (Fig. 2) and TEM micrographs (Fig. 3a,b) it appears chain-like constricted resulting in an undulated surface in the 3-D reconstruction (Fig. 1b). During interphase the main products of dictyosomes in Micrasterias are large mucilage vesicles which origin from the edges of the outermost trans-cisternae and are also found accumulated around the dictyosomes (Fig. 4, Suppl. animation). Additionally smaller secretory vesicles are present. These findings confirm earlier results obtained from serial sectioning of HP frozen or chemically fixed Micrasterias cells and TEM (Meindl et al., 1992). The trans-most cisterna is frequently separated from the dictyosome and appears circular (Fig. 2). Depending on how it is oriented it may be assigned to a TGN. Like in other plant cells (Faso et al., 2009; Moreau et al., 2007) almost all dictyosomes are in close spatial relationship to an ER cisterna at their cis-side (Figs. 2, 3b, 5, 6, Suppl. animation) and transitorial vesicles between these two compartments of the endomembrane system are visible. For the first time FIB-serial sectioning and 3-D reconstruction shows that the dictyosomes in Micrasterias are almost entirely engulfed by a huge ER sheath which is not limited to the cis-Golgi side but also envelopes the trans-Golgi area to a large extent (Figs. 5 and 6, Suppl. animation). Frequently the membranes of the trans-cisternae are in close contact with the ER membranes either directly or via vesicular connections (Fig. 6). This finding yields a new picture of the close spatial connection of the ER-Golgi machinery which became only possible by the capability of FIB/SEM tomography of providing 3-D analysis of large volumes particularly in z-direction. Although single ER cisternae have been observed in vicinity to the dictyosomal trans-cisternae in TEM as well (Fig. 3b), the presence of such a huge enwrapping “trans-ER” sheath would have been never deducible from 2-D TEM micrographs. The fact that several dictyosomes of a cell can be serial sliced and visualized by FIB/SEM at a time, enables comparison of different Golgi-ER associations thus yielding additional information on the generality of these observations.
Fig.1.
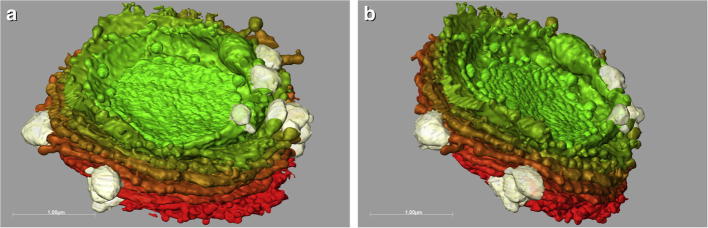
(a), and (b) Different 3-D views of the trans-side of a dictyosome of M. denticulata reconstructed from FIB/SEM series. Cisternal rims are lacerated, outermost-trans-cisterna are shorter than others and undulated. Secretory vesicles (grey) are connected to edges of trans-cisternae. From red to green: cis- to trans-cisternae.
Fig.2.
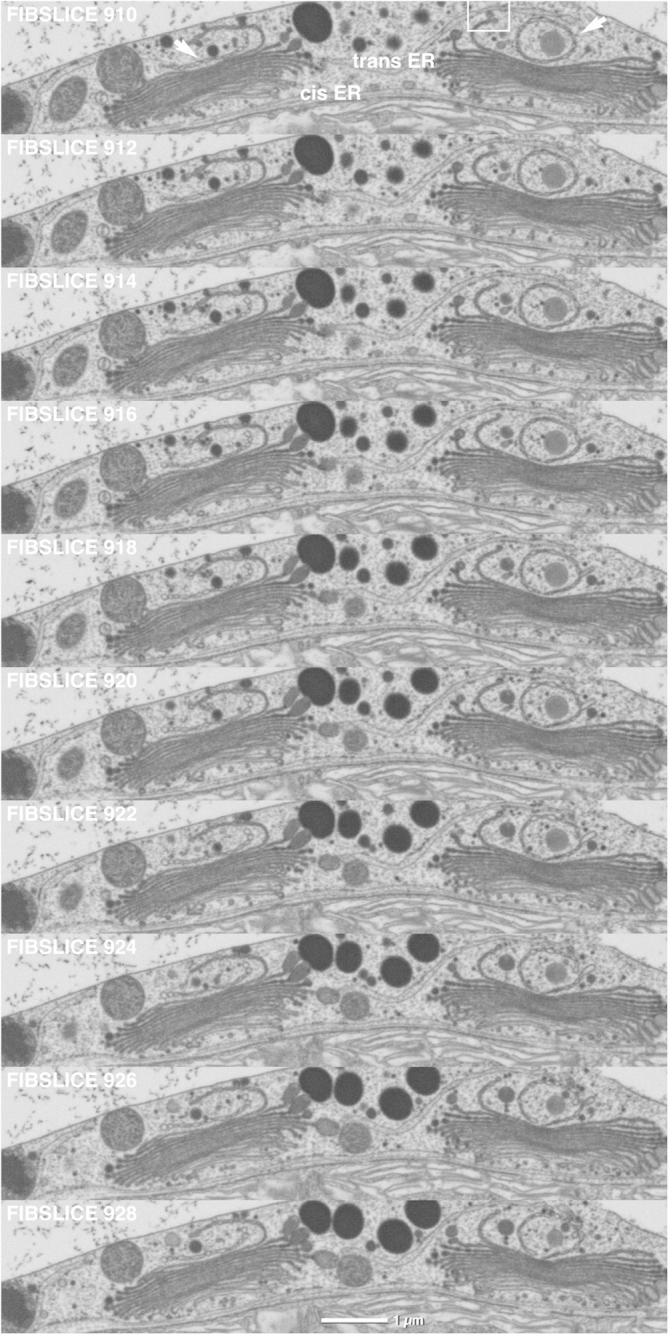
FIB/SEM series of two dictyosomes of an interphase Micrasterias cell. Trans-most Golgi cisternae are shorter and involute respectively circular (arrows). ER cisternae present at both cis- and trans-side of the right dictyosome. Framed area indicates vesicular contact between a trans-Golgi cisterna and the trans-ER. Small secretory vesicles and large mucilage vesicles are visible around the left dictyosome. 10 nm slices, every second image shown.
Fig.3.
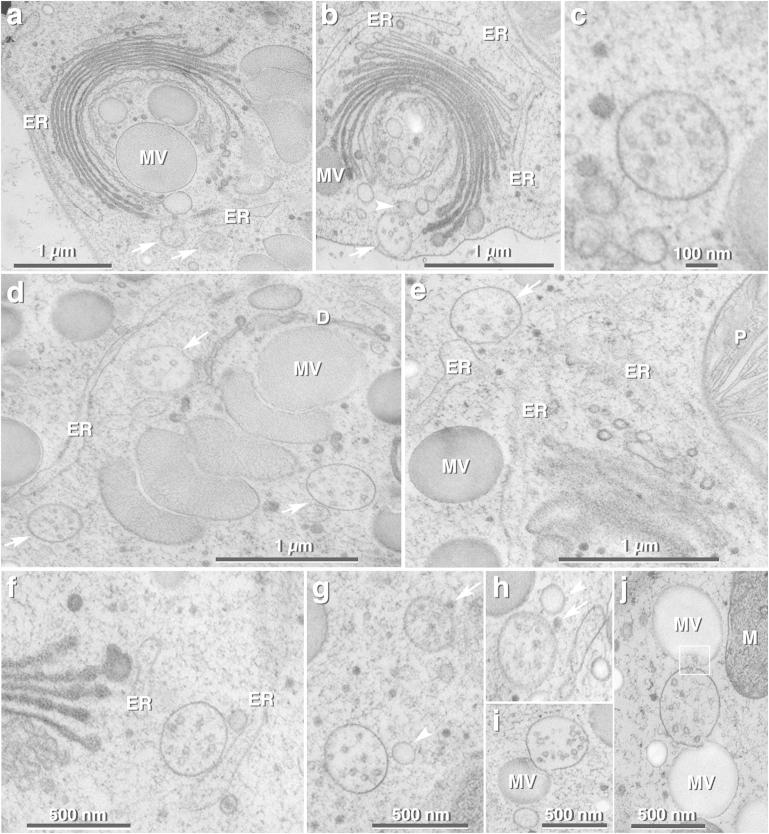
TEM micrographs of ultrathin sections of high-pressure frozen and freeze substituted interphase cells of M. denticulata. (a) Dictyosomes with adjacent ER cisterna at cis- and trans-side. Trans-most cisterna is chain-like constricted. Large mucilage vesicles (MV) are in contact with rims of trans-cisternae. Two MVBs (arrows) are located in close proximity to the trans-Golgi side and to a trans-ER cisterna. (b) MVB (arrow) is in contact with vacuolar membrane. translucent vesicles (arrow head) are in contact with trans-dictyosomal cisterna and MVB. ER = endoplasmic reticulum. (c) Representative MVB of Micrasterias with intraluminal vesicles. (d) Trans-side of dictyosome (D) with stack of mucilage vesicles (MV). Three MVBs (arrows) are in close proximity to the dictyosome and to a trans-ER cisterna. ER = endoplasmic reticulum. (e) Dictyosome and parts of the enwrapping ER. Contact between MVB (arrow) and part of a trans-ER cisterna visible. MV = mucilage vesicle; ER = endoplasmic reticulum; P = Plastid. (f) Part of a dictyosome and MVB attached to an ER cisterna. ER = endoplasmic reticulum. (g,h) MVBs in spatial contact with small electron-dense vesicle (arrow) and with larger translucent vesicle (arrow head). (i,j) MVBs attached to mucilage vesicles (MV). (j) Intraluminal vesicle of MVB discharged into upper mucilage vesicle (framed area). M = mitochondrion.
Fig.4.
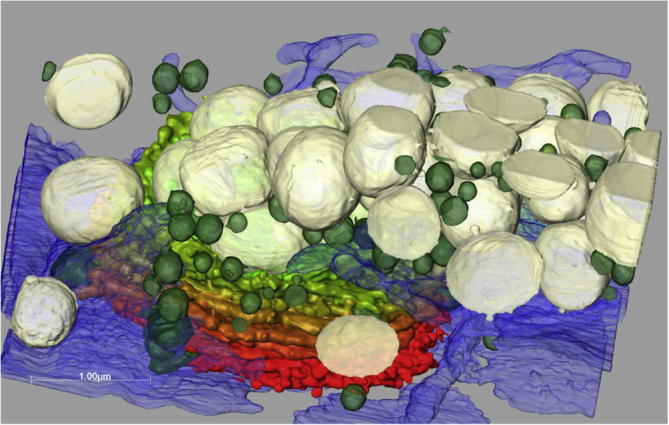
3-D reconstruction of interphase dictyosome of Micrasterias with enwrapping ER system (blue) and different vesicles populations. Small secretory vesicles are shown in green, mucilage vesicles in grey. Dictyosomal cis-side in red, trans-side in green.
Fig.5.
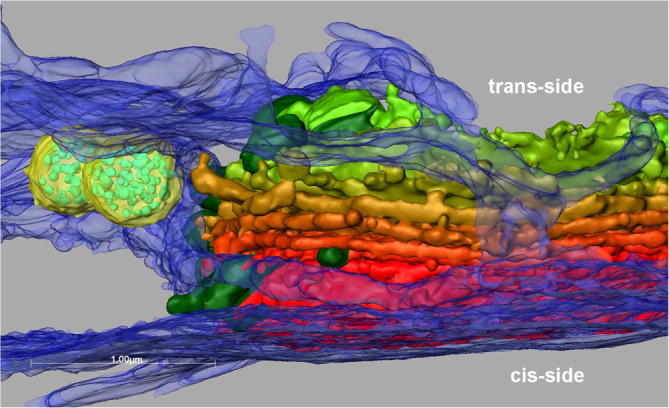
3-D reconstruction of dictyosome and ER envelope (blue) at cis- and trans-side of the dictyosome. Two MVBs (yellow) are visible in proximity of the dictyosomal trans-cisternae.
Fig.6.
FIB/SEM series of dictyosome in contact to a cis- and a trans-ER cisterna. Trans-ER covers the trans-side of the dictyosome almost entirely. At least two of the trans-cisternae (framed areas) are in contact with the trans-ER either directly or via vesicular connections. The trans-most cisterna is vesicular and disconnected from Golgi stack appearing as TGN. Large mucilage vesicles (MV) are visible in close vicinity to dictyosome. 10 nm slices, every 10th image shown.
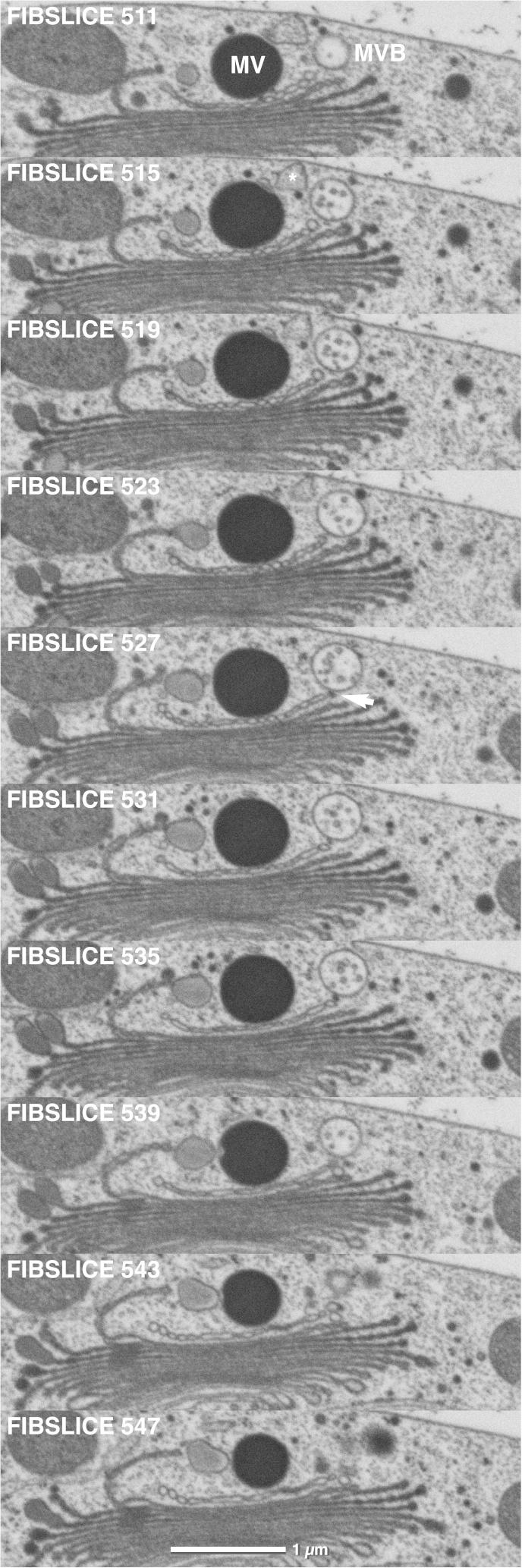
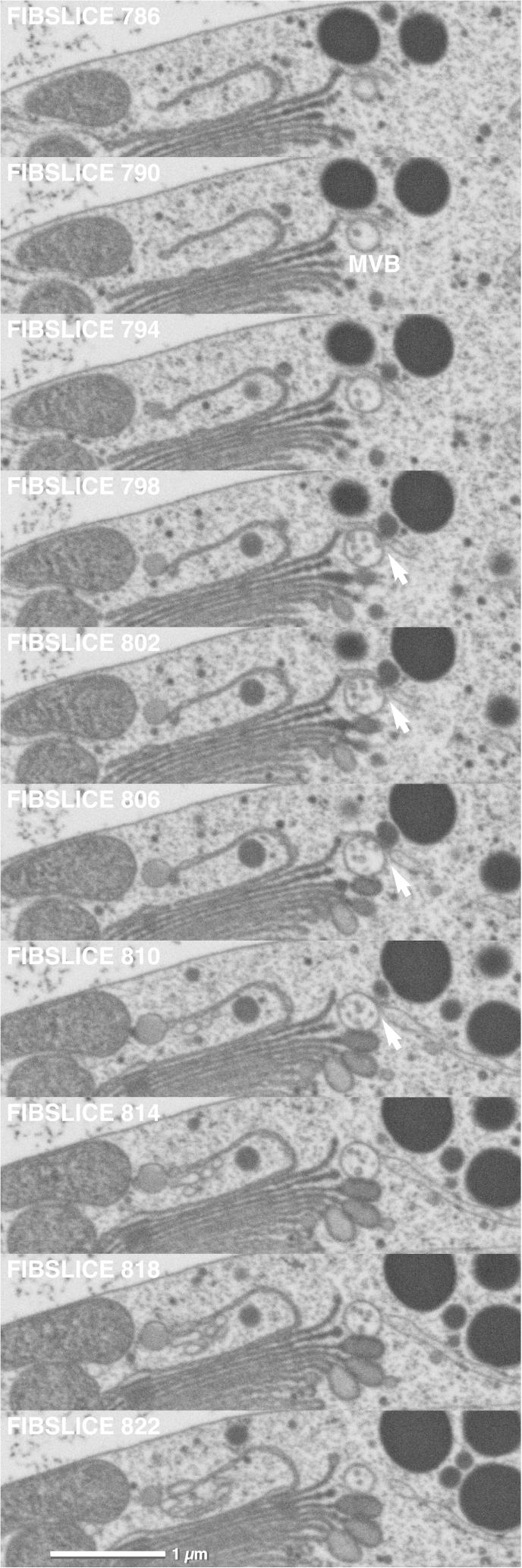
MVBs in Micrasterias are mostly found in close association to dictyosomes both in interphase cells (Figs. 7 and 8) and during cell growth (not shown). As in higher plant cells (Lam et al., 2007; Otegui and Spitzer, 2008; Otegui et al., 2006; Scheuring et al., 2011; Wang et al., 2011) they appear always spherical and contain intraluminal vesicles with a diameter of about 50 nm in Micrasterias (Fig. 3c). Their maximum diameter averages 400 nm. Several MVBs may be found in close vicinity of one dictyosome (Fig. 3d). All previous studies on MVB maturation mostly done in protein storage tissue, have indicated that they originate from either the TGN or from dictyosomal derived vesicles that fuse to form a MVB (see references above). Our study employing FIB/SEM analysis provides evidence that MVB formation in Micrasterias is not only a function of the trans-Golgi region but involves a “trans-ER” as well. Most of the MVBs investigated in Micrasterias were in spatial contact to both, an ER and a trans-dictyosomal cisterna. Examples of these contacts are visible in the series of Figs. 7 and 8. Occasionally direct contacts existed exclusively between MVB and ER although the MVB was located in close vicinity to trans-Golgi cisternae (Fig. 9a,b, Suppl. animation). The contact between the membranes of the ER and the MVB appears to be a direct one: membranes of ER and MVBs are frequently attached to each other (Fig. 3e,f). Connections to dictyosomal derived tubular structures indicating that MVBs originate from the TGN as observed in Arabidopsis root tips (Scheuring et al., 2011) were never found in Micrasterias. Two different vesicle populations were frequently seen in contact with MVBs: small electron-dense vesicles with a diameter of approx. 50 nm corresponding to the intraluminal vesicles of the MVBs both in size and in electron density and larger, electron translucent vesicles with an average diameter of 130 nm (Fig. 3g,h). Electron translucent vesicles were frequently found to be in contact to the rims of trans-cisternae suggesting that they originate from the dictyosomes (Fig. 3b) whereas the origin of the small vesicles is unclear. Contacts between the MVBs and the dictyosomal cisternae may occur either via these vesicles, or directly between the dictyosomal membrane and the membrane of the MVB (Fig. 7). Most contacts between MVBs and dictyosomes were observed with the outermost trans-cisterna or with one of the other trans-cisternae, only rarely with medium cisternae. MVBs with no visible contact to either the ER or a dictyosome were also rarely found by FIB/SEM tomography. However, it cannot be excluded that the corresponding endomembrane system in these cases was located in a larger distance above or below the MVB and was simply not included in the respective series.
Fig.7.
FIB/SEM series of MVB in contact with trans-most dictyosomal cisterna via small vesicles (arrow). Maturating and ripe mucilage vesicle in contact to each other and close to trans-Golgi cisternae. 10 nm slices, every 4th image shown.
Fig.8.
FIB/SEM series of MVB in direct spatial contact to both a trans-dictyosomal cisterna and a trans-ER cisterna (arrow). The MVB also contacts one of the maturating mucilage vesicles.
Fig.9.
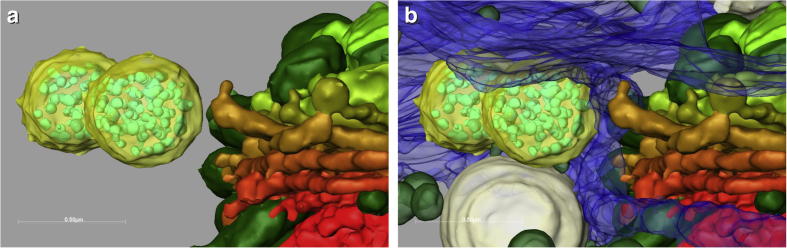
(a,b). 3-D reconstruction of spatial relationship between trans-Golgi cisternae and MVBs (yellow) without (a) and with (b) enwrapping ER system (blue). The reconstructions show that the membranes of the MVBs are in direct contact with the membranes of the ER but not with the Golgi cisternae.
In addition to their structural connections to trans-Golgi cisternae and the ER, MVBs were observed to be in contact with each other (Figs. 5 and 9a,b) and with vacuoles (Fig. 3b). Occasionally MVBs were found tightly attached to membranes of large mucilage vesicles (Fig. 3i) and their transluminal vesicles seemed to be discharged into the mucilage vesicle (Fig. 3j). Frequently contacts to several compartments existed simultaneously.
4. Discussion
Technical advances by FIB/SEM analysis and subsequent 3-D reconstruction provides new insight into architecture of interphase dictyosomes and into formation of MVBs in the unicellular green alga Micrasterias. The most important new result is the 3-D visualization of a huge ER sheath at the trans-side of the dictyosomes which together with the common cis-Golgi associated ER system known from different plant cells (among others see Hawes and Satiat-Jeunemaitre (2005), Moreau et al. (2007)) almost entirely enwraps the dictyosomes in Micrasterias. Neither TEM serial sectioning nor electron tomography would be able to provide information on the magnitude of this ER envelope. Due to the relatively thick sections (60–70 nm) information is lost in TEM and only parts of this “trans-ER system” are depicted. On the other hand, electron tomography is limited to a relatively small cytoplasmic volume and would not allow calculating such large cytoplasmic areas. Additionally our FIB/SEM series indicate that this “trans-ER” sheath may also be involved in MVB formation which has been so far attributed solely to trans-Golgi compartments and their derivatives in higher plant cells (e.g. (Scheuring et al., 2011; Tse et al., 2004; Wang et al., 2011)).
The presence of a “trans-ER” system is well known from mammalian secretory cells where it represents a specialized ER cisterna in close association with the trans-most Golgi cisterna (reviewed by Mogelsvang et al. (2004)). In higher plant cells indications for a “trans-ER” possibly involved in secretion in concert with the dictyosomes, have been obtained from early thick-sectioning techniques in mung bean cells revealing direct connections between the ER and trans-dictyosome cisternae (Harris and Oparka, 1983). However, in the recent literature referring preferentially to cryo-fixed samples or to studies in living cells, reports of the presence of a trans-Golgi-ER association are missing (see reviews by Faso et al. (2009), Hawes and Satiat-Jeunemaitre (2005)). From an evolutionary point of view it is interesting that this trans-Golgi-ER association appears in an alga such as Micrasterias which is closely related to higher plants (Wodniok et al., 2011). Moreover, the FIB/SEM series show that direct structural contacts between the “trans-ER” and the trans-most Golgi cisterna – as postulated by early studies in mammalian and plant cells (Hand and Oliver, 1977; Harris and Oparka, 1983; Novikoff et al., 1971) – do in fact occur in Micrasterias.
The presence of structural connections of MVBs not only to trans-Golgi cisternae and their derivatives but also to a “trans-ER” may point towards a particular way of degradation. According to the early GERL hypothesis of Novikoff et al. (1971) postulating that newly synthesized lysosomal enzymes are directly delivered from the ER to the trans-most Golgi cisterna in neurons, the ER in Micrasterias may supply MVBs with lytic enzymes whereas the cargo proteins are secreted through the Golgi apparatus. In this way the MVBs would become lytic compartments and starting points for degradation as postulated in early TEM studies (Tanchak and Fowke, 1987). In fact an increase in the number of MVBs has been observed in Micrasterias upon exposure to environmental stress such as high salinity or cadmium exposure (Affenzeller et al., 2009a; Andosch et al., 2012), indicating their involvement in stress induced degradation. Golgi-derived vesicles resembling the “dense vesicles” carrying storage proteins in cotyledons or embryos of legumes (Hinz et al., 1999; Otegui and Spitzer, 2008; Robinson et al., 1998) and electron-dense small vesicles with the same size as the interluminal vesicles have been found to be in contact with MVBs in Micrasterias and are interpreted to contribute to their formation. Our data on frequent, close structural contacts between different compartments suggest that discharge of MVBs occurs both into vacuoles or lytic compartments but also into mucilage vesicles. Mucilage vesicles are secreted throughout the cell cycle of Micrasterias (Oertel et al., 2004) and were recently found to represent vehicles for heavy metal detoxification (Volland et al., 2011). Heavy metals are sequestered in mucilage vesicles and removed from the cell by mucilage secretion. In the same way degrading proteins inside MVBs may be disposed via the secretion pathway. Cytochemical and immuno-TEM studies including cells after stress exposure will be required to verify this hypothesis.
Acknowledgments
We gratefully acknowledge the excellent technical assistance by Silvia Dobler and Ancuela Andosch and the valuable suggestions and support by Max Scheungrab during the manual segmentation for the 3-D reconstructions. The study was financially supported by the Austrian Science Fund project 21035-B16 to U.L-M.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jsb.2013.10.003.
Contributor Information
Gerhard Wanner, Email: wanner@lrz.uni-muenchen.de.
Tillman Schäfer, Email: tillman.schaefer@gmx.de.
Ursula Lütz-Meindl, Email: ursula.meindl@sbg.ac.at.
Appendix A. Supplementary data
Animation of a 3-D reconstruction of an interphase dictyosome of Micrasterias denticulata. Trans-side of dictyosome in light green, ER in blue, mucilage vesicles in light yellow. The dictyosome is attached to a cis-ER sheath and is covered by a trans-ER. Two multivesicular bodies (yellow) containing intraluminal vesicles are attached to each other and to a trans-ER sheath, but not to Golgi cisternae. Numerous large mucilage vesicles are located above the trans-ER.
References
- Affenzeller M.J., Darehshouri A., Andosch A., Lütz C., Lütz-Meindl U. Salt stress-induced cell death in the unicellular green alga Micrasterias denticulata. J. Exp. Bot. 2009;60:939–954. doi: 10.1093/jxb/ern348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Affenzeller M.J., Darehshouri A., Andosch A., Lütz C., Lütz-Meindl U. PCD and autophagy in the unicellular green alga Micrasterias denticulata. Autophagy. 2009;5:854–855. doi: 10.4161/auto.8791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aichinger N., Lütz-Meindl U. Organelle interactions and possible degradation pathways visualized in high-pressure frozen algal cells. J. Microsc. 2005;219:86–94. doi: 10.1111/j.1365-2818.2005.01496.x. [DOI] [PubMed] [Google Scholar]
- Andosch A., Affenzeller M.J., Lütz C., Lütz-Meindl U. A freshwater green alga under cadmium stress: ameliorating calcium effects on ultrastructure and photosynthesis in the unicellular model Micrasterias. J. Plant Physiol. 2012;169:1489–1500. doi: 10.1016/j.jplph.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Bassham D.C., Laporte M., Marty F., Moriyasu Y., Ohsumi Y., Olsen L.J., Yoshimoto K. Autophagy in development and stress responses of plants. Autophagy. 2006;2:2–11. doi: 10.4161/auto.2092. [DOI] [PubMed] [Google Scholar]
- Darehshouri A., Affenzeller M., Lütz-Meindl U. Cell death upon H2O2 induction in the unicellular green alga Micrasterias. Plant Biol. 2008;10:732–745. doi: 10.1111/j.1438-8677.2008.00078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohoe B.S., Mogelsvang S., Staehelin L.A. Electron tomography of ER, Golgi and related membrane systems. Methods. 2006;39:154–162. doi: 10.1016/j.ymeth.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Eder M., Lütz-Meindl U. Pectin-like carbohydrates in the green alga Micrasterias characterized by cytochemical analysis and energy filtering TEM. J. Microsc. 2008;231:201–214. doi: 10.1111/j.1365-2818.2008.02036.x. [DOI] [PubMed] [Google Scholar]
- Eder M., Tenhaken R., Driouich A., Lütz-Meindl U. Occurrence and characterization of arabinogalactan-like proteins and hemicelluloses in Micrasterias (Streptophyta) J. Phycol. 2008;44:1221–1234. doi: 10.1111/j.1529-8817.2008.00576.x. [DOI] [PubMed] [Google Scholar]
- Faso C., Boulaflous A., Brandizzi F. The plant Golgi apparatus: last 10 years of answered and open questions. FEBS Lett. 2009;583:3752–3757. doi: 10.1016/j.febslet.2009.09.046. [DOI] [PubMed] [Google Scholar]
- Hand A.R., Oliver C. Relationship between Golgi apparatus, Gerl, and secretory granules in acinar cells of rat exorbital lacrimal gland. J. Cell Biol. 1977;74:399–413. doi: 10.1083/jcb.74.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris N., Oparka K.J. Connections between dictyosomes, ER and GERL in cotyledons of mung bean (Vigna radiata L.) Protoplasma. 1983;114:93–102. [Google Scholar]
- Hawes C. Cell biology of the plant Golgi apparatus. New Phytol. 2005;165:29–44. doi: 10.1111/j.1469-8137.2004.01218.x. [DOI] [PubMed] [Google Scholar]
- Hawes C., Satiat-Jeunemaitre B. The plant Golgi apparatus-going with the flow. Biochim. Biophys. Acta. 2005;1744:466–480. doi: 10.1016/j.bbamcr.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Hayashi-Nishino M., Fujita N., Noda T., Yamaguchi A., Yoshimori T., Yamamoto A. A subdomain of the endoplasmic reticulum forms a cradle for autophagosome formation. Nat. Cell Biol. 2009;11:1433–1437. doi: 10.1038/ncb1991. [DOI] [PubMed] [Google Scholar]
- Hinz G., Hillmer S., Bäumer M., Hohl I.I. Vacuolar storage proteins and the putative vacuolar sorting receptor BP-80 exit the Golgi apparatus of developing pea cotyledons in different transport vesicles. Plant Cell. 1999;11:1509–1524. doi: 10.1105/tpc.11.8.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang I., Robinson D.G. Transport vesicle formation in plant cells. Curr. Opin. Plant Biol. 2009;12:660–669. doi: 10.1016/j.pbi.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Kang B.H., Staehelin L.A. ER-to-Golgi transport by COPII vesicles in Arabidopsis involves a ribosome-excluding scaffold that is transferred with the vesicles to the Golgi matrix. Protoplasma. 2008;234:51–64. doi: 10.1007/s00709-008-0015-6. [DOI] [PubMed] [Google Scholar]
- Kang B.H., Nielsen E., Preuss M.L., Mastronarde D., Staehelin L.A. Electron tomography of RabA4b- and PI-4Kbeta1-labeled trans Golgi network compartments in Arabidopsis. Traffic. 2011;12:313–329. doi: 10.1111/j.1600-0854.2010.01146.x. [DOI] [PubMed] [Google Scholar]
- Knott G., Marchman H., Wall D., Lich B. Serial section scanning electron microscopy of adult brain tissue using focused ion beam milling. J. Neurosci. 2008;28:2959–2964. doi: 10.1523/JNEUROSCI.3189-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam S.K., Siu C.L., Hillmer S., Jang S., An G.H., Robinson D.G., Jiang L.W. Rice SCAMP1 defines clathrin-coated, trans-Golgi-located tubular-vesicular structures as an early endosome in tobacco BY-2 cells. Plant Cell. 2007;19:296–319. doi: 10.1105/tpc.106.045708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner C., Kerschbaum H.H., Lütz-Meindl U. Nitric oxide suppresses growth and development in the unicellular green alga Micrasterias denticulata. J. Plant Physiol. 2009;166:117–127. doi: 10.1016/j.jplph.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Lütz-Meindl U., Brosch-Salomon S. Cell wall secretion in the green alga Micrasterias. J. Microsc. 2000;198:208–217. doi: 10.1046/j.1365-2818.2000.00699.x. [DOI] [PubMed] [Google Scholar]
- Lütz-Meindl U., Aichinger N. Use of energy-filtering transmission electron microscopy for routine ultrastructural analysis of high-pressure-frozen or chemically fixed plant cells. Protoplasma. 2004;223:155–162. doi: 10.1007/s00709-003-0033-3. [DOI] [PubMed] [Google Scholar]
- Marty F. Cytochemical studies on GERL, provacuoles, and vacuoles in root meristematic cells of Euphorbia. Proc. Natl. Acad. Sci. U.S.A. 1978;75:852–856. doi: 10.1073/pnas.75.2.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty F. Plant vacuoles. Plant Cell. 1999;11:587–600. doi: 10.1105/tpc.11.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meindl U. Micrasterias cells as a model system for research on morphogenesis. Microbiol. Rev. 1993;57:415–433. doi: 10.1128/mr.57.2.415-433.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meindl U., Lancelle S., Hepler P. Vesicle production and fusion during lobe formation in Micrasterias visualized by high-pressure freeze fixation. Protoplasma. 1992;170:104–114. [Google Scholar]
- Mogelsvang S., Marsh B.J., Ladinsky M.S., Howell K.E. Predicting function from structure: 3D structure studies of the mammalian Golgi complex. Traffic. 2004;5:338–345. doi: 10.1111/j.1398-9219.2004.00186.x. [DOI] [PubMed] [Google Scholar]
- Moreau P., Brandizzi F., Hanton S., Chatre L., Melser S., Hawes C., Satiat-Jeunemaitre B. The plant ER-Golgi interface: a highly structured and dynamic membrane complex. J. Exp. Bot. 2007;58:49–64. doi: 10.1093/jxb/erl135. [DOI] [PubMed] [Google Scholar]
- Novikoff P.M., Novikoff A.B., Quintana N., Hauw J.J. Golgi apparatus, GERL, and lysosomes of neurons in rat dorsal root ganglia, studied by thick section and thin section cytochemistry. J. Cell Biol. 1971;50:859–886. doi: 10.1083/jcb.50.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel A., Aichinger N., Hochreiter R., Thalhamer J., Lütz-Meindl U. Analysis of mucilage secretion and excretion in Micrasterias (Chlorophyta) by means of immune electron microscopy and digital time lapse video microscopy. J. Phycol. 2004;40:711–720. [Google Scholar]
- Otegui M.S., Spitzer C. Endosomal functions in plants. Traffic. 2008;9:1589–1598. doi: 10.1111/j.1600-0854.2008.00787.x. [DOI] [PubMed] [Google Scholar]
- Otegui M.S., Herder R., Schulze J., Jung R., Staehelin L.A. The proteolytic processing of seed storage proteins in Arabidopsis embryo cells starts in the multivesicular bodies. Plant Cell. 2006;18:2567–2581. doi: 10.1105/tpc.106.040931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D.G., Jiang L., Schumacher K. The endosomal system of plants: charting new and familiar territories. Plant Physiol. 2008;147:1482–1492. doi: 10.1104/pp.108.120105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D.G., Bäumer M., Hinz G., Hohl I. Vesicle transfer of storage proteins to the vacuole: the role of the Golgi apparatus and multivesicular bodies. J. Plant Physiol. 1998;152:659–667. [Google Scholar]
- Salomon S., Meindl U. Brefeldin A induces reversible dissociation of the Golgi apparatus in the green alga Micrasterias. Protoplasma. 1996;194:231–242. [Google Scholar]
- Scheuring D., Viotti C., Krüger F., Künzl F., Sturm S., Bubeck J., Hillmer S., Frigerio L., Robinson D.G., Pimpl P., Schumacher K. Multivesicular bodies mature from the trans-Golgi network/early endosome in Arabidopsis. Plant Cell. 2011;23:3463–3481. doi: 10.1105/tpc.111.086918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlösser U.G. Sammlungen von Algenkulturen. Ber. Deutsch. Bot. Ges. 1982;95:181–276. [Google Scholar]
- Schroeder-Reiter E., Wanner G. Chromosome centromeres: structural and analytical investigations with high resolution scanning electron microscopy in combination with focused ion beam milling. Cytogenet. Genome Res. 2009;124:239–250. doi: 10.1159/000218129. [DOI] [PubMed] [Google Scholar]
- Schroeder-Reiter E., Perez-Willard F., Zeile U., Wanner G. Focused ion beam (FIB) combined with high resolution scanning electron microscopy: a promising tool for 3D analysis of chromosome architecture. J. Struct. Biol. 2009;165:97–106. doi: 10.1016/j.jsb.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Schroeder-Reiter E., Sanei M., Houben A., Wanner G. Current SEM techniques for de- and re-construction of centromeres to determine 3D CENH3 distribution in barley mitotic chromosomes. J. Microsc. 2012;246:96–106. doi: 10.1111/j.1365-2818.2011.03592.x. [DOI] [PubMed] [Google Scholar]
- Staehelin L.A., Moore I. The plant Golgi-apparatus – structure, functional-organization and trafficking mechanisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1995;46:261–288. [Google Scholar]
- Staehelin L.A., Giddings T.H., Jr., Kiss J.Z., Sack F.D. Macromolecular differentiation of Golgi stacks in root tips of Arabidopsis and Nicotiana seedlings as visualized in high pressure frozen and freeze-substituted samples. Protoplasma. 1990;157:75–91. doi: 10.1007/BF01322640. [DOI] [PubMed] [Google Scholar]
- Tanchak M.A., Fowke L.C. The morphology of multivesicular bodies in soybean protoplasts and their role in endocytosis. Protoplasma. 1987;138:173–182. [Google Scholar]
- Tse Y.C., Mo B., Hillmer S., Zhao M., Lo S.W., Robinson D.G., Jiang L. Identification of multivesicular bodies as prevacuolar compartments in Nicotiana tabacum BY-2 cells. Plant Cell. 2004;16:672–693. doi: 10.1105/tpc.019703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villinger C., Gregorius H., Kranz C., Höhn K., Münzberg C., von Wichert G., Mizaikoff B., Wanner G., Walther P. FIB/SEM tomography with TEM-like resolution for 3D imaging of high-pressure frozen cells. Histochem. Cell Biol. 2012;138:549–556. doi: 10.1007/s00418-012-1020-6. [DOI] [PubMed] [Google Scholar]
- Viotti C., Bubeck J., Stierhof Y.D., Krebs M., Langhans M., van den Berg W., van Dongen W., Richter S., Geldner N., Takano J., Jürgens G., de Vries S.C., Robinson D.G., Schumacher K. Endocytic and secretory traffic in Arabidopsis merge in the trans-Golgi network/early endosome, an independent and highly dynamic organelle. Plant Cell. 2010;22:1344–1357. doi: 10.1105/tpc.109.072637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volland S., Andosch A., Milla M., Stöger B., Lütz C., Lütz-Meindl U. Intracellular metal compartmentalization in the green algal model system Micrasterias denticulata (Streptophyta) measured by transmission electron microscopy–coupled electron energy loss spectroscopy. J. Phycol. 2011;47:565–579. doi: 10.1111/j.1529-8817.2011.00988.x. [DOI] [PubMed] [Google Scholar]
- Wang J., Tse Y.C., Hinz G., Robinson D.G., Jiang L. Storage globulins pass through the Golgi apparatus and multivesicular bodies in the absence of dense vesicle formation during early stages of cotyledon development in mung bean. J. Exp. Bot. 2011;63:1367–1380. doi: 10.1093/jxb/err366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodniok S., Brinkmann H., Glöckner G., Heidel A.J., Philippe H., Melkonian M., Becker B. Origin of land plants: do conjugating green algae hold the key? BMC Evol. Biol. 2011;11:104. doi: 10.1186/1471-2148-11-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylä-Anttila P., Vihinen H., Jokitalo E., Eskelinen E.L. 3D tomography reveals connections between the phagophore and endoplasmic reticulum. Autophagy. 2009;5:1180–1185. doi: 10.4161/auto.5.8.10274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Animation of a 3-D reconstruction of an interphase dictyosome of Micrasterias denticulata. Trans-side of dictyosome in light green, ER in blue, mucilage vesicles in light yellow. The dictyosome is attached to a cis-ER sheath and is covered by a trans-ER. Two multivesicular bodies (yellow) containing intraluminal vesicles are attached to each other and to a trans-ER sheath, but not to Golgi cisternae. Numerous large mucilage vesicles are located above the trans-ER.