Abstract
We have developed a method to extract and separate phytochelatins (PCs)—metal(loid) complexes using parallel metal(loid)-specific (inductively coupled plasma-mass spectrometry) and organic-specific (electrospray ionization-mass spectrometry) detection systems—and use it here to ascertain the nature of arsenic (As)-PC complexes in plant extracts. This study is the first unequivocal report, to our knowledge, of PC complex coordination chemistry in plant extracts for any metal or metalloid ion. The As-tolerant grass Holcus lanatus and the As hyperaccumulator Pteris cretica were used as model plants. In an in vitro experiment using a mixture of reduced glutathione (GS), PC2, and PC3, As preferred the formation of the arsenite [As(III)]-PC3 complex over GS-As(III)-PC2, As(III)-(GS)3, As(III)-PC2, or As(III)-(PC2)2 (GS: glutathione bound to arsenic via sulphur of cysteine). In H. lanatus, the As(III)-PC3 complex was the dominant complex, although reduced glutathione, PC2, and PC3 were found in the extract. P. cretica only synthesizes PC2 and forms dominantly the GS-As(III)-PC2 complex. This is the first evidence, to our knowledge, for the existence of mixed glutathione-PC-metal(loid) complexes in plant tissues or in vitro. In both plant species, As is dominantly in non-bound inorganic forms, with 13% being present in PC complexes for H. lanatus and 1% in P. cretica.
Phytochelatins (PCs) are induced in a wide range of plant species by the oxy-anions arsenate [As(V)] and selenate and a range of cations such as Ag+, Cd2+, Cu2+, Hg2+, and Pb2+ (Grill et al., 1985). They are synthesized from reduced glutathione (GSH) by the transpeptidation of γ-glutamyl-cysteinyl dipeptides through the action of the constitutive enzyme PC synthase (Schmöger et al., 2000; Vatamaniuk et al., 2000). PCs have the common structure (γ-Glu-Cys)nGly, where n = 2 - 11, although PC2 and PC3 are the most common (Cobbett, 2000; Cobbett and Goldsbrough, 2002). There is strong evidence that PCs complex metal(loid) ions, for which they have high affinity, in plant extracts (Mehra et al., 1996a, 1996b; Bae and Mehra, 1997; Leopold and Gunther, 1997; Leopold et al., 1998; Pickering et al., 1999; Satofuka et al., 2001; Cruz et al., 2002; Doreak and Krezel, 2003).
Little is known of the nature of metal(loid) ion-PC complexes in planta. Preliminary experiments with cadmium and copper using HPLC using chromatography with metal-specific detectors (coupled to inductively coupled plasma [ICP]-mass spectrometry [MS], UV detection, or off-line atomic absorption spectrometry; Maitani et al., 1996; Mehra et al., 1996a; Leopold and Gunther, 1997; Leopold et al., 1998; Scarano and Morelli, 2002) did not give convincing results for the complex formation. More basic preparative scale chromatography with fraction collectors have shown that presumed PC complexes have eluted from columns intact (Sneller et al., 1999; Schmöger et al., 2000). Off-line analysis of PCs in collected fractions is performed by HPLC after derivatization, losing all structural information regarding the nature of metal(loid) ion-PC complexes. Because no previous studies have been conducted with organic- and metal-specific detectors in parallel, previous studies, particularly those investigating complex cell extracts, simply indicate that the metal ion studied is complexed in plant extracts and that these complexes probably involve PCs.
More detailed studies that give an indication of the complexes actually formed have been conducted with purified PCs in vitro. Pickering et al. (1999) analyzed purified PC3 mixed with Cd by x-ray absorption spectroscopy, finding a predominantly tetrahedral coordination of Cd by sulfur (S), with evidence of polynuclear Cd clusters with PC3. Complex binding chemistries, dependent on pH and Cd:PC molar ratios, also have been observed in in vitro experiments using NMR and polarographic detection (Cruz et al., 2002; Doreak and Krezel, 2003). Evidence for metal(loid) ion-PC formation also has been derived from in vitro studies using optical spectroscopic approaches (Mehra et al., 1996a, 1996b). Schmöger et al. (2000) are the only group to provide organic mass spectra of a metal(loid)-PC complex, and this was for arsenic (As). They identified the presence of As-(PC2)2 complexes in a mixture of arsenite [As(III)] and PC2 by directly injecting the mixture into an electrospray ionization (ESI)-MS. Although in vitro studies can show the existence of metal(loid) ion-PC complexes, they cannot predict what complexes form in planta because the metal-(loid) ions are in a complex cell environment, with a range of PCs and other biomolecules with which they have affinity, such as glutathione, which itself can form complexes with metal(loid) ions in vitro (Raab et al., 2004).
PCs have a high affinity for As(III), not As(V). As is mainly taken into terrestrial plants as As(V) or As(III) (Abedin et al., 2002; Meharg and Hartley-Whitaker, 2002), followed by rapid reduction [through As(V) reductases or via reducing agents such as glutathione and ascorbic acid] of As(V) to As(III) (Meharg and Hartley-Whitaker, 2002). PCs are thought to have a fundamental role in As detoxification in plants (Sneller et al., 1999; Pickering et al., 2000; Schmöger et al., 2000; Hartley-Whitaker et al., 2001), although evidence of As-PC complexes in plant extracts is by inference only using preparative scale chromatographic columns with fraction collection, followed by individual analysis of PCs and As (Sneller et al., 1999; Schmöger et al., 2000), limited HPLC-ICP-MS investigations (Maitani et al., 1996), or in vivo observations using x-ray absorption spectroscopy (XAS), which has shown that at least some of the As in plant cells is present as As(III) coordinated with S (Pickering et al., 2000). Studies on the plants Holcus lanatus (Hartley-Whitaker et al., 2001), Silene vulgaris (Sneller et al., 1999), and Rauvolfia serpentina (Schmöger et al., 2000) give molar ratios of As to PC-thiol (SH) (SH: thiol-groups of PC) groups of approximately 1:1, 1:4, and 1:2.7, respectively. In the As-hyperaccumulating fern Pteris vittata, only PC2 is synthesized on As exposure (Zhao et al., 2003). As is present in the fern mainly as uncoordinated inorganic forms with enough PC present to theoretically complex, assuming complete complexation of the PC as As(III)-(PC2)2, only 1% to 3% of the As (Zhao et al., 2003).
To date, no publication has reported intact metal-(loid)-PC speciation in plant extracts to identify the exact nature of As-PC species present in plant tissues. This has been primarily because of limitations in technology, namely the failure to use mild acid extraction procedures and the use of parallel metal-specific (ICP-MS) and organic-specific (ESI-MS) detection systems interfaced with a suitable chromatographic column and buffer condition. Presented here are the first unequivocal speciations, to our knowledge, of metal(loid)-PC complexes using HPLC-(ICP-MS)-(ESI-MS). As-PC complexes were extracted from the As-tolerant grass H. lanatus (Meharg and Macnair, 1992) and the As-hyperaccumulating fern Pteris cretica (Meharg, 2003).
RESULTS
Chromatography and Spectra of PCs and As-PC Complexes Produced in Vitro
Chromatographic and detector conditions have been explored and optimized in a study of, arsenicglutathion, As(III)-GS3 complex formed in vitro (Raab et al., 2004). Using a reverse-phase C18 column and a gradient of 1% (v/v) formic acid and methanol as the mobile phase, the As(III)-glutathione complex was found to be stable and eluted from the column. Stability was cross-checked by comparing speciation ascertained by 1H-NMR. The As(III)-glutathione complex was not stable for anionic ion-exchange columns under mobile phase conditions suitable for both ICP-MS and ESI-MS detectors. Although the As(III)-glutathione complex was stable with an acid mobile phase (pyridine pH 2.5) using size exclusion and cation exchange chromatography, chromatographic separation was poor. The same chromatographic reverse-phase C18 column and mobile phase conditions that successfully separated As(III)-glutathione complex with parallel ICP-MS and ESI-MS detectors also provided excellent stability and separation of As(III)-PC complexes.
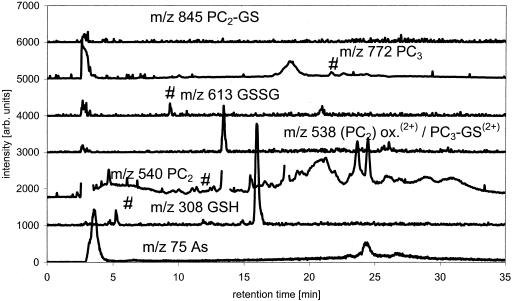
Optimization of chromatography and detection of As-PC complexes by HPLC-(ICP-MS)-(ESI-MS) was performed using a mixed GSH, PC2, and PC3 solution in 1% (v/v) formic acid. Spectra were first obtained for the PCs not reacted with As(III). The mixture contained 36% glutathione, 50% PC2, and 14% PC3 (percentage on molar basis calculated from -SH equivalents). PC2, PC3, and GSH gave strong signals at their [M + H]+ masses of 540, 772, and 308 (spectra not shown). The oxidized forms PC2 (two S-S bridges) and oxidized glutathione (GSSG) were detectable at mass-to-charge ratio (m/z) 538, which is [M + 2H]2+, and m/z 613 [M + H]+ and 307 [GSSG + 2H]2+, respectively. None of the oxidized forms of PC3 were detectable (spectra not shown). PC2 oxidized at one S by GSH was also detectable at m/z 845 and 423 [M + 2H]2+.
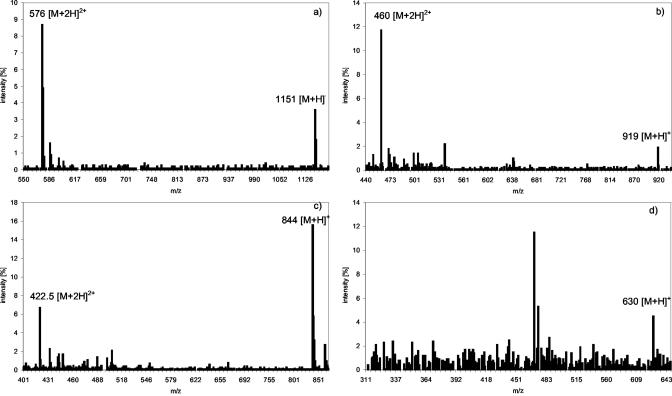
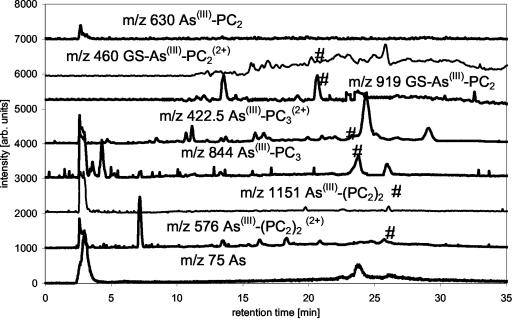
The mixed glutathione-PC solution was then reacted with a surplus of As(III) in the presence of 1% (v/v) formic acid. On adding As(III) to the PC solution, the following complex species could be formed theoretically: As(III)-GS3, As(III)-(PC2)2, GS-As(III)-PC2, As(III)-PC3, As(III)-PC2, and GS-As(III)-PC3 (GS: glutathione bound to arsenic via sulphur of cysteine). The molecular masses expected from these compounds are shown in Table I. The complexes actually detected were As(III)-(PC)2, GS-As(III)-PC2, As(III)-PC3, and As(III)-PC2, showing a preference for the formation of the double charged ion [M + 2H]2+ under the acidic conditions investigated. As(III)-(PC2)2 showed a main signal at m/z 576 [M + 2H]+2 and the expected [M + H]+ signal at m/z 1,151 as minor signal. Signals at m/z 919 [M + H]+ and dominant signal at m/z 460 [M + 2H]2+ were from GS-As(III)-PC2. As(III)-PC3, the complex formed in the greatest quantity, showed a strong signal at m/z 844 [M + H]+ and a minor one at m/z 422.5 [M + 2H]2+, respectively. The amount of As(III)-PC2 formed was very small and resulted in a signal at m/z 630. Corresponding to [PC2-As(OH)+H]+, no double charged form of this molecule was detectable. The spectra are shown in Figure 1.
Table I.
GSH, PCs and their arsenic complexes, molecular masses, and retention time
| Chemical Species | Formula | m/z | Retention Times |
|---|---|---|---|
| min | |||
| PC2 | C18H29N5O10S2 | 540 | 13.0 |
| PC2 oxidized | C36H54N10O20S4 | 1,075/538 | 13.8 |
| PC3 | C26H41N7O14S3 | 772/386.6 | 22.1 |
| PC3 oxidized (1 S-S bridge) | C52H80N14O28S6 | 1,541/771 | Co-eluting with PC3 reduced ? |
| GSH | C10H17N3O6S | 308 | 5.2 |
| GSSG | C20H32N6O12S2 | 613 | 9.6 |
| (PC2)-(GS) | C28H44N8O16S3 | 845/423 | 16.9 |
| As(III)-(PC2)2 | C36H55N10O20S4As | 1,151/576 | 29.5 |
| GS-As(III)-PC2 | C28H43N8O16S3As | 919/460 | 22.8 |
| As(III)-PC3 | C26H38N7O14S3As | 844/422.5 | 24.2 |
| As(III)-(GS)3 | C30H48N9O18S3As | 994/497.5 | 16.8 |
| PC3-GS | C36H56N10O20S4 | 1,077/539 | Co-eluting with PC2 oxidized ? |
| As(III)-PC2 | C18H27N5O10S2AsOH | 630 | 20.1 |
| GS-As(III)-PC3 | C36H55N10O20S4As | 1,151/576 | Co-eluting with As(III)-(PC2)2? |
Figure 1.
Spectra of As(III)-(PC2)2 (a), GS-As(III)-PC2 (b), As(III)-PC3 (c), and As(III)-PC2 (d) taken from a separation of the partially purified PC mixture, separation with a gradient of 1% (v/v) formic acid/methanol on a C18 ODS 5 column, ESI-MS parameters fragmenter voltage of 100 V, and a capillary voltage of 4,000 V in positive scan mode m/z 120 to 1,400.
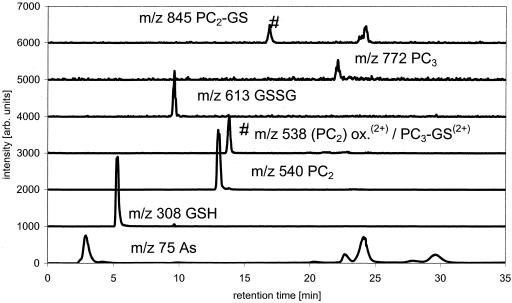
Good separation for GSH, PC2, PC3, their oxidized forms and the As(III)-containing complexes was achieved as shown by the ESI-MS and ICP-MS data. Besides reduced GSH, PC2, and PC3, the sample contained some oxidized GSSG, oxidized (PC2)2, and a mixed complex of PC2 and one GSH (Fig. 2).
Figure 2.
Separation of PC's ESI-MS data of PC species and As trace, separation with MeOH/formic acid gradient on a C18 ODS2 column, m/z 75 (As) measured by parallel use of ICP-MS (*, unknown species; and #, species identified as mentioned in trace).
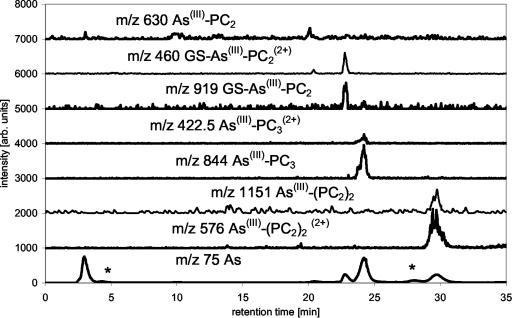
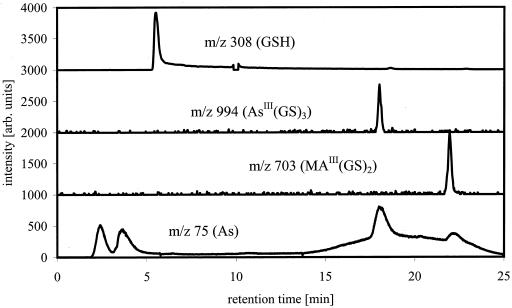
The ICP-MS data showed that besides unbound As(III), six other As-containing species were present in the solution. Combination of the results from ICP-MS (elemental information) and ESI-MS (molecular information) showed that As(III)-PC3 is the dominantly formed As(III)-PC complex, despite the higher availability of PC2 in the mixture. As(III)-(PC2)2 and GS-As(III)-PC2 complexes were formed at about the same rate and identified by their molecular ions and the bound As (Fig. 3). A small amount of As(III)-PC2 was detectable as well. It was not yet possible to identify two other As-containing species in this solution detected by ICP-MS because there were no corresponding detectable signals for the ESI-MS, due to the lower sensitivity of ESI-MS compared with ICP-MS. One of these unknowns might be GS-As(III)-PC3. As(III)-(GS)3 did not form in the solution as shown by the lack of the [M + H]+ peak, despite the fact that about 20% of the thiol groups in the solution are contributed by GSH. The separation of synthesized As(III)-(GS)3 using the same chromatographic conditions showed that the complex would be separable if present in solution and would show a signal in ESI-MS and ICP-MS at 16.8 min (Fig. 4).
Figure 3.
Separation of As-PC complexes ESI-MS data of As-PC species, m/z 75 (As) measured by parallel use of ICP-MS, separation with MeOH/formic acid gradient on a C18 ODS2 column (*, unknown species; #, species identified as mentioned in trace).
Figure 4.
As(III)-GS3 and methylarsinous acid-GSH (MA(III)-GS2), m/z traces of GSH and As(III)-GS3 and MA(III)-GS2 measured by ESI-MS and m/z 75 (As) measured by ICP-MS. Separation, MeOH/formic acid gradient on C18 ODS2 column, the broad m/z 75 peak under the eluting As(III)-GS3 and MA(III)-GS2 signals is a result of the presence of γ-Glutamylcysteinylglycinyl)di-methylthioarsinite (DMA(III)-GS) in the mixture, which is not stable under these chromatographic conditions (*, unknown species; #, species identified as mentioned in trace).
As-PC Complexes in H. lanatus Extracts
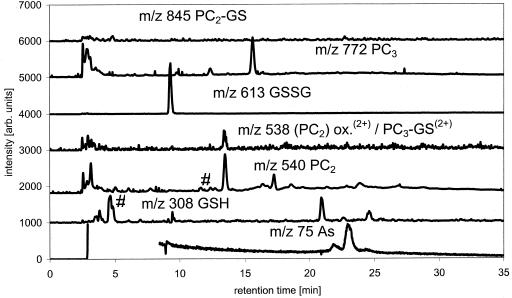
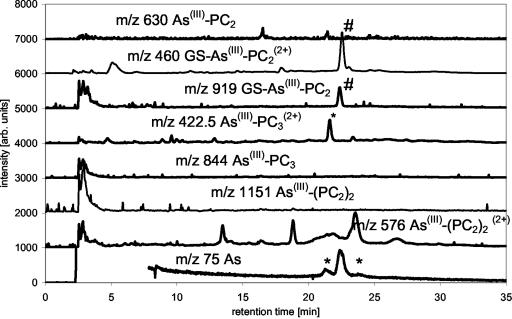
An intact turf of As(V)-tolerant H. lanatus was obtained from As mine spoil and maintained in a greenhouse for 2 years before experiments commenced. The extract of shoots clipped from the turf contained about 2.5 μg As g sample fresh weight-1, when extracted in 1% (v/v) formic acid for 24 h at 1°C. This is approximately 70% of the total As present in the H. lanatus leaves (Table II). Ninety-six percent of the As present in the extracts eluted from the column. GSH, PC3, and PC2 were all present in the extract as determined by ESI-MS by comparison of the retention times and ESI-MS spectra with the purified standard mixture (Fig. 5). No attempts were made to quantify the species via the ESI-MS signals. The As trace of the ICP-MS showed that there are four organic-bound As species beside unbound inorganic As present in the H. lanatus extracts. The As(III)-PC3 complex was identified positively using ESI-MS at m/z 844 [M + H]+ and 422.5 [M + 2H]2+ and ICP-MS m/z 75 (As; Fig. 6). The GS-As(III)-PC2 complex was identified mainly by comparison of the retention times of the As species measured by ICP-MS because the ESI-MS peak was too small to provide a reliable mass spectrum. Whether As(III)-(PC2)2 is present in H. lanatus or not is uncertain because the retention time of the As peak and the retention times of ESI-MS signals of m/z 1,151 and 576 did not fit with the retention times of the standard.
Table II.
Total arsenic concentrations in digest and extract and recoveries
| Name of Plant Sample | As (Total) | As (Extractable) | Extraction Efficiency | As (Recovered after HPLC) | Recovery |
|---|---|---|---|---|---|
| μg As g plant-1 | % | μg As g plant-1 | % | ||
| H. lanatus 1 | 4.2 | 4.3 | 102 | 2.7 | 63 |
| H. lanatus 2 | 2.9 | 2.2 | 76 | 2.1 | 95 |
| P. cretica 1 | 73.9 | 70.2 | 95 | 68.6 | 98 |
| P. cretica 2 | 74.0 | 74.3 | 100 | 72.2 | 97 |
Figure 5.
H. lanatus extract, m/z traces of GSH and PCs measured by ESI-MS and m/z 75 (As) measured by ICP-MS. Separation, MeOH/formic acid gradient on C18 ODS2 column (*, unknown species; #, species identified as mentioned in trace).
Figure 6.
H. lanatus extract, m/z traces of As-PC complexes measured by ESI-MS and m/z 75 (As) measured by ICP-MS. Separation, MeOH/formic acid gradient on C18 ODS2 column (*, unknown species; #, species identified as mentioned in trace).
For quantification of the As complexes, the ICP-MS data were used based on the ICP-MS response to sodium dimethylarsinic acid [DMA(V)] assuming that every As species gives the same sensitivity. The As concentrations of the different species were determined in two plants and showed that most of the extractable As is in the inorganic form (approximately 78% = 1.5 μg As g fresh weight plant-1), about 10% of the As is bound in the PC3 complex (approximately 230 ng As g-1), and about 3% is present in the GS-As(III)-PC2 complex (approximately 60 ng As g-1). The rest is present in two species not identified yet, one eluting shortly after inorganic As (approximately 3%) and the other later than As(III)-PC3 but before As(III)-(PC2)2 (approximately 5%; Table III). Previous experiments have shown that small amounts of PC4 are synthesized by H. lanatus (Hartley-Whitaker et al., 2001). Because we did not have any purified PC4, it could not be confirmed if the unidentified peaks corresponded to As-PC4 complexes. The peak eluting shortly after the inorganic As may be DMA(V), which is often detected as minor trace in plant extracts (Meharg and Hartley-Whitaker, 2002). The retention time of a DMA(V) standard is identical to the retention time in the sample, and there is also a signal at m/z 139, the [M + H]+ of DMA(V).
Table III.
Amount of arsenic bound to different PCs and unbound arsenic in percentage of total elutable arsenic U1 and U2 stand for the two species for which no structure was ascribed.
| Sample Name | inorganic As | As-PC2 | As-PC2-GS | As-PC3 | U1 | U2 |
|---|---|---|---|---|---|---|
| H. lanatus 1 | 68.1 | - | 2.8 | 10.2 | 6.1 | - |
| H. lanatus 2 | 65.2 | - | 2.5 | 10.4 | 4.8 | - |
| P. cretica 1 | 99.9 | 0.02 | 0.06 | - | - | 0.01 |
| P. cretica 2 | 99.9 | 0.02 | 0.02 | - | - | 0.01 |
As-PC Complexes in P. cretica Extracts
Leaves including spores and stems were taken from P. cretica growing on soil containing 100 mg kg-1 As for over 1 year. Tissues were extracted as for H. lanatus. The total As concentration in the extracts was about 150 μg As g fresh weight-1 of the plant, with inorganic As contributing about 99% of the total As. The chromatographic recovery was 99% of the total extractable As (Table II). The main As-PC complex in P. cretica is the GS-As(III)-PC2 complex (approximately 36 ng As g-1) and two minor species As not identified yet (Table III). No signal at the retention time of As(III)-(PC2)2 was detectable with the ICP-MS. The presences or absences of the complexes were confirmed by the ESI-MS data of the same separation. The ESI-MS data of the same measurements showed that the P. cretica extract contained large amounts of oxidized and reduced GSH but that there was no As species bound in the form of the As(III)-(GS)3 complex (Figs. 7 and 8).
Figure 7.
P. cretica extract, m/z traces of GSH and PCs measured by ESI-MS and m/z 75 (As) measured by ICP-MS. Separation, MeOH/formic acid gradient on C18 ODS2 column (*, unknown species; #, species identified as mentioned in trace).
Figure 8.
P. cretica extract, m/z traces of As-PC complexes measured by ESI-MS and m/z 75 (As) measured by ICP-MS. Separation, MeOH/formic acid gradient on C18 ODS2 column (*, at m/z 422.5 this signal is not identical with As(III)-PC3(2+) analysis of retention time, and spectra show it to be an unknown organic species; *, unknown species; #, species identified as mentioned in trace).
Repeated runs of P. cretica extracts were made (10 in total), all showing the same result. We have only presented two representative chromatographic runs.
Stability of Complexes during Storage
Samples of both model plants were used to study the stability of the As-PC complexes in solution and in the intact plant during storage. The extracted complexes are stable for about 3 d in 1% (v/v) formic acid when stored at 1°C; after 4 weeks, the complexes were no longer detectable whether they were stored at 1°C or -20°C. Storage of the intact plant material at -20°C for 4 weeks before powdering and extraction also destroyed the As-PC complexes (data not shown). The stability of synthesized As(III)-GS3 complex during this kind of extraction procedure was found to be approximately 50%. As-PC complexes are more stable than the As(III)-GS3 complex in 1% (v/v) formic acid. No changes in signal intensity were observed in the partially purified standard after storing it for up to 2 months at 1°C.
DISCUSSION
We have developed a method that enables the detection and quantification of individual As-PC complexes in plant extracts using HPLC coupled to ESI-MS and ICP-MS in parallel, which enabled us to provide the first report, to our knowledge, of how a metal(loid) is complexed by PCs in plant extracts. The separation of purified As-PC complexes and plant extracts showed that As-PC complexes in different forms, until now more deduced than definitively detected (Sneller et al., 1999, Pickering et al., 2000; Schmöger et al., 2000), do exist. Combining a separation method developed for the measurement of As(III)-(GS)3 (Raab et al., 2004) with the low detection limit of an ICP-MS and the molecular information delivered by an ESI-MS, the unequivocal identification of As(III)-(PC2)2, GS-As(III)-PC2, As(III)-PC3, and As(III)-PC2 in the standard preparation and in extracts of As-containing plants were obtained, revealing for the first time, to our knowledge, the stoichiometry of As-PC complexes and the presence of mixed metal(oid)-GS-PC2 complexes.
The few studies that had investigated previously the nature of As-PC complexes mainly used preparative scale size exclusion chromatography to separate the inorganic As from the “organic” As with the use of off-line separate detection of the PCs, and As (Schmöger et al., 2000; Sneller et al., 1999), without any possibility to detect specific As-PC complexes. For the specific detection of unbound PCs, the complexes were destroyed and the PCs determined by Ellman's reagent. Only one, to our knowledge, limited HPLC-ICP-MS has been published for As (Maitani et al., 1996). Because the authors of this paper did not run standards, the interference of the presence of As-PC complex (only one peak was observed) is weak.
The current study using HPLC with parallel ICP-MS and ESI-MS detection, which enables the separation of a mixture of GSH, PC2 and PC3 complexes with As(III), showed that the complex formation is not only driven by the availability of the ligand but by kinetic and steric considerations. The formation of As(III)-PC3 was preferred over the formation of the As(III)-(PC2)2 complex by a factor of 2.5 in a mixture of purified PC2 and PC3, where PC2 was present at higher concentrations (3.6:1 PC2:PC3 in -SH equivalents). The mixed complex of GS-As(III)-PC2 was about one-half as likely to be formed as the As(III)-(PC2)2 complex. The amount of unbound PC3 in the presence of As(III) was also very small compared with PC2. Although we have observed As(III)-(GS)3 complexes in vitro (Raab et al., 2004), none were detected when GSH was in the presence of PCs, either in vitro or in plant extracts.
It is possible that As associated with PCs dissociates during the plant extraction procedure or that the reverse happens: on cellular disruption, As-PC complexes form. Any technique investigating the nature of plant complexes that is chromatographically based will have similar uncertainties. Because the complexes were stable for 24 h, and our observations in vitro fit well with what is observed in plant extracts, the extraction procedure shows what complexes are present in the plant, rather than their absolute concentrations. Extraction was conducted under acidic conditions (pH 2.0) for which it is known that As-PC complexes are more stable. At cytoplasmic and vacuolar pHs, speciation may differ. The only approach used to study As-PC complexes in intact plant tissues that gives molecular information is XAS. XAS can only predict the atomic environment in which the As exists, i.e. the atoms to which it is bound or coordinated. It cannot give information concerning the actual chemical formulae of the complexes. When used to investigate As speciation in Brassica juncea, XAS showed that virtually all the As was trihedrally coordinated with S, presumably as PC complexes (Pickering et al., 2000). XAS of H. lanatus leaves (A. Raab, J. Feldmann, and A.A. Meharg, unpublished data) indicates the dominant presence of inorganic As, consistent with our findings presented here. XAS data published by Lombi et al. (2002) for the As-hyperaccumulating fern P. vittata showed only the presence of inorganic As. Similar spectra, which only observed inorganic As, have been obtained for P. cretica (A. Raab, J. Feldmann, and A.A. Meharg, unpublished data). As XAS is a relatively insensitive technique, signals from small amounts of S coordination will be swamped by large amounts of inorganic As species.
P. cretica, an As hyperaccumulator (Meharg 2003), contains about 20 times more total As than H. lanatus adapted to high-As concentrations in soil. Nevertheless, the amount of As complexed by PCs is a factor of eight higher in H. lanatus. Both species show a different composition of GSH and PC's. H. lanatus produces both PC2 and PC3, as also shown previously by Hartley-Whitaker et al. (2001), but contains a relatively small amount of GSH. In contrast, P. cretica, a hyperaccumulator of As, only produces PC2, identical to the As-hyperaccumulating fern P. vittata (Zhao et al., 2003), and contains a high amount of reduced and oxidized GSH. In H. lanatus, most of the complexed As is present in the form of the As(III)-PC3 with a minor component of GS-As(III)-PC2, whereas in P. cretica, which does not have PC3, the GS-As(III)-PC2 complex is the main complex.
The only previous study attempting to speciate As-PC complexes was that of Schmöger et al. (2000), who mixed As(III) and purified PC2 together and directly injected (i.e. not conducting chromatographic separation) the mixture into an ESI-MS detector. Though they detected the As(III)-(PC2)2 complex, we have shown here that under cellular conditions, this complex is not favored. When PC3 is present, As(III) is preferentially coordinated with this complex because the three -SH Cys groups form a more stable trihedral coordination than either GS-As(III)-PC2 or As(III)-(PC2)2.
The results of this study show that the As-tolerant grass H. lanatus and the As-hyperaccumulating fern P. cretica (and related As-hyperaccumulating ferns such as P. vittata) have evolved different mechanisms for coping with high internal levels of As. H. lanatus excludes As from its roots via suppression of As(V) uptake from soils (Meharg and Macnair, 1992). However, although As(V) uptake is at a reduced rate in tolerant clones of H. lanatus compared with non-tolerant clones, over the lifespan of tolerant clones, high levels of As build up in its tissue. As uptake strongly induces the synthesis of PCs in both tolerant and non-tolerant H. lanatus, with both PC2 and PC3 being the dominant PCs produced (Hartley-Whitaker et al., 2001). Once inside the plant, As(V) is, in the main, reduced to As(III), either through the action of reducing agents such as glutathione or ascorbic acid, or more likely through the action of As(V) reductase (Meharg and Hartley-Whitaker, 2002). Given the much greater prevalence of As(III)-PC3 in excess PC2, in plant extracts, and in vitro, this suggests that the PC2 is the intermediate in the production of PC3. Only small traces of PC4 have been identified previously in H. lanatus (Hartley-Whitaker et al., 2001). The present study predicts that 13% of the As is complexed by PCs in H. lanatus. However, GSH also complexes with PC2, making simple estimates of the theoretical total amount of As that could be complexed with PCs difficult. That only 13% of the As was PC complexed suggests that the bulk of the As is inorganic, non-complexed species, i.e. As(V) and As(III). It is probable, given the toxicity of As(V) and As(III) to cellular metabolism (Meharg and Hartley-Whitaker, 2002), that most of the inorganic As is present in the vacuole.
The PC complexation in P. cretica contrasts strongly with H. lanatus, where in the fern only 1% of the As is PC complexed and only PC2 is synthesized. Although synthesis of PC2 is induced on As exposure in hyperaccumulating ferns (Zhao et al., 2003), the actual molar ratio of As to PC -SH is small at 0.09 in shoots (Zhao et al., 2003), theoretically complexing 4.5% of the As in a GS-As(III)-PC2 complex for P. vittata. Lombi et al. (2002) have produced evidence that As is stored primarily in the vacuole of P. vittata, and Wang et al. (2002) have shown that this As is primarily As(III), with some As(V). The high amount of reduced and oxidized GSH in P. cretica in this study shows that the low concentration of PCs is not limited by the synthesis of glutathione but potentially by a low activity of PC synthase. The PC synthase in hyperaccumulating ferns may differ significantly from the synthases investigated to date in other plant species in terminating at synthesis of PC2.
The low quantities of PCs produced on As exposure in P. vittata (Zhao et al., 2003) and the results of this study suggest that PCs do not have a major role in As storage in plant tissues because most of the As in H. lanatus, P. cretica, and P. vittata is in toxic inorganic forms. As hypothesized by Zhao et al. (2003), PCs may act to shuttle As through the cytoplasm in a relatively nontoxic form, and this may be their major role in both hyperaccumulating and non-hyperaccumulating plants. There is evidence for a Cd-PC transporter in the vacuolar membrane (Salt and Rauser, 1995; Cobbett, 2000; Cobbett and Goldsbrough, 2002). No studies have been published showing an As-PC vacuolar membrane transporter, although one may exist. The results of the present study suggest that if As-PC transporters do exist that they may differ between plants depending on the dominant As-PC complex present.
In conclusion, we have developed an HPLC-(ICP-MS)-(ESI-MS) method that has provided, to our knowledge, the first unequivocal speciation in plant extracts of metal(loid)-PCs. We applied the method to the study of As in tolerant and hyperaccumulating plants. The same basic methodologies could be applied to other elements to understand their PC speciation. The technique has shown for the first time, to our knowledge, that glutathione forms mixed complexes with PCs in plant tissues and that As(III)-PC3 is the most stable complex out of the possible complexes that PC2, PC3, and GSH theoretically allow.
MATERIALS AND METHODS
Plant Culture and Extraction
An intact turf of As(V)-tolerant Holcus lanatus was obtained from As mine spoil and maintained in a greenhouse for 2 years before experiments commenced. Pteris cretica Mayii, an As hyperaccumulator (Meharg, 2003), was grown in John Innes potting compost no. 3 contaminated with 100 mg kg-1 dry weight As(V), added as NaH2AsO4, for over 1 year. Both plant species were grown in an unheated greenhouse with no supplemental lighting or heating. The soils were not fertilized.
For both plants, fresh cut leaves were ground within 2 h after harvest under liquid nitrogen and then extracted with 1% (v/v) formic acid. The grounded leaf suspension was shaken and than stored for 12 h at 1°C, filtered (0.45-μm syringe filter, Sulpelco, Dorset, UK), and analyzed for the As species at once. In another experiment, the intact plants were stored at -20°C for 1 month before extraction, or the extracts were stored at -20°C or 1°C.
Chemicals
All reagents used were of analytical grade or better quality. Deionized water (18 mΩ Elga Ltd., High Wycomb, Bucks, UK) was used throughout the experiments. As arsenic trioxide (As2O3) (BDH, Leics, UK), sodium arsenate (Na2HAsO3-7 H2O; BDH) and DMA(V) (Strem, Newburyport, MA) were used for the preparation of standard solutions and for the synthesis of the complexes. For the preparation of the mobile phase solutions, formic acid (100% [v/v] p.a., BDH) and methanol (p.a., Fisher, Loughborough, Leicestershire, UK) were used. A mixture of PCs, containing 36% (v/v) GSH, 49.7% (v/v) PC2, and 14.3% (v/v) PC3 (percentage calculated on a molar basis), used as a standard was a gift from Henk Schat (Vrije Universiteit, Amsterdam). The lyophilized PC mixture was dissolve in 1% (v/v) formic acid, and a portion was mixed with a surplus of As(III). Two plant samples were extracted and prepared for H. lanatus and 10 samples for P. cretica. Each extract was run in duplicate through the chromatographic procedure.
Analytical Methods
The HP1100 HPLC system (Agilent Technologies, Stockport, Cheshire, UK) with cooled auto-sampler and a Peltier controlled column compartment was used throughout the experiments. The column compartment was set to 30°C, and the auto-sampler was cooled to 4°C. The HPLC parameters used throughout the experiments were 1 mL min-1 flow for the mobile phase and 100 μL of sample volume. A Spherisorb S5 ODS2 column (250 × 4.6 mm) reverse-phase column (Waters, Elstree, Hertfordshire, UK) was used throughout the study because it had been shown to be suitable for separation of As(III)-glutathione complexes (Raab et al., 2004). A gradient of 1% (v/v) formic acid and methanol was used (0–20 min, 0%–13% [v/v] methanol; 20–30 min, 13%–0% [v/v] methanol; and 30–40 min, 0% [v/v] methanol). Post-column, the flow was split in a ratio of 1:5 (1 part into the ICP-MS, 5 parts into the ESI-MS) using a microsplitter (Upchurch, Luton, Bedfordshire, UK). The separation method was adapted from previous experiments with the As(III)-glutathione complex (Raab et al., 2004).
The HP1100 series LC/MSD instrument (Agilent Technologies, Stockport, Cheshire, UK) was used as a molecule-specific detector for post-column detection of the As-PC complexes by their molecular peaks, [M + H]+ or [M + 2H] 2+. The MSD was used in the positive ionization mode from m/z 120 to m/z 1,400 or in the single ion mode with the API-electrospray head. The settings chosen were: capillary voltage of 4,000 V, nebulizer pressure of 40 psi, drying gas flow of 12 L min-1 at 350°C, quadrupole temperature 100°C, and fragmenter voltage of 100 V for positive ionization mode. The mass calibration was controlled regularly and, when necessary, optimized using the calibration solution supplied by Agilent (m/z 112–2,233).
An ICP-MS 7500 (Agilent Technologies) was used for element-specific detection of As. The instrument was equipped with a microconcentric nebulizer (flow rate < 100 μL min-1), a Peltier cooled spray chamber, and oxygen as additional plasma gas. The instrument was used in the soft extraction mode with 2% (v/v) oxygen. The instrument settings were checked daily for As sensitivity and optimized when necessary. The elements monitored were As (m/z 75), tellurium (m/z 130), S (m/z 34), sulfur oxide (m/z 48), copper (m/z 63 and 65), zinc (m/z 64), and Cd (m/z 112).
Acknowledgments
Henk Schat, Vrije Universiteit, Amsterdam generously supplied the PC mixture used in this study.
Article, publication date, and citation information can be found at http://www.plantphysiol.org/cgi/doi/10.1104/pp.103.033506.
This work was supported by the Biotechnology and Biological Science Research Council (grant no. I/REI 18479) and by the College of Engineering and Physical Sciences, Aberdeen, Aberdeenshire, UK.
References
- Abedin MJ, Feldmann J, Meharg AA (2002) Uptake kinetics of arsenic species in rice (Oryza sativa L.) plants. Plant Physiol 128: 1120-1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae W, Mehra RK (1997) Metal-binding characteristics of a phytochelatin analog (Glu-Cys)(2)Gly. J Inorg Biochem 68: 201-210 [Google Scholar]
- Cobbett CS (2000) Phytochelatins and their roles in heavy metal detoxification. Plant Physiol 123: 825-832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbett C, Goldsbrough P (2002) Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu Rev Plant Biol 53: 159-182 [DOI] [PubMed] [Google Scholar]
- Cruz BH, Diaz-Cruz JM, Sestakova I, Velek J, Arino C, Esteban M (2002) Differential pulse voltammetric study of the complexation of Cd(II) by the phytochelatins (gamma-Glu-Cys)(2)Gly assisted by multivariate curve resolution. J Electroanal Chem 520: 111-118 [Google Scholar]
- Doreak V, Krezel A (2003) Correlation of acid-base chemistry of phytochelatins PC2 with its coordination properties towards the toxic metal ion Cd (II). Dalton Trans 11: 2253-2259 [Google Scholar]
- Grill E, Winnacker EL, Zenk MH (1985) Phytochelatins: the principal heavy-metal complexing peptide of higher plants. Science 230: 674-676 [DOI] [PubMed] [Google Scholar]
- Hartley-Whitaker J, Ainsworth G, Vooijs R, Ten Bookum W, Schat H, Meharg AA (2001) Phytochelatins are involved in differential arsenate tolerance in Holcus lanatus. Plant Physiol 126: 299-306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold I, Gunther D (1997) Investigation of the binding properties of heavy-metal-peptide complexes in plant cell cultures using HPLC-ICP-MS. Fresenius J Anal Chem 359: 364-370 [Google Scholar]
- Leopold I, Gunther D, Neumann D (1998) Application of high performance liquid chromatography: inductively coupled plasma mass spectrometry to the investigation of phytochelatins complexes and their role in heavy metal detoxification in plants. Analusis Mag 26: 28-33 [Google Scholar]
- Lombi E, Zhao F-J, Fuhrmann M, Ma LQ, McGrath SP (2002) Arsenic distribution and speciation in the fronds of the hyperaccumulator Pteris vittata. New Phytol 156: 195-203 [DOI] [PubMed] [Google Scholar]
- Maitani T, Kubota H, Sato K, Yamada T (1996) The composition of metals bound to class III metalothionein (phytochelatins and its desglycyl peptide) induced by various metals in root cultures of Rubia tinctorum. Plant Physiol 110: 1145-1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meharg AA (2003) Variation in arsenic accumulation: hyperaccumulation in ferns and their allies. New Phytol 157: 25-31 [DOI] [PubMed] [Google Scholar]
- Meharg AA, Hartley-Whitaker J (2002) Arsenic uptake and metabolism in arsenic resistant and non-resistant plant species: Tansley Review. New Phytol 154: 29-43 [Google Scholar]
- Meharg AA, Macnair MR (1992) Suppression of the phosphate uptake system: a mechanism of arsenate tolerance in Holcus lanatus L. J Exp Bot 43: 519-524 [Google Scholar]
- Mehra RK, Miclat J, Kodati R, Abdullah R, Hunter TC, Mulchandani P (1996a) Optical spectroscopic and reverse-phase HPLC analyses of Hg(II) binding to phytochelatins. Biochem J 314: 73-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra RK, Tran K, Scott GW, Mulchandani P, Saini SS (1996b) Ag(I): binding to phytochelatins. J Inorg Biochem 61: 125-142 [DOI] [PubMed] [Google Scholar]
- Pickering IJ, Prince RC, George MJ, Rauser WE, Wickramasinghe WA, Watson AA, Dameron CT, Dance IG, Fairlie DP, Salt DE (1999) X-ray absorption spectroscopy of cadmium phytochelatin and model systems. Biochem Biophys Acta 1429: 351-364 [DOI] [PubMed] [Google Scholar]
- Pickering IJ, Prince RC, George MJ, Smith RD, George GN, Salt DE (2000) Reduction and co-ordination of arsenic in Indian mustard. Plant Physiol 122: 1171-1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab A, Meharg AA, Jaspars M, Genney DR, Feldmann J (2004) Arsenic-glutathione complexes: their stability in solution and during separation by different HPLC modes. J Atomic Absorp Spec 19: 183-190 [Google Scholar]
- Salt DE, Rauser WE (1995) MgATP-dependent transport of phytochelatins across the tonoplast of oat roots. Plant Physiol 107: 1293-1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satofuka H, Fukui T, Takagi M, Atomi H, Imanaka T (2001) Metal-binding properties of phytochelatins-related peptides. J Inorg Biochem 86: 595-602 [DOI] [PubMed] [Google Scholar]
- Scarano G, Morelli E (2002) Characterization of cadmium- and lead-phytochelatins complexes formed in marine microalga in response to metal exposure. Biometals 15: 145-151 [DOI] [PubMed] [Google Scholar]
- Schmöger MEV, Oven M, Grill E (2000) Detoxification of arsenic by phytochelatins in plants. Plant Physiol 122: 793-801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneller FEC, van Heerwaarden LM, Kraaijeveld-Smit FJL, Ten Bookum WM, Koevoets PLM, Schat H, Verkleij JAC (1999) Toxicity of arsenate in Silene vulgaris, accumulation and degradation of arsenate-induced phytochelatins. New Phytol 144: 223-232 [Google Scholar]
- Vatamaniuk OK, Mari S, Lu YP, Rea PA (2000) Mechanism of heavy metal ion activation of phytochelatin (PC) synthase: blocked thiols are sufficient for PC synthase-catalyzed transpeptidation of glutathione and related thiol peptides. J Biol Chem 275: 31451-31459 [DOI] [PubMed] [Google Scholar]
- Wang J, Zhao F-J, Meharg AA, Raab A, Feldmann J, McGrath SP (2002) Mechanisms of arsenic hyperaccumulation in Pteris vittata: uptake kinetics, interactions with phosphate, and arsenic speciation. Plant Physiol 130: 1552-1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao FJ, Wang JR, Barker JHA, Schat H, Bleeker PM, McGrath SP (2003) The role of phytochelatins in arsenic tolerance in the hyperaccumulator Pteris vittata. New Phytol 159: 403-410 [DOI] [PubMed] [Google Scholar]