Abstract
Acclimation to changing environments, such as increases in light intensity, is necessary, especially for the survival of sedentary organisms like plants. To learn more about the importance of ascorbate in the acclimation of plants to high light (HL), vtc2, an ascorbate-deficient mutant of Arabidopsis, and the double mutants vtc2npq4 and vtc2npq1 were tested for growth in low light and HL and compared with the wild type. The vtc2 mutant has only 10% to 30% of wild-type levels of ascorbate, vtc2npq4 has lower ascorbate levels and lacks non-photochemical quenching of chlorophyll fluorescence (NPQ) because of the absence of the photosystem II protein PsbS, and vtc2npq1 is NPQ deficient and also lacks zeaxanthin in HL but has PsbS. All three genotypes were able to grow in HL and had wild-type levels of Lhcb1, cytochrome f, PsaF, and 2-cysteine peroxiredoxin. However, the mutants had lower electron transport and oxygen evolution rates and lower quantum efficiency of PSII compared with the wild type, implying that they experienced chronic photooxidative stress. The mutants lacking NPQ in addition to ascorbate were only slightly more affected than vtc2. All three mutants had higher glutathione levels than the wild type in HL, suggesting a possible compensation for the lower ascorbate content. These results demonstrate the importance of ascorbate for the long-term acclimation of plants to HL.
During the course of their life cycle, plants are exposed to a varying light environment, such as slow seasonal changes and a sudden increase in light intensity because of an opening in the leaf canopy. Plants have been evolving to cope with this changing light environment in a way that not only enables them to harvest light optimally but also to protect themselves from excess light. Excess absorbed light is dangerous to plants because it can lead to the enhanced production of reactive oxygen species (ROS), such as hydrogen peroxide (H2O2), superoxide, hydroxyl radicals, and singlet oxygen (Niyogi, 1999), which can damage many cellular components (Foyer, 1997), including PSII and PSI.
Much is already known about how plants acclimate to high light (HL). Important responses include a reduction in the size of the light-harvesting complex and an increase in the rate of photosynthesis, which correlates with increases in ATP synthase, electron transport components, and Calvin-Benson cycle enzymes (Anderson and Osmond, 1987; Walters and Horton, 1994). Other acclimation responses include changes in morphology. Leaves that have developed in HL (sun leaves) are generally thicker because of more cell layers with a higher leaf mass per unit area than leaves that have developed in low light (LL; shade leaves; Björkman, 1981; Pearcy, 1998).
One very fast response to an increased light intensity is non-photochemical quenching of chlorophyll (Chl) fluorescence (NPQ), which dissipates excess energy as heat (Müller et al., 2001). NPQ is composed of three parts: qT, qI, and qE. qT is caused by state transitions, and in vascular plants, it is generally the smallest component of NPQ. qI is photo-inhibitory quenching and can be distinguished from other forms of quenching by its very slow relaxation times. qE is the main component of NPQ in higher plants and is also called feedback de-excitation. qE is a thermal dissipation process that functions to minimize the production of triplet Chls and prevent overreduction of PSII electron acceptors (Li et al., 2002b). For qE to occur, there are three requirements: zeaxanthin, which is also increased in response to HL (Demmig et al., 1987; Demmig-Adams et al., 1989), a proton gradient across the thylakoid membrane (for review, see Müller et al., 2001), and the PSII protein PsbS (Li et al., 2000). Arabidopsis mutants affected in each of these requirements have been isolated. The npq1 mutant has a mutation in the violaxanthin de-epoxidase gene and, therefore, does not accumulate zeaxanthin in HL (Niyogi et al., 1998). The npq4-1 mutant lacks the PsbS protein (Li et al., 2000). Several mutants that are impaired in the production of the proton gradient have been isolated because of their low qE phenotype (Shikanai et al., 1999).
Antioxidants are molecules that function to reduce oxidative stress by scavenging or quenching ROS. The main antioxidants in the chloroplast are ascorbate (vitamin C) and glutathione, which are water-soluble, and α-tocopherol (vitamin E) and carotenoids, which are in the chloroplast membranes. Zeaxanthin and lutein have been shown through mutant studies in Arabidopsis and Chlamydomonas reinhardtii to be involved in the protection of the photosynthetic membrane (Niyogi et al., 1997, 1998, 2001; Havaux and Niyogi, 1999; Baroli et al., 2003). The small molecule antioxidants are supported by a network of antioxidant enzymes; for example, ascorbate peroxidase (APX), superoxide dismutase, and glutathione reductase, which are involved in utilizing and recycling antioxidants (Foyer, 1997). Many of these enzymes are found in several isoforms or with several cofactors. In general, genes encoding antioxidant enzymes are up-regulated when light stress occurs (Rossel et al., 2002).
Ascorbate is found in concentrations of up to 25 mm (Smirnoff, 2000a), and 30% to 40% of a plant cell's ascorbate is found in the chloroplast (Foyer et al., 1983). Ascorbate is important as a cofactor of violaxanthin de-epoxidase (Hager, 1969), for the synthesis of Hyp-rich glycoproteins in the cell wall, and for its role in the cell cycle (Smirnoff, 2000a). Until now, no viable plant that totally lacks ascorbate has been identified, possibly because many of the intermediates of the ascorbate biosynthesis pathway also have important functions (Lukowitz et al., 2001). However, Arabidopsis mutants with lower ascorbate content have been isolated (Conklin et al., 2000). The first two mutants, vtc1 (Conklin et al., 1996) and vtc2 (Jander et al., 2002), were isolated because of their sensitivity to ozone, whereas additional vtc mutants were isolated using a colorimetric assay for lowered ascorbate content (Conklin et al., 2000). All ascorbate mutants have been shown to have somewhat reduced NPQ levels (Noctor et al., 2000; Smirnoff, 2000b), probably because of substrate limitation of violaxanthin de-epoxidase, the enzyme that converts violaxanthin to zeaxanthin in conditions of excess light (Müller-Moulé et al., 2002). The vtc2 mutant, which accumulates only 10% to 30% of the wild-type level of ascorbate (Jander et al., 2002; Müller-Moulé et al., 2002), is also sensitive to HL and shows bleaching when transferred from LL to HL (Müller-Moulé et al., 2003).
We were interested in elucidating the importance of various photoprotective mechanisms for HL growth. In this paper, we studied the growth of a series of ascorbate-deficient single and double mutants under conditions of HL. The vtc2 single mutant only lacks ascorbate, the double mutant vtc2npq4 also lacks qE, and the double mutant vtc2npq1 lacks ascorbate, zeaxanthin, and qE. Here, we show that these ascorbate-deficient strains were not able to acclimate fully to HL and exhibited symptoms of chronic photooxidative stress, despite showing higher glutathione levels when grown in HL. The double mutants, on the other hand, were only slightly more affected than vtc2.
RESULTS
Ascorbate-Deficient Mutants Were Smaller and Had Fewer Leaves
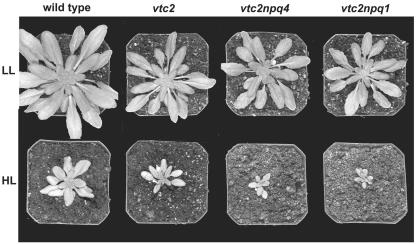
All genotypes matured much faster when grown in HL (1,800 μmol photons m-2 s-1) at a photon flux density (PFD) that was nearly equivalent to full sun-light. As shown in Figure 1, the HL-grown plants were only one-third of the size of plants grown in LL (180 μmol photons m-2 s-1) at the time of sampling. Because of the difference in flowering time, HL-grown plants were sampled at the age of 4 weeks, whereas LL-grown plants were sampled at the age of 6 weeks. HL-grown plants had thicker leaves than LL-grown plants because of additional cell layers and larger cells, but the ascorbate-deficient mutants all had thinner leaves than the wild type when grown in LL or in HL (data not shown).
Figure 1.
Images of wild-type and mutant plants grown in LL and HL. All plants were grown in a short-day photoperiod (10 h of light, 14 h of dark). LL plants were 6 weeks old, and HL plants were 4 weeks old.
All genotypes showed a decrease in rosette diameter and leaf number when grown in HL (Table I). Ascorbate-deficient mutants were 30% smaller than the wild type and had 20% fewer leaves in both light regimes (Table I). There was no difference in the number of leaves between vtc2, vtc2npq4, and vtc2npq1 in LL or HL, although there were small differences in the rosette diameter between the ascorbate-deficient mutants when grown in LL. Per leaf area, the leaf mass of HL-grown leaves was nearly twice that of LL-grown leaves (data not shown) because of higher leaf thickness. Stomatal index, which is known to be responsive to environmental effects on photosynthesis (Gray et al., 2000; Brownlee, 2001), was also higher for leaves of HL-grown plants of all genotypes but not different between the mutants and the wild type (Table I).
Table I.
Rosette diameter, leaf no., and stomatal indices of LL- and HL-grown plants Data shown are the means ± se (n = 38-57 for diameter and leaf no., n = 3 for stomatal index). The stomatal index (no. of stomata/total epidermal cells) combines both the adaxial and the abaxial leaf sides.
| Parameter | Wild Type | vtc2 | vtc2npq4 | vtc2npq1 |
|---|---|---|---|---|
| Rosette diameter (cm) in LL | 12.4 ± 0.4 | 8.4 ± 0.3 | 9.8 ± 0.4 | 9.1 ± 0.3 |
| Rosette diameter (cm) in HL | 4.3 ± 0.3 | 3.0 ± 0.2 | 3.5 ± 0.4 | 2.6 ± 0.2 |
| No. of leaves in LL | 42.8 ± 1.3 | 34.9 ± 1.2 | 34.7 ± 1.5 | 34.6 ± 1.5 |
| No. of leaves in HL | 20.4 ± 0.6 | 16.8 ± 0.6 | 16.3 ± 0.4 | 15.7 ± 0.5 |
| Stomatal index in LL | 0.25 ± 0.02 | 0.25 ± 0.04 | 0.21 ± 0.02 | 0.23 ± 0.03 |
| Stomatal index in HL | 0.38 ± 0.08 | 0.34 ± 0.02 | 0.35 ± 0.04 | 0.38 ± 0.04 |
Ascorbate-Deficient Mutants Exhibited Symptoms of Chronic Photooxidative Stress When Grown in HL
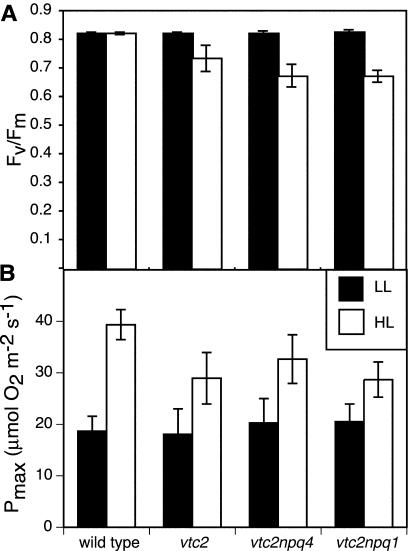
The maximum efficiency of PSII photochemistry was measured as the fluorescence parameter in the dark-adapted state Fv/Fm. In LL, all genotypes showed a value of 0.82, which is the typical value for uninhibited Arabidopsis plants. When grown in HL, wild-type plants maintained an Fv/Fm of 0.82, whereas all ascorbate-deficient mutants appeared to have a chronically lower PSII efficiency (Fig. 2A), with Fv/Fm values of 0.73 (vtc2) or 0.67 (vtc2npq4 and vtc2npq1).
Figure 2.
Photosynthesis parameters of LL- and HL-grown plants. A, Fv/Fm. Data are the means ± se (n = 6). B, Maximum oxygen evolution rate (Pmax). Oxygen evolution was measured at a saturating PFD of 1,790 μmol photons m-2 s-1 for HL-grown plants and 1,380 μmol photons m-2 s-1 for LL-grown plants. Data are the means ± sd (n = 4-6).
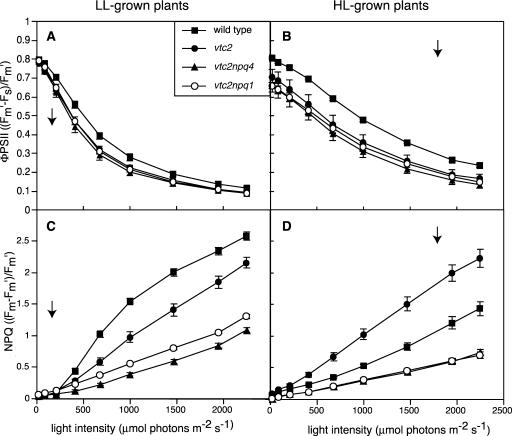
Similarly, photosynthesis rates in ascorbate-deficient mutants did not show full acclimation to HL. Photosynthesis rates were determined by two methods. We used oxygen evolution to determine the whole-chain electron transport activity and used Chl fluorescence measurements to determine the efficiency of PSII electron transport (ΦPSII) as a function of incident PFD. As can be seen in Figure 2B, all genotypes exhibited a higher Pmax when grown in HL. The Pmax for the wild type was 2-fold higher in HL- compared with LL-grown plants, whereas the ascorbate-deficient mutants only had a 1.6-fold higher rate when grown in HL compared with LL growth. The ΦPSII measured at all light intensities was also higher in HL-grown plants of all genotypes (Fig. 3, A and B). The Chl fluorescence analysis revealed a substantial difference between the HL-grown mutants and the wild type, consistent with the measurements of oxygen evolution. In LL-grown plants, there was a smaller difference in ΦPSII between the mutants and the wild type; however, there was no difference found between the four genotypes in terms of Pmax.
Figure 3.
Light response curves for Chl fluorescence parameters. Data are the means ± se (n = 6). A and B, ΦPSII. C and D, NPQ. Arrows indicate growth PFD of measured plants.
To learn more about the photoprotective response to HL, we also measured NPQ in LL- and HL-grown plants. As can be seen in Figure 3C, when grown in LL, wild-type plants exhibited the highest amount of NPQ (2.5), followed by the vtc2 mutant. Both double mutants reached NPQ levels of approximately 1 at the highest PFD, with the vtc2npq4 mutant having less NPQ than the vtc2npq1 mutant. The NPQ measured in the two double mutants is of a different type than that in the wild type or vtc2 because only state transitions (qT) and photo-inhibitory quenching (qI) are contributing to it, whereas most of the NPQ in the wild type and the vtc2 mutant is qE. HL-grown plants exhibited less NPQ, except for the vtc2 mutant that still had the same high levels as when grown in LL (Fig. 3D).
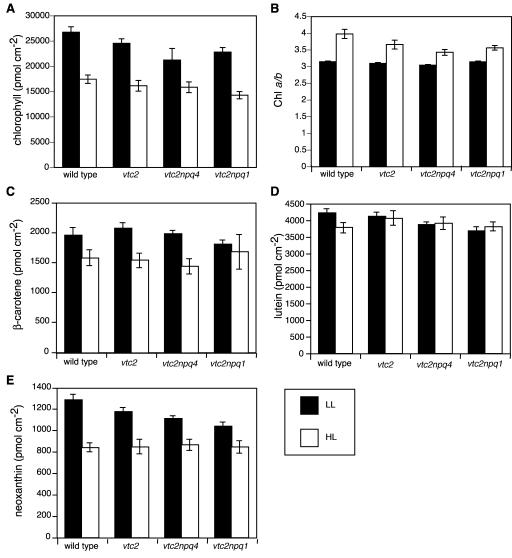
Ascorbate-Deficient Plants Had a Lower Pigment Content in HL
Total Chl per leaf area was 30% lower in HL-grown plants compared with LL in all four genotypes, with the two double mutants having less Chl in LL and HL than the wild type (Fig. 4A). In addition, the Chl a to b ratio was higher in all genotypes when grown in HL, but the ascorbate-deficient mutants had lower Chl a to b ratios than the wild type (Fig. 4B). β-Carotene levels were also lower in HL-grown plants when compared with LL-grown plants, except for the vtc2npq1 mutant, in which there were no differences (Fig. 4C). There was no difference in lutein content between LL- and HL-grown ascorbate-deficient mutants, but the wild type had slightly lower amounts of lutein when grown in HL (Fig. 4D). Neoxanthin levels were lower for the ascorbate-deficient mutants compared with the wild type when grown in LL but were the same when grown in HL (Fig. 4E). In comparison with LL-grown plants, the HL-grown plants had lower levels of neoxanthin.
Figure 4.
Pigment levels of LL- and HL-grown plants. Data are the means ± se (n = 12). A, Total Chl a and b. B, Chl a to b ratio. C, β-Carotene. D, Lutein. E, Neoxanthin.
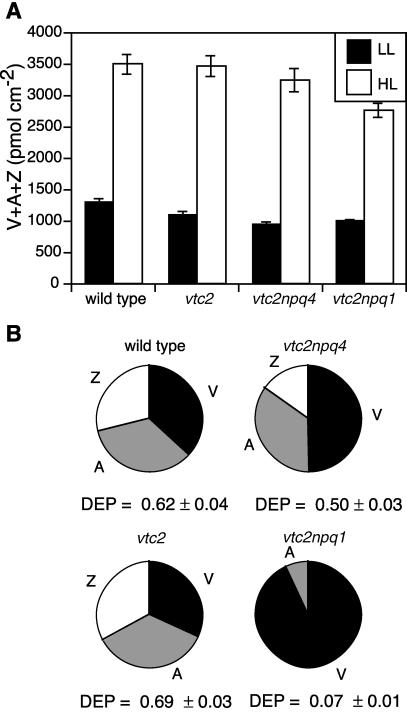
The xanthophyll cycle pool size was the same for HL-grown genotypes, except for vtc2npq1, which had slightly lower values, and it was about three times higher than in LL-grown plants (Fig. 5A). On the other hand, all ascorbate-deficient mutants had a somewhat smaller xanthophyll cycle pool than the wild type when grown in LL. In LL, the xanthophyll cycle pool was not much de-epoxidized in any of the four genotypes ([A + Z]/[V + A + Z] < 0.045; data not shown). When grown in HL, the vtc2 mutant had 69% of its pool de-epoxidized (Fig. 5B). Both the vtc2npq4 mutant and the wild type had only 50% and 62% of their pools de-epoxidized, respectively. The vtc2npq1 mutant, because of its impairment in violaxanthin de-epoxidase, had less than 10% of its pool in an unepoxidized state.
Figure 5.
Xanthophyll cycle pigments. Data are the means ± se (n = 12). A, Total xanthophyll cycle pool (V + A + Z). B, Composition of the xanthophyll cycle pool in HL. DEP, De-epoxidation state [(A + Z)/(V + A + Z)].
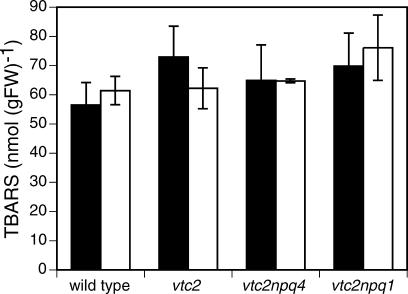
Plants Grown in HL Did Not Have Higher Lipid Peroxidation
Plants grown in HL showed the same degree of lipid peroxidation as did plants grown in LL (Fig. 6). Lipid peroxidation was measured as the amount of thiobarbituric acid-reactive substances (TBARS). The TBARS method has been shown to give a good estimate of lipid peroxidation and correlates well with other types of lipid peroxidation measurements, such as thermoluminescence (Müller-Moulé et al., 2003). Both LL- and HL-grown plants had levels of between 55 and 75 nmol malondialdehyde g fresh weight-1.
Figure 6.
Lipid peroxidation of LL- and HL-grown plants. Data are the means ± se (n = 12).
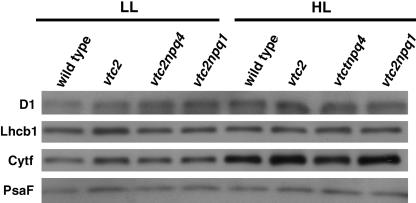
The Ascorbate-Deficient Mutants Had the Same Levels of D1, Lhcb1, Cytochrome f, and PsaF Compared with the Wild Type
To test if thylakoid protein complexes were affected in the ascorbate-deficient mutants, we tested levels of several proteins. Because photosynthetic function is dependent on Chl, we normalized protein levels based on Chl content. The photosynthetic electron transport chain is comprised of three major protein complexes: PSII, the cytochrome b6/f complex, and PSI. We wanted to test at least one protein of each complex to detect differences between LL- and HL-grown plants and between genotypes. The PSII reaction center protein D1 is known to be the primary target of photodamage (Aro et al., 1993) and, therefore, was interesting for analysis. Lhcb1 is a light-harvesting antenna protein of PSII, whose antenna generally is smaller in sun plants (Anderson and Osmond, 1987; Walters and Horton, 1994). The cytochrome f protein level generally increases in sun leaves (Anderson and Osmond, 1987; De la Torre and Burkey, 1990; Walters and Horton, 1994) and should correlate with the amount of the cytochrome b6/f complex in the membrane; similarly, the PsaF protein levels should correlate with PSI levels. LL-grown mutants had the same levels of all the tested proteins as the wild type (Fig. 7). The wild-type and the ascorbate-deficient mutants showed a major increase in the cytochrome f protein levels when grown in HL; however, there were no genotypic differences (Fig. 7). No major differences were found between genotypes or light treatment for the D1, Lhcb1, and the PsaF proteins (Fig. 7).
Figure 7.
Immunoblot analysis of photosynthetic proteins in LL- and HL-grown plants. Thylakoid samples were loaded on an equal Chl basis. Shown are representative blots from two experiments done.
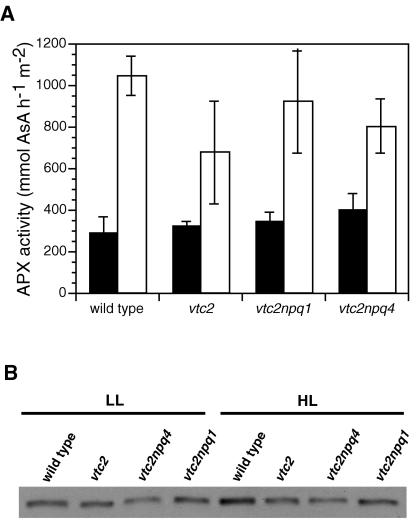
The Ascorbate-Deficient Mutants Had the Same APX Activity and 2-Cys Peroxiredoxin (2-CP) Protein Levels as the Wild Type
We also tested the activity of APX, which uses ascorbate to reduce hydrogen peroxide to water. APX is an important enzyme that is found in several compartments and replaces catalase in the chloroplast. Because there might be substrate limitation in the ascorbate-deficient mutants, we were interested to see if the ascorbate-deficient mutants were impaired in APX activity. However, there were no differences between the mutants in LL or HL, although the data were somewhat more variable in the HL-grown mutants (Fig. 8A).
Figure 8.
Antioxidant enzymes. A, APX activity. B, 2-CP. Total soluble protein samples were loaded on an equal protein basis.
2-CP is an important antioxidant enzyme that is found in the chloroplast and reduces lipid hydroperoxides (Baier and Dietz, 1999b). No difference in protein level was found between the genotypes in LL; however, the wild type had a somewhat higher level of 2-CP compared with the mutants when grown in HL (Fig. 8B).
The Ascorbate-Deficient Mutants Had Higher Glutathione Levels in HL
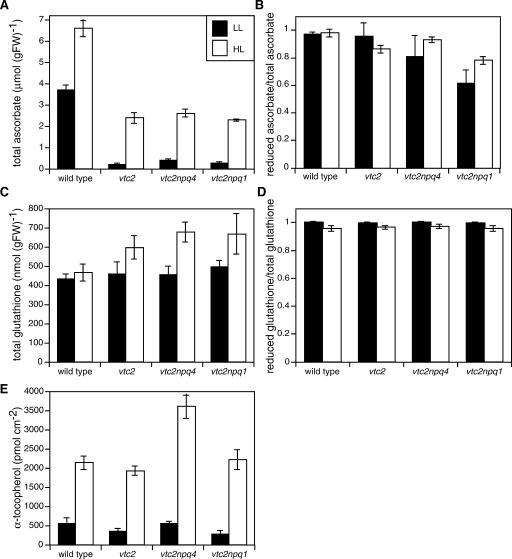
HL-grown plants had higher levels of antioxidants when compared with LL-grown plants. In the wild type, both ascorbate and α-tocopherol contents were higher in HL (Fig. 9, A and E), whereas the amount of glutathione was not changed (Fig. 9C). Also, the ascorbate redox state was the same in LL and HL (96% reduced; Fig. 9B), whereas the glutathione pool was slightly more oxidized in HL (Fig. 9D).
Figure 9.
Antioxidants in LL- and HL-grown plants. Data are the means ± se (n = 6 for A-D, n = 12 for E). A, Total ascorbate. B, Reduction state of the ascorbate pool. C, Total glutathione. D, Reduction state of the glutathione pool. E, α-Tocopherol.
In LL and HL, the ascorbate content of the ascorbate-deficient mutants was only 5% to 10% and 35% to 39%, respectively, of the wild-type value (Fig. 9A). Thus, the ascorbate level of the mutants was 7- to 14-fold higher in HL than in LL, compared with approximately 2-fold higher levels in the wild type. The redox state of ascorbate (reduced ascorbate/total ascorbate) was not different between the wild type and the mutants in LL except for vtc2npq1, which had a lower ratio of 0.6 (Fig. 9B). In HL, both vtc2 and vtc2npq1 had a lower redox state than the wild type. The vtc2npq4 double mutant had a somewhat higher ascorbate level in LL and a slightly higher ascorbate redox state in HL than the other mutants.
Total glutathione content was 30% higher in the ascorbate-deficient mutants when grown in HL (Fig. 9C), whereas there was no difference in the wild type. For all genotypes, there was a very small difference in the reduced to total glutathione ratio between LL- and HL-grown plants. When grown in HL, all four genotypes showed a slightly more oxidized pool (0.95 in HL versus 0.99 in LL).
In LL, the wild type and the vtc2npq4 had the same α-tocopherol levels, whereas vtc2 and vtc2npq1 had somewhat lower levels (Fig. 9E). When grown in HL, plants had approximately 4-fold higher α-tocopherol levels, and there were no clear differences between wild type, vtc2, and vtc2npq1. The vtc2npq4 mutant, on the other hand, had 7-fold higher α-tocopherol levels when grown in HL.
DISCUSSION
Ascorbate-deficient mutants were able to grow to maturity at a PFD of 1,800 μmol photons m-2 s-1. No visible signs of damage were apparent at this HL intensity. The ascorbate-deficient mutants had similar levels of representative thylakoid proteins (Fig. 7) and did not bleach, produce anthocyanins, or show lipid peroxidation (Figs. 1 and 6), although they exhibited symptoms of photooxidative stress such as lower Fv/Fm (Fig. 2A), Pmax (Fig. 2B), and ΦPSII (Fig. 3B). The growth in HL was surprising because under standard LL conditions, the vtc2 mutants have been shown to have 10% to 25% of the wild-type ascorbate content (Conklin et al., 2000; Müller-Moulé et al., 2002). Because ascorbate is one of the major antioxidants in plant cells, one might have expected to see a more negative effect on growth in HL, maybe even lethality. Transfer of ascorbate-deficient mutants from LL to HL results in dramatic bleaching and lipid peroxidation (Müller-Moulé et al., 2003).
When grown in HL, however, both the wild-type and the ascorbate-deficient mutants had higher ascorbate levels than in LL. In fact, the levels of ascorbate increased relatively more in the mutants than in the wild type, so that the HL-grown mutants had approximately 40% of the wild-type ascorbate levels (Fig. 9A). Thus, we conclude that an ascorbate content of 10% or 40% of the wild type is enough for Arabidopsis plants to survive when grown in LL or HL, respectively.
The relatively higher ascorbate content of the HL-grown mutants could be because of an increased ascorbate synthesis. The mutants exhibit lower Fv/Fm values in HL and might experience higher photooxidative stress than the wild type when grown in HL, which might cause a stronger acclimation response in regard to producing more antioxidants. The relatively higher ascorbate levels could also be because of developmental variation in ascorbate content in the mutants. For example, the ascorbate content of LL-grown vtc2 mutants decreases from 30% to 10% of wild-type levels in “mature” leaves between 2 and 6 weeks of age (Conklin et al., 2000).
However, how is it possible that less than one-half the wild-type ascorbate levels is sufficient for growth in HL? One likely explanation is that the ascorbate-deficient plants compensated for the lack of ascorbate by increasing other photoprotective mechanisms or the level of other antioxidants. First of all, ascorbate-deficient mutants showed an increase in another water-soluble antioxidant, glutathione, which had 30% higher levels in HL than the wild type (Fig. 9C). The increase in glutathione provides evidence for overlap in the antioxidant functions of ascorbate and glutathione in vivo. In contrast, there were no differences in the levels of the lipid-soluble antioxidants, carotenoids (Figs. 4 and 5) and α-tocopherol (Fig. 9E), except for a smaller xanthophyll cycle pool size in the vtc2npq1 mutant and higher α-tocopherol levels in vtc2npq4 (Fig. 9E). There were also no differences in the antioxidant enzymes APX and 2-CP (Fig. 8).
Even though the ascorbate-deficient mutants were able to grow in HL, they did not show a full acclimation to it as compared with the wild type. There were signs of photooxidative stress and impairment of cell components when the mutants were grown in HL. Mutant plants, except vtc2npq4, had a more oxidized ascorbate pool when grown in HL. The vtc2npq4 mutant also had a higher total antioxidant content in HL than the vtc2 and vtc2npq1 mutants because of a higher α-tocopherol content (Fig. 9E). Higher levels of α-tocopherol and a more reduced ascorbate pool were observed in the npq4 single mutant grown under identical HL conditions (T. Golan, P. Müller-Moulé, and K.K. Niyogi, unpublished data). Although in LL the mutants had the same Fv/Fm and Pmax as the wild type and only a small difference in ΦPSII, these photosynthetic parameters were all lower than in the wild type in HL (Figs. 2 and 3, A and B). Unexpectedly, the growth of the two double mutants was not affected much more by the HL than the growth of vtc2. Both double mutants had somewhat lower Fv/Fm values than vtc2 (Fig. 2A). Also, the vtc2npq1 mutant also had a lower xanthophyll cycle pool size (Fig. 5A) and a lower ascorbate redox state (Fig. 9B). This shows that the lack of qE or of zeaxanthin did have an effect in HL, but the effect was relatively minor in comparison with the lack of ascorbate, and the double mutants could still grow in HL despite their deficiencies as described above.
An increased need for dissipation of excess energy because of the lowered ΦPSII was apparent in the vtc2 mutant. The vtc2 mutant had the same amount of NPQ at different PFDs, irrespective of growth in HL or LL, whereas the wild type had lower NPQ when grown in HL (Fig. 3, C and D). Fitting with this observation is the higher de-epoxidation state in vtc2 compared with the wild type (Fig. 5B).
It is somewhat surprising that the mutants did not have the same or even a higher Pmax and ΦPSII than the wild type because increased electron transport would provide a means of channeling excess excitation energy into harmless or productive paths. Therefore, it is likely that photosynthesis in vtc2 plants is limited by other factors; for example, Rubisco. A recent publication on vtc1 showed that this mutant had a reduction of about 20% in rbcL transcripts, Rubisco protein, and total activity compared with the wild type grown at 200 μmol photons m-2 s-1 (Pastori et al., 2003). It is also known that some of the Calvin cycle enzymes such as glyceraldehyde-3-P-dehydrogenase, Fru-1,6-bisphosphatase, and Rubisco can be inhibited by ROS, which would cause a lowering of the Calvin cycle activity and, hence, photosynthesis (Kaiser, 1979; Mehta et al., 1992; Garcia-Ferris and Moreno, 1994).
It is also possible that there was incomplete acclimation to HL in the mutants because of a defect in a signaling pathway, which might involve ascorbate. Both the redox states of ascorbate and glutathione might play a role in signaling HL stress (Potters et al., 2002). It is also possible that the VTC2 gene product itself might have a signaling function, although the mutant was able to acclimate to HL in terms of relative ascorbate content. The vtc2-1 mutant used in this study has a mutation in the putative gene At4g26850, which changes the 3′ splice site of the predicted intron 5 (Jander et al., 2002). It is still unknown what function the vtc2 gene has (Jander et al., 2002), and neither biosynthetic nor regulatory function can be excluded.
In both light conditions, the ascorbate-deficient mutants had the same stomatal index as the wild type, fewer and thinner leaves with more cell layers, and a smaller rosette diameter than the wild type (Table I; Fig. 1). Because leaf thickening is a characteristic feature of HL acclimation in the wild type, it is possible that the failure of the mutants to make this morphological change contributed to the symptoms of photooxidative stress that were observed. In LL, however, the decreased growth of ascorbate-deficient mutants does not seem to be attributable to increased oxidative stress, because in previous studies vtc1 displayed slower shoot growth even in high CO2 where oxidative stress is minimized (Veljovic-Jovanovic et al., 2001). Rather, the negative effect on growth and the difference in cell morphology is likely because of the role of ascorbate in cell elongation and division (Smirnoff, 2000a) or because of a change in hormone content. The ascorbate-deficient mutant vtc1 has a higher amount of abscisic acid, which is a hormone involved in growth regulation (Pastori et al., 2003).
To summarize, we have shown that ascorbate-deficient mutants are able to grow in HL. This likely is because of compensation by other antioxidants and photoprotective mechanisms, when plants were grown in HL from seedling age on. The mutants had higher glutathione levels than the wild type and possibly other increased protective mechanisms not considered here. Also, the increased photosynthetic rate combined with the smaller antenna size might have lowered the amount of excess light plants were actually experiencing, thereby reducing the amount of ROS produced. When plants were transferred from LL to HL, ascorbate-deficient mutants showed a much higher sensitivity to HL (Müller-Moulé et al., 2003) because of an increased need for scavenging of ROS. In conclusion, it is clear that ascorbate plays an important role in the acclimation of plants to HL.
MATERIALS AND METHODS
Plant Material and Growth Conditions
All genotypes used were of the Arabidopsis ecotype Columbia-0. The two double mutants were constructed by crossing a twice-backcrossed vtc2-1 with a four times-backcrossed npq1-2 (vtc2npq1) or with a four times-backcrossed npq4-1 (vtc2npq4). The double mutants were identified based on measurements of NPQ, ascorbate content, and lack of either zeaxanthin (vtc2npq1) or the PsbS protein (vtc2npq4). The plants were grown in a 10-h-light (20°C)/14-h-dark (18°C) cycle with a PFD of 180 μmol photons m-2 s-1 (LL) or a PFD of 1,800 μmol photons m-2 s-1 (HL) in the same growth chamber with high-intensity discharge lighting (E15, Conviron, Winnipeg, MN, Canada). Measurements were performed on rosette leaves from plants at the same developmental stage (before the induction of flowering), which corresponded to an age of 6 weeks for LL-grown plants and 4 weeks for HL-grown plants. Samples were taken 4 h into the light period except for the fluorescence measurements. Leaf temperature was 21.1°C ± 0.5°C in LL and 24.9°C ± 1.4°C in HL.
Fluorescence Measurements
Standard modulated Chl fluorescence measurements (for review, see Maxwell and Johnson, 2000) were performed using overnight dark-adapted plants and an FMS2 instrument (Hansatech, King's Lynn, UK). A total of six samples (three independently grown sets of plants with two samples each) were measured. Dark-adapted plants were subjected to a saturating light pulse and then illuminated during a programmed series of 5-min periods of increasing light intensities. Between steps in actinic PFD, the minimum fluorescence in the light-adapted state (Fo′) was determined during a 1-s period of far-red illumination. NPQ was calculated as (Fm - Fm′)/Fm′ and ΦPSII as (Fm′ - Fs)/Fm′.
Quantification of Pigments and Tocopherols
A total of 12 samples (three independently grown sets of plants with four samples each) were measured by HPLC as previously described by Müller-Moulé et al. (2002). The results were normalized to leaf area to allow comparison with previously published studies.
Ascorbate Assay
Total and reduced ascorbate were determined by a spectrophotometric method using the absorption at 265 nm by reduced ascorbate (Conklin et al., 1996). Six samples total from two sets (HL-grown plants) or one set (LL-grown plants) were measured.
Glutathione Assay
Glutathione was determined by HPLC followed by electrochemical detection (ESA Coularray, ESA Inc., Chelmsford, MA). Leaf disc samples (six total from two sets; HL-grown plants) or one set (LL-grown plants) were taken and immediately frozen in liquid nitrogen. The frozen discs were ground to a fine powder and extracted with 300 μL of 2% (w/v) metaphosphoric acid/2 mm EDTA, followed by 30 s of vortexing. The extract was centrifuged at 15,300g for 3 min, and 20 μL of the filtered supernatant was subjected to HPLC and separated on a TSK-GEL ODS-80Tm column (XXXX, XXXX, XX) at 30°C with a flow rate of 1 mL min-1 for 20 min. The mobile phase consisted of 0.2 m potassium phosphate (pH 3), 100 mg L-1 1-pentanesulfonic acid, and 1% (w/v) acetonitrile. The eight channels of the electrochemical detector were set at -200, -100, 0, +450, +50, +750, +880, and +900 mV. GSH was detected at +650 and +750 mV with +650 mV being the major channel. Oxidized glutathione was detected at +650, +750, and 880 mV with +750 mV being the major channel.
Lipid Peroxidation Measurements
Lipid peroxidation was determined as the level of TBARS (Hodges et al., 1999), and data were expressed on a fresh weight basis. A total of 12 samples (three independently grown sets of plants with four samples each) were measured.
Oxygen Evolution Measurements
Oxygen evolution was measured in detached leaves with a leaf disc electrode system (LD2/2, Hansatech) in response to a series of increasing PFDs at saturating CO2. Seven leaf discs from different plants were used for each measurement for the LL-grown plants. For the HL-grown plants, seven whole leaves from different plants were used. For normalization of data for the HL-grown plants, leaf area was determined using the NIH Image program (National Institutes of Health, Bethesda, MD). The saturating PFD for oxygen evolution was determined for plants grow under each light condition by a light response curve, and the maximum rate of oxygen evolution was determined at a saturating PFD (1,380 μmol photons m-2 s-1 for LL-grown plants and 1,790 μmol photons m-2 s-1 for HL-grown plants). The measurements were repeated four to six times with plant samples from one set.
Immunoblot Analysis
Leaves were harvested and frozen immediately in liquid nitrogen. Thylakoids were isolated from these leaves as described before (Li et al., 2002a), and Chl concentrations were determined. For the detection of photosynthetic proteins, thylakoids were dissolved in solubilization buffer (250 mm Tris-HCl [pH 6.8], 3.5% [w/v] SDS, 10% [v/v] glycerol, 1 m urea, and 5% [v/v] beta-mercaptoethanol), and samples were loaded on a Chl basis (0.5 nmol Chl lane-1). For the detection of 2-CP, young developing leaves were used because 2-CP accumulates over time in older leaves (Baier and Dietz, 1999a). Total soluble proteins were extracted as described (Baier and Dietz, 1999a), and because these extracts did not contain Chl, they were loaded on a protein basis (10 μg protein lane-1). All protein samples were loaded on precast polyacrylamide gels (10%-20% [w/v] gradient) with Tris-Gly buffer (NOVEX, San Diego). Proteins were blotted to a polyvinylidene difluoride membrane and detected using an enhanced chemiluminescence western blotting kit (Amersham Pharmacia Biotech, Piscataway, NJ). Antibody binding and detection were performed simultaneously on the LL and HL samples, which were on two different membranes. Antibodies against the following proteins were used: D1 (kindly provided by Prof. Anastasios Melis, University of California, Berkeley, CA), Lhcb1 (kindly provided by Prof. Stefan Jansson, Umeå University, Umeå, Sweden), cytochrome f (kindly provided by Prof. Richard Malkin, University of California, Berkeley, CA), PsaF (kindly provided by Prof. Anna Haldrup, The Royal Veterinary and Agricultural University, Frederiksburg, Denmark), and 2-CP (kindly provided by Dr. Margarete Baier, and Prof. Karl-Josef Dietz, University of Bielefeld, Bielefeld, Germany).
APX Activity Measurements
Two leaf discs were taken and immediately frozen in liquid nitrogen. The leaf discs were ground to a fine powder in 1.5 mL of extraction buffer using a mortar and pestle. A previously described APX assay was used (Grace and Logan, 1996), but 1.3 mm ascorbate were added to the extraction buffer, and the assay was done as a plate assay using a SPECTRAmax PLUS microplate spectrophotometer (Molecular Devices, Sunnyvale, CA). Eight samples were measured simultaneously with nonspecific rates measured in neighboring wells. A total of three samples were measured for each time point.
Acknowledgments
We thank Barry Logan for helping with the APX assay and Anastasios Melis, Andrew Staehelin, Stefan Jansson, Richard Malkin, Anna Haldrup, Margarete Baier, and Karl-Josef Dietz for providing antibodies. We also thank Xiao-Ping Li for critical reading of this manuscript.
Article, publication date, and citation information can be found at http://www.plantphysiol.org/cgi/doi/10.1104/pp.103.032375.
This work was supported by the U.S. Department of Agriculture National Research Initiative (grant no. 98-35306-6600) and by the Director, Office of Science, Office of Basic Energy Sciences, Chemical Sciences Division, of the U.S. Department of Energy (contract no. DE-AC03-765F00098).
References
- Anderson JM, Osmond CB (1987) Shade-sun responses: compromises between acclimation and photoinhibition. In DJ Kyle, CB Osmond, CJ Arntzen, eds, Photoinhibition, Vol 9. Elsevier, Amsterdam, pp 1-38 [Google Scholar]
- Aro E-M, Virgin I, Andersson B (1993) Photoinhibition of photosystem II: inactivation, protein damage and turnover. Biochim Biophys Acta 1143: 113-134 [DOI] [PubMed] [Google Scholar]
- Baier M, Dietz K-J (1999a) Protective function of chloroplast 2-cysteine peroxiredoxin in photosynthesis: evidence from transgenic Arabidopsis. Plant Physiol 119: 1407-1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier M, Dietz K-J (1999b) Alkyl hydroperoxide reductases: the way out of the oxidative breakdown of lipids in chloroplasts. Trends Plant Sci 4: 166-168 [DOI] [PubMed] [Google Scholar]
- Baroli I, Do AD, Yamane T, Niyogi KK (2003) Zeaxanthin accumulation in the absence of a functional xanthophyll cycle protects Chlamydomonas reinhardtii from photooxidative stress. Plant Cell 15: 992-1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkman O (1981) Responses to different quantum flux densities. In O Lange, Nobel PS, Osmond CB, Ziegler H, eds, Physiological Plant Ecology I: Responses to the Physical Environment. Springer-Verlag, Berlin, pp 57-107
- Brownlee C (2001) The long and short of stomatal density signals. Trends Plant Sci 6: 441-442 [DOI] [PubMed] [Google Scholar]
- Conklin PL, Saracco SA, Norris SR, Last RL (2000) Identification of ascorbic acid-deficient Arabidopsis thaliana mutants. Genetics 154: 847-856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin PL, Williams EH, Last RL (1996) Environmental stress sensitivity of an ascorbic acid-deficient Arabidopsis mutant. Proc Natl Acad Sci USA 93: 9970-9974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Torre W, Burkey K (1990) Acclimation of barley to changes in light intensity: photosynthetic electron transport activity and components. Photosynth Res 24: 127-136 [DOI] [PubMed] [Google Scholar]
- Demmig B, Winter K, Krueger A, Czygan F-C (1987) Photoinhibition and zeaxanthin formation in intact leaves. Plant Physiol 84: 218-224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig-Adams B, Winter K, Krueger A, Czygan F-C (1989) Light response of carbon dioxide assimilation dissipation of excess excitation energy and zeaxanthin content of sun and shade leaves. Plant Physiol 90: 881-886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer C, Rowell J, Walker D (1983) Measurement of ascorbate content of spinach leaf protoplasts and chloroplasts during illumination. Planta 157: 239-244 [DOI] [PubMed] [Google Scholar]
- Foyer CH (1997) Oxygen metabolism and electron transport in photosynthesis. In JG Scandalios, ed, Oxidative Stress and the Molecular Biology of Antioxidant Defenses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp 587-621
- Garcia-Ferris C, Moreno J (1994) Oxidative modification and breakdown of ribulose-1,5-bisphosphate carboxylase/oxygenase induced in Euglena gracilis by nitrogen starvation. Planta 193: 208-215 [Google Scholar]
- Grace SC, Logan BA (1996) Acclimation of foliar antioxidant systems to growth irradiance in three broad-leaved evergreen species. Plant Physiol 112: 1631-1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JE, Holroyd GH, van der Lee FM, Bahrami AR, Sijmons PC, Woodward FI, Schuch W, Hetherington AM (2000) The HIC signalling pathway links CO2 perception to stomatal development. Nature 408: 713-716 [DOI] [PubMed] [Google Scholar]
- Hager A (1969) Lichtbedingte pH-erniedrung in einem chloroplasten-kompartiment als ursache der enzymatischen violaxanthin-zeaxanthin-umwandlung; beziehung zur photophosphorylierung. Planta 89: 224-243 [DOI] [PubMed] [Google Scholar]
- Havaux M, Niyogi KK (1999) The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism. Proc Natl Acad Sci USA 96: 8762-8767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges DM, DeLong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207: 604-611 [DOI] [PubMed] [Google Scholar]
- Jander G, Norris SR, Rounsley SD, Bush DF, Levin IM, Last RL (2002) Arabidopsis map-based cloning in the post-genome era. Plant Physiol 129: 440-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser W (1979) Reversible inhibition of the Calvin cycle and activation of oxidative pentose phosphate cycle in isolated chloroplasts by hydrogen peroxide. Planta 145: 377-382 [DOI] [PubMed] [Google Scholar]
- Li X-P, Björkman O, Shih C, Grossman AR, Rosenquist M, Jansson S, Niyogi KK (2000) A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 403: 391-395 [DOI] [PubMed] [Google Scholar]
- Li X-P, Müller-Moulé P, Gilmore AM, Niyogi KK (2002b) PsbS-dependent enhancement of feedback de-excitation protects photosystem II from photoinhibition. Proc Natl Acad Sci USA 99: 15222-15227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X-P, Phippard A, Pasari J, Niyogi KK (2002a) Structure-function analysis of photosystem II subunit S (PsbS) in vivo. Funct Plant Biol 29: 1131-1139 [DOI] [PubMed] [Google Scholar]
- Lukowitz W, Nickle TC, Meinke DW, Last RL, Conklin PL, Somerville CR (2001) Arabidopsis cyt1 mutants are deficient in a mannose-1-phosphate guanylyltransferase and point to a requirement of N-linked glycosylation for cellulose biosynthesis. Proc Natl Acad Sci USA 98: 2262-2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell K, Johnson GN (2000) Chlorophyll fluorescence: a practical guide. J Exp Bot 51: 659-668 [DOI] [PubMed] [Google Scholar]
- Mehta RA, Fawcett TW, Porath D, Mattoo AK (1992) Oxidative stress causes rapid membrane translocation and in-vivo degradation of ribulose-1,5-bisphosphate carboxylase-oxygenase. J Biol Chem 267: 2810-2816 [PubMed] [Google Scholar]
- Müller P, Li X-P, Niyogi KK (2001) Non-photochemical quenching: a response to excess light energy. Plant Physiol 125: 1558-1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Moulé P, Conklin PL, Niyogi KK (2002) Ascorbate deficiency can limit violaxanthin de-epoxidase activity in vivo. Plant Physiol 128: 970-977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Moulé P, Havaux M, Niyogi KK (2003) Zeaxanthin deficiency enhances the high light sensitivity of an ascorbate-deficient mutant of Arabidopsis. Plant Physiol 133: 748-760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi KK (1999) Photoprotection revisited: genetic and molecular approaches. Annu Rev Plant Physiol Plant Mol Biol 50: 333-359 [DOI] [PubMed] [Google Scholar]
- Niyogi KK, Björkman O, Grossman AR (1997) The roles of specific xanthophylls in photoprotection. Proc Natl Acad Sci USA 94: 14162-14167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi KK, Grossman AR, Björkman O (1998) Arabidopsis mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy conversion. Plant Cell 10: 1121-1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi KK, Shih C, Chow WS, Pgoson BJ, DellaPenna D, Björkman O (2001) Photoprotection in a zeaxanthin- and lutein-deficient double mutant of Arabidopsis. Photosynth Res 67: 139-145 [DOI] [PubMed] [Google Scholar]
- Noctor G, Veljovic-Jovanovic S, Foyer CH (2000) Peroxide processing in photosynthesis: antioxidant coupling and redox signalling. Philos Trans R Soc Lond B Biol Sci 355: 1465-1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastori GM, Kiddle G, Antoniw J, Bernard S, Veljvic-Jovanovic S, Verrier PJ, Noctor G, Foyer CH (2003) Leaf vitamin C contents modulate plant defense transcripts and regulate genes that control development through hormone signaling. Plant Cell 15: 939-951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearcy RW (1998) Acclimation to sun and shade. In A Raghavendra, ed, Photosynthesis. Cambridge University Press, Cambridge, UK
- Potters G, De Gara L, Asard H, Horemans N (2002) Ascorbate and glutathione: guardians of the cell cycle, partners in crime? Plant Physiol Biochem 40: 537-548 [Google Scholar]
- Rossel JB, Wilson IW, Pogson BJ (2002) Global changes in gene expression in response to high light in Arabidopsis. Plant Physiol 130: 1109-1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikanai T, Munekage Y, Shimizu K, Endo T, Hashimoto T (1999) Identification and characterization of Arabidopsis mutants with reduced quenching of chlorophyll fluorescence. Plant Cell Physiol 40: 1134-1142 [DOI] [PubMed] [Google Scholar]
- Smirnoff N (2000a) Ascorbic acid: metabolism and functions of a multi-facetted molecule. Curr Opin Plant Biol 3: 229-235 [PubMed] [Google Scholar]
- Smirnoff N (2000b) Ascorbate biosynthesis and function in photoprotection. Philos Trans R Soc Lond B Biol Sci 355: 1455-1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veljovic-Jovanovic SD, Pignocchi C, Noctor G, Foyer CH (2001) Low ascorbic acid in the vtc-1 mutant of Arabidopsis is associated with decreased growth and intracellular redistribution of the antioxidant system. Plant Physiol 127: 426-435 [PMC free article] [PubMed] [Google Scholar]
- Walters RG, Horton P (1994) Acclimation of Arabidopsis thaliana to the light environment: changes in composition of the photosynthetic apparatus. Planta 195: 248-256 [DOI] [PubMed] [Google Scholar]