Abstract
The pore-forming toxin listeriolysin O (LLO), which is produced by Listeria monocytogenes, mediates bacterial phagosomal escape and facilitates bacterial multiplication during infection. This toxin has recently gained attention because of its confirmed role in the controlled and specific modulation of the immune response. Currently, cancer immunotherapies are focused on conquering the immune tolerance induced by poorly immunogenic tumor antigens and eliciting strong, lasting immunological memory. An effective way to achieve these goals is the co-administration of potent immunomodulatory adjuvant components with vaccine vectors. LLO, a toxin that belongs to the family of cholesterol-dependent cytolysins (CDCs), exhibits potent cell type-non-specific toxicity and is a source of dominant CD4+ and CD8+ T cell epitopes. According to recent research, in addition to its effective cytotoxicity as a cancer immunotherapeutic drug, the non-specific adjuvant property of LLO makes it promising for the development of efficacious anti-tumor vaccines.
Keywords: Listeria, LLO, pore-forming toxin, cytotoxicity, immunogenicity, anti-tumor vaccine, cancer immunotherapy
Introduction
In the past five decades, traditional cancer therapeutic procedures, including surgery, radiation, and chemotherapy, have been in use, but there have been bottlenecks to further reducing the relapse rate and improving the prognosis of patients with progressive disease. During this time, developments in tumor immunology broadened our knowledge of the interactions between tumor cells, the immune system and the tumor microenvironment. These developments promoted the development of an alternative, immune-based, anti-cancer therapeutic strategy. Compared with chemotherapeutics, the use of anti-tumor vaccines to enhance host immune responses against tumor tissues has the advantage of bypassing the intrinsic drug resistance of tumor cells and avoiding the toxic effects of long-term dosing. Prophylactic and therapeutic anti-tumor vaccines are based on the existence of tumor-associated antigens (TAAs), which are recognized by the immune system and induce an effective response. However, most of these TAAs are endogenous antigens with low immunogenicity and, thus, tolerance is easily induced. These TAAs are usually overexpressed in tumor cells or have structural and functional mutations that distinguish them from wild-type proteins. In addition, tumors exposed to various stressors that affect cell survival, have developed a number of immunosuppressive mechanisms to evade host immune surveillance and elimination. Thus, an efficient vaccine vector system to deliver TAAs would be able to prime a strong and tumor-specific immune response and break the tolerance barrier. To date, a series of strongly immunogenic adjuvant molecules, including cytokines, chemokines, co-stimulatory molecules, unmethylated cytosine-phosphate-guanine (CpG) sequences, chemical compounds and bacterial components, have been used to construct anti-tumor vaccines. The major modalities of cancer vaccines include plasmid DNA, modified viruses, peptide epitopes, proteins, treated whole tumor cells, dendritic cells, activated autologous lymphocytes, engineered bacterial vehicles and embryonic stem cells (ESCs).1
There is a distant evolutionary relationship between bacteria and humans. Bacterial infection often results in a rapid and intense host immune response, which overcomes the immunological unresponsiveness of immune ignorance or tolerance. This phenomenon has encouraged the development of bacterial vectors of tumor antigens for cancer treatment.2 In fact, the adoption of bacteria as a nonspecific immunostimulatory agent can be traced back over 100 y, when Coley’ toxins were invented to cure a malignant tumor.3 Currently, Bacillus Calmette-Guérin (BCG) is successfully used to treat bladder cancer, and the weekly intravesicular administration of BCG can prevent tumor recurrence in almost 60% of patients.4,5 The consensus regarding this bacterial anti-tumor vaccine is that the bacteria’s pathogen-associated molecular pattern (PAMP) can act as an adjuvant for mounting an effective immune response against the expressed tumor antigens. The interaction between PAMPs and pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs) and nucleotide-binding oligomerization domain-like receptors (NLRs), found in antigen-presenting cells (APCs) plays a pivotal role in the activation of innate and adaptive immunity.
During the past two decades, several kinds of bacteria have been confirmed to be efficient as vaccine vectors for cancer immunotherapy or infectious diseases, such as Mycobacterium (BCG), Escherichia coli, Listeria, Salmonella, Saccharomyces, Shigella, Lactococcus, and Yersinia. Among the different genera of bacteria, Listeria monocytogenes (Lm) may be a more effective vector than other bacteria due to its unique life cycle and some relevant virulence factors. To date, Some of Lm-based anti-tumor vaccines have gone through phase I/II clinical studies.
L. monocytogenes is a widespread, food-borne, Gram-positive bacterium that is responsible for sporadic severe infections in humans and other animal species.6,7 This pathogen is a facultative intracellular microorganism that is able to enter and multiply in a wide variety of eukaryotic cells,8-10 including macrophages,11 epithelial cells,12 endothelial cells,13 splenocytes14 and hepatocytes.10L. monocytogenes invades cells through either direct phagocytosis or binding to host cells through virulence factors called internalins, which include internalin A (InlA) and internalin B (InlB).14 Once in the blood circulation, the mostly disseminated bacteria are rapidly phagocytosed by macrophages and other phagocytic cells that are predominantly found in the liver (Kupffer cells) and spleen (resident macrophages).15 Upon uptake, the vast majority of bacteria are killed and degraded within the phagolysosome, but approximately 5–10% of the bacteria can escape into the cytosol because the pore-forming toxin listeriolysin O (LLO), and sometimes bacterial phosphatidylinositol-phospholipase C (PI-PLC) and phosphatidylcholine-phospholipase C (PC-PLC) in synergy with LLO lyse the primary and secondary vacuoles.16-20 Thus, because of LLO, L. monocytogenes possesses the ability to escape phagosomal compartments and live in the cytoplasm,16-18 which explains why this bacterium is particularly effective as a vector for the delivery of tumor antigens for cancer immunotherapy. Moreover, this bacterium replicates in the cytoplasm before moving to the periphery of the cell and forming pseudopod-like structures that are recognized and internalized by adjacent cells, in which the cycle is subsequently repeated.21 Therefore, L. monocytogenes infection induces a weak humoral immune response and strong cell-mediated immunity that is dominated by CD4+ and CD8+ T cells.15,22-25 In addition, the infected cells and associated immune cells produce a broad range of cytokines and chemokines, such as IL-1, IL-6, IL-12, CC chemokine ligand 2 (CCL2), tumor necrosis factor (TNF)-α and interferon (IFN)-β, which activate APCs, inducing an innate immune response and promoting a T-helper 1 (Th1) cell-mediated immune response.15,22-26 These characteristics of L. monocytogenes have accelerated the development of Lm-based cancer vaccines that induce tumor antigen-specific CD4+ and CD8+ T cell responses. In recent years, genetic manipulations have created a large number of mutant and attenuated Listeria monocytogenes strains that carry tumor antigens, and numerous preclinical studies have been performed in animal models of cancer and infectious disease.27-30 The most striking achievements have been attained through the use of live attenuated Lm-vectored immunotherapy against human papilloma virus (HPV)-associated tumors. Advaxis Inc. created an Lm-LLO-E7 anti-tumor vaccine (patented as ADXS11–001) by fusing the E7 protein with a non-hemolytic truncated LLO fragment and conducted Phase I/II clinical trials on HPV-associated cancers, including cervical intraepithelial neoplasia, cervical cancer, and HPV-positive head and neck cancer.31,32 It is anticipated that studies on Lm-based cancer immunotherapies will be ongoing if the outcomes of the current clinical trials are able to validate the safety and efficacy of the Lm-vectored anti-tumor vaccine observed in preclinical studies.
However, it is hard to accept the idea of using a live and potentially pathogenic microbe as a vaccine vector to cure malignant neoplasms, even though the live vectors created for the clinical trials are highly attenuated and easily treatable in the case of deleterious events. In addition, the administration of Lm-vectored vaccines in immune-compromised or -suppressed patients, including the very young, the elderly and pregnant women, can have serious consequences, such that the use of these vaccines may be inappropriate for these populations.33 To circumvent this problem, there may be many methods to ameliorate the effect of Lm-based vaccination to avoid potential impairment, such as the adoption of heterologous immunization regimens that involve priming with a DNA vaccine and subsequent boosting with Listeria. However, we may be neglecting one important advantage of Lm-based anti-tumor vaccines, the virulence factor LLO. It is likely that some characteristics of LLO make it adequate for use in cancer immunotherapy.
In fact, early studies have concluded that LLO may represent the dominant antigen during the immune response to L. monocytogenes,34-36 which implies that LLO may be a strong immunogenic molecule. In the last decades, numerous studies have revealed that LLO is a multifunctional molecule37-44 and is the dominant source of CD4+ and CD8 T+ cell epitopes,45-54 which implies that LLO likely has promise in cancer immunotherapy. Of note, preclinical trials showed that when two vaccines were constructed from Listeria strains that produced the E7 tumor antigen, one that expressed E7 alone and one that secreted the Lm-LLO-E7 fusion protein, the second vaccine effectively cured the majority of tumor-bearing mice and exhibited significantly higher efficacy.55
Structure and Related Functions
LLO is required for L. monocytogenes pathogenesis and belongs to the family of cholesterol-dependent cytolysins (CDCs), which are pore-forming toxins produced by various bacterial species.56-60 LLO, which is synthesized as a precursor, is composed of 529 amino acid residues with a typical signal peptide in the N-terminus (Fig. 1B),61 and the putative propeptide is approximately 58 kD. After its signal sequence is removed, the mature protein is secreted into the extracellular space as water-soluble monomers that can bind to host cell membranes, oligomerize, and form a large β-barrel pore through the bilayer plasmalemma.56,62,63 LLO is unique among the CDCs because its activity is optimized at an acidic pH and normally repressed at a neutral pH; thus, this molecule is capable of acting in an acidic vacuolar compartment to mediate the escape of the bacterium into the host cytosol.64,65 An early study by Jones and Portnoy showed that the expression of perfringolysin O (PFO), which is a pore-forming toxin from Clostridium perfringens, in an LLO-deficient strain of L. monocytogenes restored hemolytic activity and promoted partial phagosomal escape in the mouse macrophage-like J774 cell line; however, PFO expression apparently damaged the infected cell and did not restore virulence to the bacterium.66 A later study by Portnoy’s group found that a single amino acid change (leucine 461 to the threonine present in PFO) could profoundly increase the hemolytic activity of LLO at a neutral pH but resulted in a 100-fold decrease in virulence in a listeriosis mouse model.65 Thus, LLO is apparently unique among the CDCs; it can disrupt the vacuolar membrane but not kill the host cell upon bacterial growth in the cytosol. These findings support the idea that L. monocytogenes has evolved to adapt to living in its host cell.
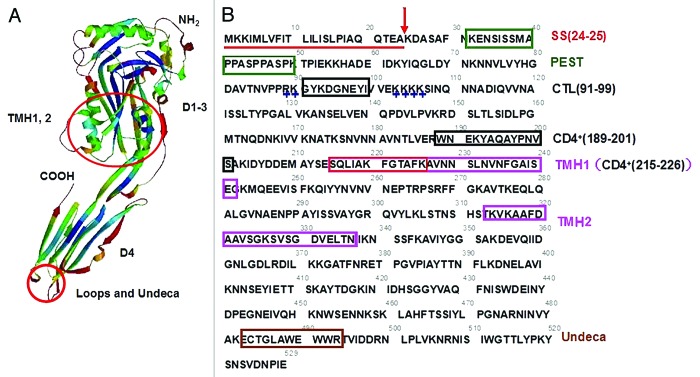
Figure 1. Structural information of the cholesterol-dependent pore-forming cytolysin listeriolysin O (LLO). (A) Putative three-dimensional model of LLO monomer based on suilysin crystal structure generated by SWISS-MODEL. Suilysin shares a sequence similarity of 44% to LLO in PDB database. The monomer of LLO contains four domains (D1–4), and the conserved undecapeptide (Undeca) and three short loops are located on the tip of Domain 4. Two transmembrane helices of TMH1,2 are made up of the two sets of α-helices in Domain 3. (B) The analyzed primary structure of LLO. The gray number above the amino acid sequence roughly represents the position of a single amino acid. SS, the signal peptide sequence of LLO showed in a red straight line and the cleavage site (residues 24–25) indicated with a red arrow indicates. PEST, a putative PEST-like motif identified in LLO showed by a dark green box. CTL(91–99), an immunodominant CTL epitope consisting of amino acids from number 91 to number 99 indicated in a black box. +, the two clusters of positively charged residues flanking the CTL epitope indicated in blue. CD4+(189–201), a characteristic immunodominant CD4+ T cell epitope consisting of amino acids from number 189 to number 201 indicated in a black box. CD4+(215–226), an immunodominant CD4+ T cell epitope contained in TMH1 region indicated with red box, consisting of amino acids from number 215 to number 226. TMH1,2, two sets of transmembrane α-helices showed in two pink boxes. Undeca, the conserved region belonging to a cytolysin family consisting of 11 amino acids.
Bioinformatics analyses have revealed that the toxin monomers of the CDC family, which consists of characteristic PFO and streptolysin O (SLO) secreted by Streptococcus pyogenes, share 40% to 80% sequence similarity, which suggests that all of these monomers may adopt similar tertiary structures and have similar modes of action. The three-dimensional (3D) structure and domains of LLO were deduced from the structures of PFO67 and intermedilysin (ILY)68 and extensive biochemical characterization. In particular, a search of the PDB protein database using the BLASTP program revealed that the recently identified cytotoxin suilysin, which originates from Streptococcus suis, has 44% identity with LLO. A conceivable 3D structure of the LLO monomer was modeled using the SwissModel Alignment Mode program based on the structure of suilysin, as shown in Figure 1A.69 In line with a previous report on the tertiary structure of LLO deduced from PFO and ILY, the monomer molecule was found to have an elongated structure and to comprise four domains. The polypeptide chain folds back and forth several times through domains 1–3, whereas Domain 4 is formed contiguously from its C-terminus (Fig. 1A).67,68,70 Three short hydrophobic loops and a highly conserved undecapeptide (ECTGLAWEWWR) are located at the top of Domain 4 (Fig. 1A).67,71 The loop region is primarily responsible for mediating the specific interaction of the CDC with cholesterol-rich membranes, and the conserved undecapeptide is required for pore formation in the target membrane.71 The undecapeptide and the three short loops at the tip of Domain 4 are involved in membrane binding and cytotoxic activity, whereas the two clusters of α-helices in Domain 3 extended from Domain 2 can transform into the transmembrane β-hairpins TMH1–2 (Fig. 1), which make up the β-barrel structure of the prepore complex to facilitate the insertion of the LLO oligomer into the host membrane.71-73 The data from other cytolysins provide a good illustration of the kinetics of the mechanism through which LLO induces perforation and the concomitant structural changes that occur in the toxin when the LLO monomer binds to cholesterol-rich membranous regions, oligomerizes and opens pores.60
A considerable body of evidence has demonstrated that the pore formed by other CDCs, such as SLO, can be removed from the plasma membrane through a mechanism involving membrane internalization, which is similar to the phenomenon by which eukaryotic cells successfully repair damaged plasma membranes and survive moderate exposure to pore-forming toxins, including the CDCs.74,75 According to a recent finding, LLO at a low concentration and under physiological conditions is necessary and sufficient to induce the formation of membrane extensions that are able to capture bacteria or inert beads coated with LLO.44 However, LLO at a higher concentration or in an acidic pH environment, similar to that found in acidic cell compartments, such as endosomes or lysosomes, exhibits a dramatic increase in hemolytic activity and cytotoxicity.64-66,44 These biological properties of LLO may indicate its promise as an immunotoxin for the elimination of tumor tissue; however, the target specificity of its tumor-killing activity needs to be determined.
A putative PEST-like motif has been identified adjacent to the N-terminus of mature LLO (Fig. 1B), and its role in LLO activity and bacterial virulence has been extensively studied by different research groups.76-79 In eukaryotic cells, several intracellular short half-life proteins often require phosphorylation for efficient poly-ubiquitination and/or degradation by the proteasome. These proteins have usually been shown to contain one or more regions rich in proline (P), glutamic acid (E), serine (S), and threonine (T), thus called the PEST motif, and these regions generally represent sites of protein-protein interactions.80-82 Portnoy and Decatur initially found that L. monocytogenes strains with a mutant LLO that lacked the PEST-like sequence entered the host cytosol but subsequently permeabilized and killed the host cell, which indicated that these strains exhibited enhanced cytotoxicity; in addition, the mutant LLO accumulated abundantly in the cytosol of the host cell.76 These researchers thus proposed that this region contributed to the biological activities of LLO, mainly through its impact on the susceptibility of LLO to intracellular proteolytic degradation.76 However, work performed by Charbit’s group showed that mutations, deletions or substitutions in this motif did not affect the secretion or hemolytic activity of LLO but significantly abolished bacterial virulence; these findings suggest that the PEST motif in LLO plays an important role in the pathogenesis of L. monocytogenes.77,78 These researchers also discovered that a high PEST score sequence was not related to the intracellular proteolytic degradation of LLO.77,78 Several years later, Decatur and coworkers found that the PEST-like region of LLO did not mediate proteasomal degradation by the host, which is contrary to their original hypothesis but consistent with the conclusions drawn by Charbit’s group.79 Decatur’s group found that the same PEST region mutants exhibited higher intracellular levels of LLO than wild-type bacteria and hypothesized that the reduced virulence of the mutants was due to the increased levels of LLO in the host cytosol, which was different from the hypothesis of impaired vacuolar escape described by Charbit’s group.79 However, a subsequent experiment performed by Decatur’s group confirmed that the discrepancy between the two studies was the result of a difference in the mutant gene copy number on the encoding plasmid. Together, these studies reveal the importance of the PEST sequence in the development of the infectious process of L. monocytogenes. However, the integrity of this region may not be necessary for the cytotoxicity of LLO.
During infection with Listeria monocytogenes, a significant CD4+ and CD8+ T cell response is directed against LLO.45,46,83,84 It has been demonstrated that LLO contains ample immunodominant epitopes of CD4+ and CD8+ T cells.45-54 To date, three immunodominant epitopes have been determined by different experiments. As shown in Figure 1B, these include one dominant cytotoxic T lymphocyte (CTL) epitope, LLO91–99 (residues 91–99), and two typical CD4+ T cell epitopes, LLO189–201 (residues 189–201), and LLO215–226 (residues 215–226).45,50,54 Although LLO is essential for phagosomal escape and cell-to-cell spread in most cell types, its membrane-perforating activity is potentially cytotoxic and must be tightly regulated to ensure that L. monocytogenes remains in its intracellular replicative niche. Several posttranscriptional mechanisms control the activity and intracellular level of LLO. In addition to an acidic pH being optimal for LLO pore formation,65 the host-mediated degradation of LLO in the cytosol is a critical determinant of the intracellular LLO level.45,49,79 Previous studies have found that the nature of the N-terminal residue of LLO does not control the rate of its intracytosolic degradation,85 but Pamer and coworkers demonstrated that the immunodominant CTL epitope (LLO91–99) is able to induce the cytosolic degradation of LLO and a specific major histocompatibility complex (MHC) class I-restricted immune response.45-53 Although a recent study found that LLO is a substrate of the ubiquitin-dependent N-end rule pathway, which recognizes LLO through its N-terminal Lys residue,55 the role of the LLO91–99 epitope is important in the ubiquitin-proteasome-mediated proteolysis pathway. During the intracellular multiplication of L. monocytogenes in infected mice, a marked Th1-based CTL response can be generated. In addition, of the abundant epitopes presented by the H-2Kd MHC class I molecule, LLO91–99 elicits a powerful dominant response.51,52,86-88 Moreover, a previous study that aimed to identify the LLO91–99 determinant that participates in bacterial pathogenesis revealed the importance of the 91–99 region in the proteolytic degradation and hemolytic activity of LLO using site-directed mutagenesis to create mutations in the epitope or the two clusters of positive charges that flank the epitope (Fig. 1B).53 Therefore, LLO91–99, as a strong immunodominant epitope that is closely correlated with the induction of LLO degradation, is able to elicit marked CTL-restricted immune responses. This finding may render LLO an attractive immunomodulatory molecule for novel anti-tumor vaccine designs.
The MHC class II-restricted T cell epitope LLO215–226 was identified early.50 In that study, the researchers used an attenuated Salmonella vaccine-Listeria infection model to analyze the capacity of the T cell epitopes of LLO to induce epitope-specific T cell responses and found that LLO 215–226 could be efficiently processed and presented to T cells as part of a Salmonella flagellin-epitope fusion protein.50 A previous study showed that endosomes obtained from resting and IFN-γ-activated macrophages containing intact LLO and LLO1–491 fragments could elicit an LLO189–201-specific CD4+ T cell response.54 Recently, a study showed that compared with tested cognate peptides, LLO tended to be one of the strongest generators of CD4+ T cell responses.89 Owing to its salient CD4+ T cell epitopes, such as LLO190–201, LLO is capable of eliciting CD4+ T cell responses at unprecedented femtomolar/picomolar ([fM]/[pM]) levels and is approximately 3000–7000 times more efficient than the homologous peptides.89 Although there was one amino acid variation along the length of the CD4+ T cell epitopes used in these two studies, there is no doubt that this region can be effectively processed in the MHC class II-restricted antigen presentation pathway.
The generation of tumor-specific CTL responses is the primary focus of anti-tumor vaccines, whose efficacy depends on the effective presentation of tumor antigens by MHC class I molecules. Thus, the interaction between LLO, which is able to disrupt acidic internalized vacuoles and efficiently enter the ubiquitin-proteasome degradation pathway, and the process of tumor antigen presentation by MHC class I molecules is an option for the development of novel anti-tumor vaccines. LLO is a strong immunogenic molecule and has the ability to promote adaptive immunity dominated by CD4+ and CD8+ T cells. In addition, several studies have documented that LLO can stimulate the innate immune system and induce cytokine production.39-41,90-96 For example, purified LLO activated NF-κB in human embryonic kidney cells (HEK293) in a MyD88- and IRAK-independent manner91 and indirectly induced the expression of interleukin (IL)-1, IL-12, and IL-18 in macrophages and IFN-γ production by natural killer (NK) cells.92-96 These findings suggest that LLO is a strong immunostimulatory factor and may act as a PAMP, which can be an effective adjuvant for tumor immunotherapy. In fact, a recently published study demonstrated that a non-hemolytic form of LLO (dtLLO) was an effective adjuvant and could act in a PAMP-like manner to facilitate a TAA-specific immune response.97 That study discovered that dtLLO, either fused to or administered as a mixture with an HPV16-E7 recombinant protein, could augment anti-tumor immune responses and facilitate tumor eradication.97 The purified dtLLO could promote the synthesis of proinflammatory cytokines, such as IL-12 and TNF-α, in mouse bone marrow-derived dendritic cells (BMDCs) similar to a PAMP and upregulate the expression of costimulatory molecules (e.g., CD40) and MHC-II on DCs.97 Thus, it can be concluded that LLO, as a unique cytotoxin with strong immunogenicity, is able to fully induce the immune system by activating both innate and adaptive immunity; thus, this molecule is an effective adjuvant for tumor immunotherapy.
Interestingly, when investigating the ability of LLO to induce cytokine expression by macrophages and NK cells, researchers found that cholesterol treatment or the use of a truncated rLLO (residues 1–416, domains 1–3) without hemolytic activity did not impair cytokine induction.92-96 These results suggest a clear dissociation between the cytotoxic properties of LLO and its immunogenicity. Recently, a study discovered that the cytotoxic effect of LLO in the pre-pore to pore transition was weakened 10- to 100-fold by mutations of two key tryptophan residues in the conserved undecapeptide; however, these mutations had no effect on the presentation of LLO to CD4+ T cells.89 The presentation of LLO to CD8+ T cells is not as robust as that observed with CD4+ T cells but is still observed in the nanomolar range.89 The reduced presentation to CD8+ T cells may be due to a damaged ability to escape from phagolysosomes and reduced degradation by proteasomes. The immunogenicity of LLO to CD4+ T cells can be maintained despite mutations, which further indicates that the immunogenicity of LLO is independent of its cytolytic activity.
The lack of association between its cytotoxic activity and its immunogenicity makes LLO unique for use in cancer immunotherapy. We can utilize either its cytolytic activity to directly kill tumor cells or its immunogenicity as an adjuvant component of anti-tumor vaccines. However, when LLO is used as a vaccine adjuvant, both its membrane-damaging ability and its immunostimulatory properties may be involved. Notably, Lee and his colleagues (1996) suggested that the delivery of therapeutic macromolecules into the cytosol can be achieved through the use of liposomes that contain LLO.98 These researchers discovered that the MHC class I-restricted presentation of peptides derived from ovalbumin (OVA) was significantly strengthened when both OVA and LLO were encapsulated in pH-sensitive liposomes.98 In addition, the use of LLO to deliver membrane-impermeable cell-killing drugs into the cytosol to directly induce tumor cell death may be an alternative option. In this review, some LLO-based cancer immunotherapeutic regimens will be discussed.
LLO-Based Immunotoxin/Immunoliposome for Killing Tumor Cells
Antibody-based therapeutic anti-tumor strategies have gradually become an important component of human cancer immunotherapy. There are some advantages associated with the use of monoclonal antibodies (mAbs) for the suppression of tumor growth and the elimination of neoplasms. Based on their intrinsic properties of high specificity and sensitivity, mAbs can block overexpressed and activated growth factor receptors on tumor cells, inhibit angiogenesis and induce tumor-targeted immune responses.99,100 In recent years, tumor-specific mAbs have been widely applied to developing tumor-targeting immunotherapies due to their ability to target therapeutic agents to tumor cells.99,100 Certain chemotherapeutic agents and several protein toxins, such as diphtheria toxin and the Pseudomonas exotoxin,101 have been conjugated to mAbs and used to specifically kill tumor cells. The underlying mechanism is known: after binding to the surface of cancer cells, mAbs are internalized into vesicles, through which cytotoxic molecules enter intracellular compartments and then exert cytotoxicity and induce cell death. However, during this process, many membrane-impermeable or protein-toxic agents are trapped in vacuoles or degraded and thus cannot effectively kill the cell because they cannot gain access to the cytosol. LLO is a pH-dependent pore-forming toxin with high cytolytic activity in acidic chambers and therefore may be able to circumvent this obstacle.
Previously, a study found that the cytotoxicity of anti-tumor immunotoxins and drugs could be enhanced by LLO.102 In the study, two immunotoxins used to kill H2987 human lung adenocarcinoma cells were constructed using a ribosome-inactivating protein ricin A chain (RA) conjugated to BR96 and L6 antibodies. The study found that LLO could significantly potentiate the cytotoxicity of BR96-RA and L6-RA by 120- and > 1340-fold, respectively.102 However, a recent study showed that LLO could act as the cytotoxic part of the immunotoxin to directly induce the death of tumor cells.103 The B3-LLO immunotoxin has been ingeniously devised: in a neutral environment, LLO is in an oxidized condition with low cytotoxicity, whereas once it is internalized into an acidic endosome compartment, the maximal activity of LLO to disrupt the phagosomal membrane and induce tumor cell death is restored.103 Thus, the LLO-based immunotoxin creates a new platform for cancer immunotherapy.
In addition, with the advancement of targeted liposome technology, some chemotherapeutic drugs are being improved to be directly delivered to the tumor mass at different high-dose levels.104,105 Membrane-permeable drugs are preferentially chosen for liposomal delivery systems because these drugs are capable of passing through the plasma membrane of the targeted tumor cells.106 However, this type of drug inevitably reaches the circulatory system, enters normal cells and leads to cytotoxicity to normal organs.107 Some other drugs, although membrane impermeable, exhibit high cytotoxicity in the cytosol.108,109 LLO appears to be a good alternative to help improve the therapeutic outcome and overcome this problem. A recent study successfully constructed an immunoliposome loaded with bleomycin, which is an efficient cytotoxic agent, to target human epidermal receptor-2 (Her-2)-overexpressing breast cancer cells using the antibody trastuzumab, and LLO was incorporated into the liposome to break down the endosomal membrane and deliver bleomycin to the cytosol.110 The results showed that treatment with the bleomycin LLO-liposome resulted in a 57,000-fold enhancement in cytotoxicity compared with free bleomycin.110
LLO-Based Anti-Tumor Vaccine Development
Over the years, the development of DNA-based vaccinations against malignancies has made significant progress compared with traditional vaccines because of to the safety, stability, and design flexibility. Currently, a major hurdle exists in the development of more effective and safer delivery systems because of the low immunogenicity of naked DNA. Thus, liposomal vectors have been extensively studied. Of these vectors, a new liposomal delivery system that consists of LPDII (anionic liposome-polycation-DNA complexes) has been designed; this system is able to deliver an adequate number of antigen genes to targeted cells, with little cytotoxicity to normal organs.111,112 However, the low transfection efficiency of anionic LPDII vectors has limited their application. Recently, one study demonstrated that an LLO-containing LPDII-DNA delivery system works effectively for DNA delivery and leads to efficient DNA priming through the adoption of a DNA prime-protein boost vaccination protocol.113 These researchers used OVA as a model antigen and found that the incorporation of LLO into the LPDII gene delivery system heightened gene expression in vitro and enhanced OVA-specific CD8+ CTL responses in vivo.113 The results of the study may imply that the design of an LLO-containing LPDII delivery system for DNA-based vaccines to stimulate protective immunity against diseases, such as cancer, has noteworthy value for future research.
Bacteria and their components, such as lipoteichoic acid (LTA), lipopolysaccharide (LPS), and CpG motifs, are some of the most potent inducers of DC maturation and can be easily sensed by the innate immune system.114,115 Similar to L. monocytogenes, a nonpathogenic recombinant E. coli strain has also proven to be a promising candidate for the delivery of tumor antigens for cancer immunotherapy. However, compared with L. monocytogenes, E. coli is less effective at inducing tumor antigen-specific CD8+ T cell responses because of its inability to escape from phagolysosomes after being phagocytosed by APCs. The use of nonpathogenic E. coli to deliver tumor antigens in humans may be accepted to some extent. How can we elevate the ability of E. coli to induce anti-tumor CTL responses? We may easily consider LLO. In fact, Radford’s group revealed that the use of a recombinant E. coli vaccine that constitutively expresses LLO and produces inducible OVA is capable of killing an OVA-expressing melanoma cell line (B16-OVA) and effectively suppressing tumor growth in challenged mice.116 However, a recombinant E. coli vaccine that only expressed OVA induce a significantly weaker anti-tumor response than a vaccine that also expressed LLO.116 Moreover, these researchers also discovered that paraformaldehyde-fixed E. coli expressing LLO was efficiently internalized by human monocyte-derived dendritic cells (MoDCs) and promoted MoDC maturation. Additionally, the use of a typical human melanoma antigen (MART1) instead of OVA in the vaccine efficiently delivered the MART127–35 antigen epitope for processing and presentation by human MoDCs.117 The anti-tumor efficacy of the paraformaldehyde-fixed E. coli vaccine is maintained, and this vaccine is significantly less harmful to humans. Similarly, another research team illustrated that an LLO-based E. coli vaccine could induce a strong immune response against a WT1-expressing leukemia tumor in vivo through enhanced CTL activity.118 Thus, LLO is able to elevate the potency of recombinant E. coli anti-tumor vaccines. It can be inferred that the combination of LLO with nonpathogenic-bacterial vaccines is a novel and efficient strategy for tumor immunotherapy. The LLO-based vaccine strategy may broaden the scope of available anti-tumor vaccines.
Many studies have reported elevated levels of CD4+CD25high regulatory T cells (Treg cells) in patients with different types of cancers.119,120 Poor prognosis and tumor relapse are often correlated with increased numbers of Treg cells in vivo.121 Therefore, an ideal cancer vaccine must both stimulate specific CTL responses and suppress the function of Treg cells. Some novel therapeutic strategies to eliminate Treg cells in cancer patients are being tested. A clinical trial investigated the ability of IL-2/diphtheria immunotoxin to target CD25high Treg cells.122 How should an anti-tumor vaccine be prepared to induce long-term tumor-specific immune memory and the functional inhibition of Treg cells? A previous discovery indicated that an LLO-based engineered E. coli vaccine could promote the generation of CD44highCD62Llow CD8+ effector memory T cells and inhibit the functions of Treg cells that expanded normally but was unable to suppress the proliferation of conventional T cells.123 Through the use of a tumor-bearing animal model, the researchers showed that E. coli LLO/OVA vaccination could generate high-avidity CTLs and functionally defective Treg cells, which led to the rejection of highly aggressive B16/OVA melanoma, compared with the results obtained with E. coli OVA.123 These studies suggest that LLO is able to boost the effectiveness of the vaccine through the inhibition of Treg cells, although the exact mechanism is not yet known. Notably, all of the above mentioned studies prepared the LLO-based E. coli vaccines using two separate plasmids for the expression of OVA/tumor antigen and LLO. In fact, Paterson’s group showed that LLO can act as an adjuvant for anti-tumor vaccines without being fused to the tumor antigen and can be expressed alone without reducing the vaccine potency.124
A heterologous prime-boost immunization strategy is currently predominantly utilized to conquer the problem of vector-pointing immune responses in cancer immunotherapy. To date, the heterologous prime-boost regimen is among the most potent strategy used to induce cellular immune responses.125 One group of researchers created an efficient integration of LLO-based E. coli vaccination and plasmid DNA vaccination to obtain a heterologous prime-boost immunization strategy that can be used to monitor the anti-tumor activity of B16-OVA tumor-bearing C57BL/6 mice by a tumor prevention or therapeutic model.126 The results showed a significantly stronger OVA-specific CD8+ T cell response and more significant tumor inhibition under the bacterial-prime/plasmid-boost setting compared with homologous and reversed sequential vaccination.126 In addition, the early suppression/depletion of Treg cells observed with anti-tumor vaccination can lead to better antigen-specific CTL responses.126 Owing to the contribution of LLO to enhanced tumor cytotoxicity, Treg cell inhibition, and memory CTL persistence, the application of LLO-based vaccines in a heterologous prime-boost immunization approach may offer novel clinical cancer therapeutic protocols.
The Lm-LLO-E7 anti-tumor vaccine patented as ADXS11–001 has been extensively studied and tested in preclinical settings and is now being used in human studies.31,32,127-129 Preclinical studies have shown that Lm-LLO-E7 is able to stimulate the expression of IL-2, IL-12, and TNF-α by DCs, promote DC maturation,127 activate both arms of the adaptive immune system,130 induce the generation of tumor antigen-specific CTLs,128 break immunological tolerance,128,129 maintain CD8+ T cell memory, block tumor reoccurrence,130 reduce Treg cells and myeloid-derived suppressor cells (MDSCs) intratumorally and diminish the tumor resistance to immune attack by antigen-specific cells.130,131 The multifaceted anti-tumor efficiency of Lm-LLO-E7 is closely related to the adjuvant properties of LLO, which include activating and augmenting anti-tumor activity, breaking TAA-associated immunological tolerance, promoting the release of inflammatory cytokines, enhancing the Th1-dominated immune response, and suppressing the effect of inhibitory immune cells and molecules.32 Paterson and coworkers conducted a series of studies to analyze the efficacy of Lm-LLO-based anti-tumor vaccines expressing different tumor-associated antigens or peptide epitopes, such as tumor vasculature antigens, vascular endothelial growth factor receptor-2/fetal liver kinase-1 (VEGFR2/Flk-1),132 endoglin (CD105),133 melanoma-associated antigen (HMW-MAA),134 38C13 murine lymphoma idiotype (Id)135 and human epidermal receptor-2 (HER-2/neu).136 The results showed that these vaccines, which target either the tumor or the tumor vasculature, could overcome tolerance and drive epitope spreading to cryptic tumor epitopes.137 The mechanism can be illustrated as follows: (1) the Lm-vectored vaccine infects APCs and primes autoreactive CD8+ T cells to kill tumor or tumor-associated vascular cells; (2) elicited CD8+ T cells attack and destroy the tumor or tumor vasculature; (3) the destruction of key cells involved in maintaining the integrity of the tumor vasculature leads to increased tumor hypoxia and apoptosis; (4) apoptotic tumor cells are phagocytosed by DCs, and the tumor proteins are cross-presented to naive CD8+ T cells; (5) newly primed CD8+ T cells targeting the cryptic tumor epitopes are generated and migrate to the inflamed tumor site; (6) resulting in a second wave of tumor cell killing.137 This type of epitope spreading could expose tumor tissue-associated antigens and fully activate the pool of antigen-responsive T cells, which can accelerate tumor mass elimination. These studies provide evidence of the advantages of Listeria as a vaccine vector for tumor immunotherapy. Of note, the adjuvant property of LLO plays an important role in the enhancement of the efficacy of these vaccines. However, further studies are required to understand how LLO affects systematic and local tumor immune responses and inhibits the function of Treg cells and MDSCs within the tumor. Because LLO is a multifunctional molecule, other mechanisms may be involved in the role of LLO in the immune response: for example, autophagy,37 which contributes to the innate immune response to microbial pathogens.
Future of LLO-Based Immunotherapy
Based on the lack of association between LLO’s cytotoxic activity and its immunogenicity, LLO could be used in a variety of applications. With the development of novel platform technologies for cancer immunotherapy, the strong immunogenicity of LLO could be applied to design significantly more effective anti-tumor vaccines. Depending on the vaccine vector, LLO could be administered as a protein, DNA, or peptide epitope. To increase the effectiveness of LLO-based vaccines, it will be necessary to simplify the vaccine composition, decrease its potential toxicity, select adequate immunization approaches and improve the delivery technology. In conclusion, although many of the underlying mechanisms have not been identified, the immunological effects of LLO as a multifunctional cytotoxin will continue to draw attention, and LLO will continue to be used to develop anti-tumor vaccines.
Acknowledgments
We are very grateful to the information provided by Cell Resource Center, Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences, School of Basic Medicine, Peking Union Medical College, and sincerely thanks to the resources of ExPASy system of SIB Bioinformatics Resource, Protein Data Bank and SWISS-MODEL Workspace.
Glossary
Abbreviations:
- LLO
listeriolysin O
- CDCs
cholesterol-dependent cytolysins
- TAAs
tumor-associated antigens
- CpG
cytosine-phosphate-guanine
- ESC
embryonic stem cell
- BCG
Bacillus Calmette-Guérin, Mycobacterium
- PAMP
pathogen-associated molecular pattern
- PRRs
pattern recognition receptors
- TLRs
Toll-like receptors
- NLRs
nucleotide-binding oligomerization domain-like receptors
- APCs
antigen-presenting cells
- Lm
Listeria monocytogenes
- L. monocytogenes
Listeria monocytogenes
- InlA
internalin A
- InlB
internalin B
- PI-PLC
phosphatidylinositol-phospholipase C
- PC-PLC
phosphatidylcholine-phospholipase C
- CCL2
CC chemokine ligand 2
- TNF
tumor necrosis factor
- IFN
interferon
- Th1 cell
T-helper 1 cell
- HPV
human papilloma virus
- PFO
perfringolysin O
- SLO
streptolysin O
- 3D
three-dimensional
- ILY
intermedilysin
- TMH
transmembrane β-hairpin
- CTL
cytotoxic T lymphocyte
- MHC
major histocompatibility complex
- [fM]/[pM]
femtomolar/picomolar
- HEK293
human embryonic kidney cells
- IL
interleukin
- NK
natural killer
- dtLLO
non-hemolytic form of LLO
- DCs
dendritic cells
- BMDCs
bone marrow-derived dendritic cells
- rLLO
truncated LLO
- OVA
ovalbumin
- mAbs
monoclonal antibodies
- RA
ribosome-inactivating protein ricin A chain
- H2987
human lung adenocarcinoma cells
- BR96-RA
L6-RA, and B3-LLO, immunotoxins
- Her-2 and HER-2/neu
human epidermal receptor-2
- LPDII
anionic liposome-polycation-DNA complexes
- LTA
lipoteichoic acid
- LPS
lipopolysaccharide
- E. coli
Escherichia coli
- B16
melanoma cell line
- MoDCs
human monocyte-derived dendritic cells
- MART1
human melanoma antigen
- Treg cells
regulatory T cells
- MDSCs
myeloid-derived suppressor cells
- VEGFR2/Flk-1
endothelial growth factor receptor-2/fetal liver kinase-1
- CD105
endoglin
- HMW-MAA
high molecular weight melanoma-associated antigen
- 38C13
murine B cell lymphoma
Disclosure of Potential Conflicts of Interest
The authors are engaging in one research of a broad-spectrum cancer vaccine project granted by Cell Resource Center of Chinese Academy of Medical Sciences. The authors declare they have no conflict of interest and no financial interest with the subject matter or materials discussed in the manuscript.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/23871
References
- 1.Yaddanapudi K, Mitchell RA, Putty K, Willer S, Sharma RK, Yan J, et al. Vaccination with embryonic stem cells protects against lung cancer: is a broad-spectrum prophylactic vaccine against cancer possible? PLoS One. 2012;7:e42289. doi: 10.1371/journal.pone.0042289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paterson Y, Maciag PC. Listeria-based vaccines for cancer treatment. Curr Opin Mol Ther. 2005;7:454–60. [PubMed] [Google Scholar]
- 3.Nauts HC, McLaren JR. Coley toxins--the first century. Adv Exp Med Biol. 1990;267:483–500. doi: 10.1007/978-1-4684-5766-7_52. [DOI] [PubMed] [Google Scholar]
- 4.Alexandroff AB, Jackson AM, O’Donnell MA, James K. BCG immunotherapy of bladder cancer: 20 years on. Lancet. 1999;353:1689–94. doi: 10.1016/S0140-6736(98)07422-4. [DOI] [PubMed] [Google Scholar]
- 5.Shelley MD, Kynaston H, Court J, Wilt TJ, Coles B, Burgon K, et al. A systematic review of intravesical bacillus Calmette-Guérin plus transurethral resection vs transurethral resection alone in Ta and T1 bladder cancer. BJU Int. 2001;88:209–16. doi: 10.1046/j.1464-410x.2001.02306.x. [DOI] [PubMed] [Google Scholar]
- 6.Farber JM, Peterkin PI. Listeria monocytogenes, a food-borne pathogen. Microbiol Rev. 1991;55:476–511. doi: 10.1128/mr.55.3.476-511.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vázquez-Boland JA, Kuhn M, Berche P, Chakraborty T, Domínguez-Bernal G, Goebel W, et al. Listeria pathogenesis and molecular virulence determinants. Clin Microbiol Rev. 2001;14:584–640. doi: 10.1128/CMR.14.3.584-640.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaillard JL, Finlay BB. Effect of cell polarization and differentiation on entry of Listeria monocytogenes into the enterocyte-like Caco-2 cell line. Infect Immun. 1996;64:1299–308. doi: 10.1128/iai.64.4.1299-1308.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuhn M, Goebel W. Identification of an extracellular protein of Listeria monocytogenes possibly involved in intracellular uptake by mammalian cells. Infect Immun. 1989;57:55–61. doi: 10.1128/iai.57.1.55-61.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dramsi S, Biswas I, Maguin E, Braun L, Mastroeni P, Cossart P. Entry of Listeria monocytogenes into hepatocytes requires expression of inIB, a surface protein of the internalin multigene family. Mol Microbiol. 1995;16:251–61. doi: 10.1111/j.1365-2958.1995.tb02297.x. [DOI] [PubMed] [Google Scholar]
- 11.MacKaness GB. Cellular resistance to infection. J Exp Med. 1962;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PubMed] [Google Scholar]
- 12.Gaillard JL, Berche P, Frehel C, Gouin E, Cossart P. Entry of L. monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from gram-positive cocci. Cell. 1991;65:1127–41. doi: 10.1016/0092-8674(91)90009-N. [DOI] [PubMed] [Google Scholar]
- 13.Drevets DA, Sawyer RT, Potter TA, Campbell PA. Listeria monocytogenes infects human endothelial cells by two distinct mechanisms. Infect Immun. 1995;63:4268–76. doi: 10.1128/iai.63.11.4268-4276.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cossart P, Toledo-Arana A. Listeria monocytogenes, a unique model in infection biology: an overview. Microbes Infect. 2008;10:1041–50. doi: 10.1016/j.micinf.2008.07.043. [DOI] [PubMed] [Google Scholar]
- 15.Bruhn KW, Craft N, Miller JF. Listeria as a vaccine vector. Microbes Infect. 2007;9:1226–35. doi: 10.1016/j.micinf.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Barry RA, Bouwer HG, Portnoy DA, Hinrichs DJ. Pathogenicity and immunogenicity of Listeria monocytogenes small-plaque mutants defective for intracellular growth and cell-to-cell spread. Infect Immun. 1992;60:1625–32. doi: 10.1128/iai.60.4.1625-1632.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bielecki J, Youngman P, Connelly P, Portnoy DA. Bacillus subtilis expressing a haemolysin gene from Listeria monocytogenes can grow in mammalian cells. Nature. 1990;345:175–6. doi: 10.1038/345175a0. [DOI] [PubMed] [Google Scholar]
- 18.Portnoy DA, Tweten RK, Kehoe M, Bielecki J. Capacity of listeriolysin O, streptolysin O, and perfringolysin O to mediate growth of Bacillus subtilis within mammalian cells. Infect Immun. 1992;60:2710–7. doi: 10.1128/iai.60.7.2710-2717.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldfine H, Wadsworth SJ. Macrophage intracellular signaling induced by Listeria monocytogenes. Microbes Infect. 2002;4:1335–43. doi: 10.1016/S1286-4579(02)00011-4. [DOI] [PubMed] [Google Scholar]
- 20.Smith GA, Marquis H, Jones S, Johnston NC, Portnoy DA, Goldfine H. The two distinct phospholipases C of Listeria monocytogenes have overlapping roles in escape from a vacuole and cell-to-cell spread. Infect Immun. 1995;63:4231–7. doi: 10.1128/iai.63.11.4231-4237.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tilney LG, Portnoy DA. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989;109:1597–608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pamer EG. Immune responses to Listeria monocytogenes. Nat Rev Immunol. 2004;4:812–23. doi: 10.1038/nri1461. [DOI] [PubMed] [Google Scholar]
- 23.Singh R, Paterson Y. Listeria monocytogenes as a vector for tumor-associated antigens for cancer immunotherapy. Expert Rev Vaccines. 2006;5:541–52. doi: 10.1586/14760584.5.4.541. [DOI] [PubMed] [Google Scholar]
- 24.Wood LM, Guirnalda PD, Seavey MM, Paterson Y. Cancer immunotherapy using Listeria monocytogenes and listerial virulence factors. Immunol Res. 2008;42:233–45. doi: 10.1007/s12026-008-8087-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Witte CE, Archer KA, Rae CS, Sauer JD, Woodward JJ, Portnoy DA. Innate immune pathways triggered by Listeria monocytogenes and their role in the induction of cell-mediated immunity. Adv Immunol. 2012;113:135–56. doi: 10.1016/B978-0-12-394590-7.00002-6. [DOI] [PubMed] [Google Scholar]
- 26.Woodward JJ, Iavarone AT, Portnoy DA. c-di-AMP secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science. 2010;328:1703–5. doi: 10.1126/science.1189801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jensen ER, Selvakumar R, Shen H, Ahmed R, Wettstein FO, Miller JF. Recombinant Listeria monocytogenes vaccination eliminates papillomavirus-induced tumors and prevents papilloma formation from viral DNA. J Virol. 1997;71:8467–74. doi: 10.1128/jvi.71.11.8467-8474.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshimura K, Jain A, Allen HE, Laird LS, Chia CY, Ravi S, et al. Selective targeting of antitumor immune responses with engineered live-attenuated Listeria monocytogenes. Cancer Res. 2006;66:1096–104. doi: 10.1158/0008-5472.CAN-05-2307. [DOI] [PubMed] [Google Scholar]
- 29.Pan ZK, Ikonomidis G, Lazenby A, Pardoll D, Paterson Y. A recombinant Listeria monocytogenes vaccine expressing a model tumour antigen protects mice against lethal tumour cell challenge and causes regression of established tumours. Nat Med. 1995;1:471–7. doi: 10.1038/nm0595-471. [DOI] [PubMed] [Google Scholar]
- 30.Craft N, Bruhn KW, Nguyen BD, Prins R, Lin JW, Liau LM, et al. The TLR7 agonist imiquimod enhances the anti-melanoma effects of a recombinant Listeria monocytogenes vaccine. J Immunol. 2005;175:1983–90. doi: 10.4049/jimmunol.175.3.1983. [DOI] [PubMed] [Google Scholar]
- 31.Maciag PC, Radulovic S, Rothman J. The first clinical use of a live-attenuated Listeria monocytogenes vaccine: a Phase I safety study of Lm-LLO-E7 in patients with advanced carcinoma of the cervix. Vaccine. 2009;27:3975–83. doi: 10.1016/j.vaccine.2009.04.041. [DOI] [PubMed] [Google Scholar]
- 32.Wallecha A, French C, Petit R, Singh R, Amin A, Rothman J. Lm-LLO-Based Immunotherapies and HPV-Associated Disease. J Oncol. 2012;2012:542851. doi: 10.1155/2012/542851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh R, Wallecha A. Cancer immunotherapy using recombinant Listeria monocytogenes: transition from bench to clinic. Hum Vaccin. 2011;7:497–505. doi: 10.4161/hv.7.5.15132. [DOI] [PubMed] [Google Scholar]
- 34.Berche P, Gaillard JL, Geoffroy C, Alouf JE. T cell recognition of listeriolysin O is induced during infection with Listeria monocytogenes. J Immunol. 1987;139:3813–21. [PubMed] [Google Scholar]
- 35.Beattie IA, Swaminathan B, Ziegler HK. Cloning and characterization of T-cell-reactive protein antigens from Listeria monocytogenes. Infect Immun. 1990;58:2792–803. doi: 10.1128/iai.58.9.2792-2803.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bouwer HG, Nelson CS, Gibbins BL, Portnoy DA, Hinrichs DJ. Listeriolysin O is a target of the immune response to Listeria monocytogenes. J Exp Med. 1992;175:1467–71. doi: 10.1084/jem.175.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyer-Morse N, Robbins JR, Rae CS, Mochegova SN, Swanson MS, Zhao Z, et al. Listeriolysin O is necessary and sufficient to induce autophagy during Listeria monocytogenes infection. PLoS One. 2010;5:e8610. doi: 10.1371/journal.pone.0008610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lam GY, Fattouh R, Muise AM, Grinstein S, Higgins DE, Brumell JH. Listeriolysin O suppresses phospholipase C-mediated activation of the microbicidal NADPH oxidase to promote Listeria monocytogenes infection. Cell Host Microbe. 2011;10:627–34. doi: 10.1016/j.chom.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kayal S, Lilienbaum A, Poyart C, Memet S, Israel A, Berche P. Listeriolysin O-dependent activation of endothelial cells during infection with Listeria monocytogenes: activation of NF-kappa B and upregulation of adhesion molecules and chemokines. Mol Microbiol. 1999;31:1709–22. doi: 10.1046/j.1365-2958.1999.01305.x. [DOI] [PubMed] [Google Scholar]
- 40.Tang P, Rosenshine I, Cossart P, Finlay BB. Listeriolysin O activates mitogen-activated protein kinase in eucaryotic cells. Infect Immun. 1996;64:2359–61. doi: 10.1128/iai.64.6.2359-2361.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weiglein I, Goebel W, Troppmair J, Rapp UR, Demuth A, Kuhn M. Listeria monocytogenes infection of HeLa cells results in listeriolysin O-mediated transient activation of the Raf-MEK-MAP kinase pathway. FEMS Microbiol Lett. 1997;148:189–95. doi: 10.1111/j.1574-6968.1997.tb10287.x. [DOI] [PubMed] [Google Scholar]
- 42.Dramsi S, Cossart P. Listeriolysin O-mediated calcium influx potentiates entry of Listeria monocytogenes into the human Hep-2 epithelial cell line. Infect Immun. 2003;71:3614–8. doi: 10.1128/IAI.71.6.3614-3618.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ribet D, Hamon M, Gouin E, Nahori MA, Impens F, Neyret-Kahn H, et al. Listeria monocytogenes impairs SUMOylation for efficient infection. Nature. 2010;464:1192–5. doi: 10.1038/nature08963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vadia S, Arnett E, Haghighat AC, Wilson-Kubalek EM, Tweten RK, Seveau S. The pore-forming toxin listeriolysin O mediates a novel entry pathway of L. monocytogenes into human hepatocytes. PLoS Pathog. 2011;7:e1002356. doi: 10.1371/journal.ppat.1002356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pamer EG, Harty JT, Bevan MJ. Precise prediction of a dominant class I MHC-restricted epitope of Listeria monocytogenes. Nature. 1991;353:852–5. doi: 10.1038/353852a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Safley SA, Cluff CW, Marshall NE, Ziegler HK. Role of listeriolysin-O (LLO) in the T lymphocyte response to infection with Listeria monocytogenes. Identification of T cell epitopes of LLO. J Immunol. 1991;146:3604–16. [PubMed] [Google Scholar]
- 47.Wipke BT, Jameson SC, Bevan MJ, Pamer EG. Variable binding affinities of listeriolysin O peptides for the H-2Kd class I molecule. Eur J Immunol. 1993;23:2005–10. doi: 10.1002/eji.1830230842. [DOI] [PubMed] [Google Scholar]
- 48.Pamer EG. Direct sequence identification and kinetic analysis of an MHC class I-restricted Listeria monocytogenes CTL epitope. J Immunol. 1994;152:686–94. [PubMed] [Google Scholar]
- 49.Villanueva MS, Sijts AJ, Pamer EG. Listeriolysin is processed efficiently into an MHC class I-associated epitope in Listeria monocytogenes-infected cells. J Immunol. 1995;155:5227–33. [PubMed] [Google Scholar]
- 50.Verma NK, Ziegler HK, Wilson M, Khan M, Safley S, Stocker BA, et al. Delivery of class I and class II MHC-restricted T-cell epitopes of listeriolysin of Listeria monocytogenes by attenuated Salmonella. Vaccine. 1995;13:142–50. doi: 10.1016/0264-410X(95)93127-U. [DOI] [PubMed] [Google Scholar]
- 51.Vijh S, Pamer EG. Immunodominant and subdominant CTL responses to Listeria monocytogenes infection. J Immunol. 1997;158:3366–71. [PubMed] [Google Scholar]
- 52.Busch DH, Pamer EG. MHC class I/peptide stability: implications for immunodominance, in vitro proliferation, and diversity of responding CTL. J Immunol. 1998;160:4441–8. [PubMed] [Google Scholar]
- 53.Lety MA, Frehel C, Raynaud C, Dupuis M, Charbit A. Exploring the role of the CTL epitope region of listeriolysin O in the pathogenesis of Listeria monocytogenes. Microbiology. 2006;152:1287–96. doi: 10.1099/mic.0.28754-0. [DOI] [PubMed] [Google Scholar]
- 54.Rodriguez-Del Rio E, Frande-Cabanes E, Tobes R, Pareja E, Lecea-Cuello MJ, Ruiz-Sáez M, et al. The intact structural form of LLO in endosomes cannot protect against listeriosis. Int J Biochem Mol Biol. 2011;2:207–18. [PMC free article] [PubMed] [Google Scholar]
- 55.Schnupf P, Zhou J, Varshavsky A, Portnoy DA. Listeriolysin O secreted by Listeria monocytogenes into the host cell cytosol is degraded by the N-end rule pathway. Infect Immun. 2007;75:5135–47. doi: 10.1128/IAI.00164-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Geoffroy C, Gaillard JL, Alouf JE, Berche P. Production of thiol-dependent haemolysins by Listeria monocytogenes and related species. J Gen Microbiol. 1989;135:481–7. doi: 10.1099/00221287-135-3-481. [DOI] [PubMed] [Google Scholar]
- 57.Tweten RK, Harris RW, Sims PJ. Isolation of a tryptic fragment from Clostridium perfringens theta-toxin that contains sites for membrane binding and self-aggregation. J Biol Chem. 1991;266:12449–54. [PubMed] [Google Scholar]
- 58.Harris RW, Sims PJ, Tweten RK. Evidence that Clostridium perfringens theta-toxin induces colloid-osmotic lysis of erythrocytes. Infect Immun. 1991;59:2499–501. doi: 10.1128/iai.59.7.2499-2501.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mitchell TJ, Andrew PW. Biological properties of pneumolysin. Microb Drug Resist. 1997;3:19–26. doi: 10.1089/mdr.1997.3.19. [DOI] [PubMed] [Google Scholar]
- 60.Tweten RK. Cholesterol-dependent cytolysins, a family of versatile pore-forming toxins. Infect Immun. 2005;73:6199–209. doi: 10.1128/IAI.73.10.6199-6209.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mainou-Fowler T, MacGowan AP, Postlethwaite R. Virulence of Listeria spp.: course of infection in resistant and susceptible mice. J Med Microbiol. 1988;27:131–40. doi: 10.1099/00222615-27-2-131. [DOI] [PubMed] [Google Scholar]
- 62.Shepard LA, Shatursky O, Johnson AE, Tweten RK. The mechanism of pore assembly for a cholesterol-dependent cytolysin: formation of a large prepore complex precedes the insertion of the transmembrane beta-hairpins. Biochemistry. 2000;39:10284–93. doi: 10.1021/bi000436r. [DOI] [PubMed] [Google Scholar]
- 63.Hotze EM, Heuck AP, Czajkowsky DM, Shao Z, Johnson AE, Tweten RK. Monomer-monomer interactions drive the prepore to pore conversion of a beta-barrel-forming cholesterol-dependent cytolysin. J Biol Chem. 2002;277:11597–605. doi: 10.1074/jbc.M111039200. [DOI] [PubMed] [Google Scholar]
- 64.Portnoy DA, Tweten RK, Kehoe M, Bielecki J. Capacity of listeriolysin O, streptolysin O, and perfringolysin O to mediate growth of Bacillus subtilis within mammalian cells. Infect Immun. 1992;60:2710–7. doi: 10.1128/iai.60.7.2710-2717.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Glomski IJ, Gedde MM, Tsang AW, Swanson JA, Portnoy DA. The Listeria monocytogenes hemolysin has an acidic pH optimum to compartmentalize activity and prevent damage to infected host cells. J Cell Biol. 2002;156:1029–38. doi: 10.1083/jcb.200201081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jones S, Portnoy DA. Characterization of Listeria monocytogenes pathogenesis in a strain expressing perfringolysin O in place of listeriolysin O. Infect Immun. 1994;62:5608–13. doi: 10.1128/iai.62.12.5608-5613.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rossjohn J, Feil SC, McKinstry WJ, Tweten RK, Parker MW. Structure of a cholesterol-binding, thiol-activated cytolysin and a model of its membrane form. Cell. 1997;89:685–92. doi: 10.1016/S0092-8674(00)80251-2. [DOI] [PubMed] [Google Scholar]
- 68.Polekhina G, Giddings KS, Tweten RK, Parker MW. Insights into the action of the superfamily of cholesterol-dependent cytolysins from studies of intermedilysin. Proc Natl Acad Sci U S A. 2005;102:600–5. doi: 10.1073/pnas.0403229101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Benkert P, Biasini M, Schwede T. Toward the estimation of the absolute quality of individual protein structure models. Bioinformatics. 2011;27:343–50. doi: 10.1093/bioinformatics/btq662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ramachandran R, Tweten RK, Johnson AE. The domains of a cholesterol-dependent cytolysin undergo a major FRET-detected rearrangement during pore formation. Proc Natl Acad Sci U S A. 2005;102:7139–44. doi: 10.1073/pnas.0500556102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Soltani CE, Hotze EM, Johnson AE, Tweten RK. Structural elements of the cholesterol-dependent cytolysins that are responsible for their cholesterol-sensitive membrane interactions. Proc Natl Acad Sci U S A. 2007;104:20226–31. doi: 10.1073/pnas.0708104105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shepard LA, Heuck AP, Hamman BD, Rossjohn J, Parker MW, Ryan KR, et al. Identification of a membrane-spanning domain of the thiol-activated pore-forming toxin Clostridium perfringens perfringolysin O: an α-helical to beta-sheet transition identified by fluorescence spectroscopy. Biochemistry. 1998;37:14563–74. doi: 10.1021/bi981452f. [DOI] [PubMed] [Google Scholar]
- 73.Ramachandran R, Tweten RK, Johnson AE. Membrane-dependent conformational changes initiate cholesterol-dependent cytolysin oligomerization and intersubunit beta-strand alignment. Nat Struct Mol Biol. 2004;11:697–705. doi: 10.1038/nsmb793. [DOI] [PubMed] [Google Scholar]
- 74.Idone V, Tam C, Andrews NW. Two-way traffic on the road to plasma membrane repair. Trends Cell Biol. 2008;18:552–9. doi: 10.1016/j.tcb.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Los FC, Kao CY, Smitham J, McDonald KL, Ha C, Peixoto CA, et al. RAB-5- and RAB-11-dependent vesicle-trafficking pathways are required for plasma membrane repair after attack by bacterial pore-forming toxin. Cell Host Microbe. 2011;9:147–57. doi: 10.1016/j.chom.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Decatur AL, Portnoy DA. A PEST-like sequence in listeriolysin O essential for Listeria monocytogenes pathogenicity. Science. 2000;290:992–5. doi: 10.1126/science.290.5493.992. [DOI] [PubMed] [Google Scholar]
- 77.Lety MA, Frehel C, Dubail I, Beretti JL, Kayal S, Berche P, et al. Identification of a PEST-like motif in listeriolysin O required for phagosomal escape and for virulence in Listeria monocytogenes. Mol Microbiol. 2001;39:1124–39. doi: 10.1111/j.1365-2958.2001.02281.x. [DOI] [PubMed] [Google Scholar]
- 78.Lety MA, Frehel C, Berche P, Charbit A. Critical role of the N-terminal residues of listeriolysin O in phagosomal escape and virulence of Listeria monocytogenes. Mol Microbiol. 2002;46:367–79. doi: 10.1046/j.1365-2958.2002.03176.x. [DOI] [PubMed] [Google Scholar]
- 79.Schnupf P, Portnoy DA, Decatur AL. Phosphorylation, ubiquitination and degradation of listeriolysin O in mammalian cells: role of the PEST-like sequence. Cell Microbiol. 2006;8:353–64. doi: 10.1111/j.1462-5822.2005.00631.x. [DOI] [PubMed] [Google Scholar]
- 80.Rogers S, Wells R, Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science. 1986;234:364–8. doi: 10.1126/science.2876518. [DOI] [PubMed] [Google Scholar]
- 81.Rechsteiner M, Rogers SW. PEST sequences and regulation by proteolysis. Trends Biochem Sci. 1996;21:267–71. [PubMed] [Google Scholar]
- 82.Meraro D, Hashmueli S, Koren B, Azriel A, Oumard A, Kirchhoff S, et al. Protein-protein and DNA-protein interactions affect the activity of lymphoid-specific IFN regulatory factors. J Immunol. 1999;163:6468–78. [PubMed] [Google Scholar]
- 83.Berche P, Gaillard JL, Geoffroy C, Alouf JE. T cell recognition of listeriolysin O is induced during infection with Listeria monocytogenes. J Immunol. 1987;139:3813–21. [PubMed] [Google Scholar]
- 84.Beattie IA, Swaminathan B, Ziegler HK. Cloning and characterization of T-cell-reactive protein antigens from Listeria monocytogenes. Infect Immun. 1990;58:2792–803. doi: 10.1128/iai.58.9.2792-2803.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lety MA, Frehel C, Beretti JL, Berche P, Charbit A. Modification of the signal sequence cleavage site of listeriolysin O does not affect protein secretion but impairs the virulence of Listeria monocytogenes. Microbiology. 2003;149:1249–55. doi: 10.1099/mic.0.26072-0. [DOI] [PubMed] [Google Scholar]
- 86.Pamer EG, Sijts AJ, Villanueva MS, Busch DH, Vijh S. MHC class I antigen processing of Listeria monocytogenes proteins: implications for dominant and subdominant CTL responses. Immunol Rev. 1997;158:129–36. doi: 10.1111/j.1600-065X.1997.tb00999.x. [DOI] [PubMed] [Google Scholar]
- 87.Vijh S, Pilip IM, Pamer EG. Noncompetitive expansion of cytotoxic T lymphocytes specific for different antigens during bacterial infection. Infect Immun. 1999;67:1303–9. doi: 10.1128/iai.67.3.1303-1309.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Finelli A, Kerksiek KM, Allen SE, Marshall N, Mercado R, Pilip I, et al. MHC class I restricted T cell responses to Listeria monocytogenes, an intracellular bacterial pathogen. Immunol Res. 1999;19:211–23. doi: 10.1007/BF02786489. [DOI] [PubMed] [Google Scholar]
- 89.Carrero JA, Vivanco-Cid H, Unanue ER. Listeriolysin o is strongly immunogenic independently of its cytotoxic activity. PLoS One. 2012;7:e32310. doi: 10.1371/journal.pone.0032310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rose F, Zeller SA, Chakraborty T, Domann E, Machleidt T, Kronke M, et al. Human endothelial cell activation and mediator release in response to Listeria monocytogenes virulence factors. Infect Immun. 2001;69:897–905. doi: 10.1128/IAI.69.2.897-905.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kayal S, Lilienbaum A, Join-Lambert O, Li X, Israël A, Berche P. Listeriolysin O secreted by Listeria monocytogenes induces NF-kappaB signalling by activating the IkappaB kinase complex. Mol Microbiol. 2002;44:1407–19. doi: 10.1046/j.1365-2958.2002.02973.x. [DOI] [PubMed] [Google Scholar]
- 92.Yoshikawa H, Kawamura I, Fujita M, Tsukada H, Arakawa M, Mitsuyama M. Membrane damage and interleukin-1 production in murine macrophages exposed to listeriolysin O. Infect Immun. 1993;61:1334–9. doi: 10.1128/iai.61.4.1334-1339.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nishibori T, Xiong H, Kawamura I, Arakawa M, Mitsuyama M. Induction of cytokine gene expression by listeriolysin O and roles of macrophages and NK cells. Infect Immun. 1996;64:3188–95. doi: 10.1128/iai.64.8.3188-3195.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nomura T, Kawamura I, Tsuchiya K, Kohda C, Baba H, Ito Y, et al. Essential role of interleukin-12 (IL-12) and IL-18 for gamma interferon production induced by listeriolysin O in mouse spleen cells. Infect Immun. 2002;70:1049–55. doi: 10.1128/IAI.70.3.1049-1055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kohda C, Kawamura I, Baba H, Nomura T, Ito Y, Kimoto T, et al. Dissociated linkage of cytokine-inducing activity and cytotoxicity to different domains of listeriolysin O from Listeria monocytogenes. Infect Immun. 2002;70:1334–41. doi: 10.1128/IAI.70.3.1334-1341.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kimoto T, Kawamura I, Kohda C, Nomura T, Tsuchiya K, Ito Y, et al. Differences in gamma interferon production induced by listeriolysin O and ivanolysin O result in different levels of protective immunity in mice infected with Listeria monocytogenes and Listeria ivanovii. Infect Immun. 2003;71:2447–54. doi: 10.1128/IAI.71.5.2447-2454.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wallecha A, Wood L, Pan ZK, Maciag PC, Shahabi V, Paterson Y. Listeria monocytogenes-Derived Listeriolysin O Has Pathogen-Associated Molecular Pattern-Like Properties Independent of Its Hemolytic Ability. Clin Vaccine Immunol. 2013;20:77–84. doi: 10.1128/CVI.00488-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee KD, Oh YK, Portnoy DA, Swanson JA. Delivery of macromolecules into cytosol using liposomes containing hemolysin from Listeria monocytogenes. J Biol Chem. 1996;271:7249–52. doi: 10.1074/jbc.271.13.7249. [DOI] [PubMed] [Google Scholar]
- 99.Schrama D, Reisfeld RA, Becker JC. Antibody targeted drugs as cancer therapeutics. Nat Rev Drug Discov. 2006;5:147–59. doi: 10.1038/nrd1957. [DOI] [PubMed] [Google Scholar]
- 100.Carter P. Improving the efficacy of antibody-based cancer therapies. Nat Rev Cancer. 2001;1:118–29. doi: 10.1038/35101072. [DOI] [PubMed] [Google Scholar]
- 101.Pastan I, Kreitman RJ. Immunotoxins for targeted cancer therapy. Adv Drug Deliv Rev. 1998;31:53–88. doi: 10.1016/S0169-409X(97)00094-X. [DOI] [PubMed] [Google Scholar]
- 102.Kerr DE, Wu GY, Wu CH, Senter PD. Listeriolysin O potentiates immunotoxin and bleomycin cytotoxicity. Bioconjug Chem. 1997;8:781–4. doi: 10.1021/bc970124+. [DOI] [PubMed] [Google Scholar]
- 103.Bergelt S, Frost S, Lilie H. Listeriolysin O as cytotoxic component of an immunotoxin. Protein Sci. 2009;18:1210–20. doi: 10.1002/pro.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Slingerland M, Guchelaar HJ, Gelderblom H. Liposomal drug formulations in cancer therapy: 15 years along the road. Drug Discov Today. 2012;17:160–6. doi: 10.1016/j.drudis.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 105.Tailor TD, Hanna G, Yarmolenko PS, Dreher MR, Betof AS, Nixon AB, et al. Effect of pazopanib on tumor microenvironment and liposome delivery. Mol Cancer Ther. 2010;9:1798–808. doi: 10.1158/1535-7163.MCT-09-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Haley B, Frenkel E. Nanoparticles for drug delivery in cancer treatment. Urol Oncol. 2008;26:57–64. doi: 10.1016/j.urolonc.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 107.Di Legge A, Trivellizzi IN, Moruzzi MC, Pesce A, Scambia G, Lorusso D. Phase 2 trial of nonpegylated doxorubicin (Myocet) as second-line treatment in advanced or recurrent endometrial cancer. Int J Gynecol Cancer. 2011;21:1446–51. doi: 10.1097/IGC.0b013e31822d754e. [DOI] [PubMed] [Google Scholar]
- 108.Mir LM, Morsli N, Garbay JR, Billard V, Robert C, Marty M. Electrochemotherapy: a new treatment of solid tumors. J Exp Clin Cancer Res. 2003;22(Suppl):145–8. [PubMed] [Google Scholar]
- 109.Safaei R, Katano K, Larson BJ, Samimi G, Holzer AK, Naerdemann W, et al. Intracellular localization and trafficking of fluorescein-labeled cisplatin in human ovarian carcinoma cells. Clin Cancer Res. 2005;11:756–67. [PubMed] [Google Scholar]
- 110.Kullberg M, Mann K, Anchordoquy TJ. Targeting Her-2+ Breast Cancer Cells with Bleomycin Immunoliposomes Linked to LLO. Mol Pharm. 2012;9:2000–8. doi: 10.1021/mp300049n. [DOI] [PubMed] [Google Scholar]
- 111.Lee RJ, Huang L. Folate-targeted, anionic liposome-entrapped polylysine-condensed DNA for tumor cell-specific gene transfer. J Biol Chem. 1996;271:8481–7. doi: 10.1074/jbc.271.14.8481. [DOI] [PubMed] [Google Scholar]
- 112.Shi G, Guo W, Stephenson SM, Lee RJ. Efficient intracellular drug and gene delivery using folate receptor-targeted pH-sensitive liposomes composed of cationic/anionic lipid combinations. J Control Release. 2002;80:309–19. doi: 10.1016/S0168-3659(02)00017-2. [DOI] [PubMed] [Google Scholar]
- 113.Sun X, Provoda C, Lee KD. Enhanced in vivo gene expression mediated by listeriolysin O incorporated anionic LPDII: Its utility in cytotoxic T lymphocyte-inducing DNA vaccine. J Control Release. 2010;148:219–25. doi: 10.1016/j.jconrel.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rescigno M, Winzler C, Delia D, Mutini C, Lutz M, Ricciardi-Castagnoli P. Dendritic cell maturation is required for initiation of the immune response. J Leukoc Biol. 1997;61:415–21. [PubMed] [Google Scholar]
- 115.Rescigno M, Citterio S, Thèry C, Rittig M, Medaglini D, Pozzi G, et al. Bacteria-induced neo-biosynthesis, stabilization, and surface expression of functional class I molecules in mouse dendritic cells. Proc Natl Acad Sci U S A. 1998;95:5229–34. doi: 10.1073/pnas.95.9.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Radford KJ, Higgins DE, Pasquini S, Cheadle EJ, Carta L, Jackson AM, et al. A recombinant E. coli vaccine to promote MHC class I-dependent antigen presentation: application to cancer immunotherapy. Gene Ther. 2002;9:1455–63. doi: 10.1038/sj.gt.3301812. [DOI] [PubMed] [Google Scholar]
- 117.Radford KJ, Jackson AM, Wang JH, Vassaux G, Lemoine NR. Recombinant E. coli efficiently delivers antigen and maturation signals to human dendritic cells: presentation of MART1 to CD8+ T cells. Int J Cancer. 2003;105:811–9. doi: 10.1002/ijc.11149. [DOI] [PubMed] [Google Scholar]
- 118.Dai MS, Nitcheu-Tefit J, Alcock S, Ramirez-Jimenez F, Chao TY, Baril P, et al. Development of an Escherichia coli expressing listeriolysin-O vaccine against Wilms tumor gene 1-expressing tumors. J Immunother. 2009;32:845–55. doi: 10.1097/CJI.0b013e3181aee259. [DOI] [PubMed] [Google Scholar]
- 119.Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, et al. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766–72. [PubMed] [Google Scholar]
- 120.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–61. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 121.Sasada T, Kimura M, Yoshida Y, Kanai M, Takabayashi A. CD4+CD25+ regulatory T cells in patients with gastrointestinal malignancies: possible involvement of regulatory T cells in disease progression. Cancer. 2003;98:1089–99. doi: 10.1002/cncr.11618. [DOI] [PubMed] [Google Scholar]
- 122.Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–33. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nitcheu-Tefit J, Dai MS, Critchley-Thorne RJ, Ramirez-Jimenez F, Xu M, Conchon S, et al. Listeriolysin O expressed in a bacterial vaccine suppresses CD4+CD25high regulatory T cell function in vivo. J Immunol. 2007;179:1532–41. doi: 10.4049/jimmunol.179.3.1532. [DOI] [PubMed] [Google Scholar]
- 124.Peng X, Treml J, Paterson Y. Adjuvant properties of listeriolysin O protein in a DNA vaccination strategy. Cancer Immunol Immunother. 2007;56:797–806. doi: 10.1007/s00262-006-0240-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dégano P, Schneider J, Hannan CM, Gilbert SC, Hill AV. Gene gun intradermal DNA immunization followed by boosting with modified vaccinia virus Ankara: enhanced CD8+ T cell immunogenicity and protective efficacy in the influenza and malaria models. Vaccine. 1999;18:623–32. doi: 10.1016/S0264-410X(99)00278-9. [DOI] [PubMed] [Google Scholar]
- 126.Dai MS, Vassaux G, Xu M, You RI, Hsieh YF, Ouisse LH, et al. Early Treg suppression by a listeriolysin-O-expressing E. coli vaccine in heterologous prime-boost vaccination against cancer. Vaccine. 2012;30:6903–11. doi: 10.1016/j.vaccine.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 127.Peng X, Hussain SF, Paterson Y. The ability of two Listeria monocytogenes vaccines targeting human papillomavirus-16 E7 to induce an antitumor response correlates with myeloid dendritic cell function. J Immunol. 2004;172:6030–8. doi: 10.4049/jimmunol.172.10.6030. [DOI] [PubMed] [Google Scholar]
- 128.Souders NC, Sewell DA, Pan ZK, Hussain SF, Rodriguez A, Wallecha A, et al. Listeria-based vaccines can overcome tolerance by expanding low avidity CD8+ T cells capable of eradicating a solid tumor in a transgenic mouse model of cancer. Cancer Immun. 2007;7:2. [PMC free article] [PubMed] [Google Scholar]
- 129.Sewell DA, Pan ZK, Paterson Y. Listeria-based HPV-16 E7 vaccines limit autochthonous tumor growth in a transgenic mouse model for HPV-16 transformed tumors. Vaccine. 2008;26:5315–20. doi: 10.1016/j.vaccine.2008.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rothman J, Wallecha A, Maciag PC, Rivera S, Shahabi V, Paterson Y. The use of a living Listeria monocytogenes as an active immunotherapy for the treatment of cancer. In: Fialho AM, eds. Emerging Cancer Therapy: Microbial Approaches and Biotechnological Tools. New York: John Wiley & Sons publishing, 2010:13-48. [Google Scholar]
- 131.Bajénoff M, Narni-Mancinelli E, Brau F, Lauvau G. Visualizing early splenic memory CD8+ T cells reactivation against intracellular bacteria in the mouse. PLoS One. 2010;5:e11524. doi: 10.1371/journal.pone.0011524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Seavey MM, Paterson Y. Anti-Angiogenesis immunotherapy induces epitope spreading to Her-2/neu resulting in breast tumor immunoediting. Breast Cancer (London) 2009;1:19–30. doi: 10.2147/bctt.s6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wood LM, Pan ZK, Guirnalda P, Tsai P, Seavey M, Paterson Y. Targeting tumor vasculature with novel Listeria-based vaccines directed against CD105. Cancer Immunol Immunother. 2011;60:931–42. doi: 10.1007/s00262-011-1002-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Maciag PC, Seavey MM, Pan ZK, Ferrone S, Paterson Y. Cancer immunotherapy targeting the high molecular weight melanoma-associated antigen protein results in a broad antitumor response and reduction of pericytes in the tumor vasculature. Cancer Res. 2008;68:8066–75. doi: 10.1158/0008-5472.CAN-08-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Neeson P, Pan ZK, Paterson Y. Listeriolysin O is an improved protein carrier for lymphoma immunoglobulin idiotype and provides systemic protection against 38C13 lymphoma. Cancer Immunol Immunother. 2008;57:493–505. doi: 10.1007/s00262-007-0388-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Singh R, Dominiecki ME, Jaffee EM, Paterson Y. Fusion to Listeriolysin O and delivery by Listeria monocytogenes enhances the immunogenicity of HER-2/neu and reveals subdominant epitopes in the FVB/N mouse. J Immunol. 2005;175:3663–73. doi: 10.4049/jimmunol.175.6.3663. [DOI] [PubMed] [Google Scholar]
- 137.Guirnalda P, Wood L, Paterson Y. Listeria monocytogenes and its products as agents for cancer immunotherapy. Adv Immunol. 2012;113:81–118. doi: 10.1016/B978-0-12-394590-7.00004-X. [DOI] [PubMed] [Google Scholar]