Abstract
Background:
Advances in the care of patients with cystic fibrosis (CF) have improved pulmonary outcomes and survival. In addition, rapid developments regarding the underlying genetic and molecular basis of the disease have led to numerous novel targets for treatment. However, clinical and basic scientific research focusing on therapeutic strategies for CF-associated chronic rhinosinusitis (CRS) lags behind the evidence-based approaches currently used for pulmonary disease.
Methods:
This review evaluates the available literature and provides an update concerning the pathophysiology, current treatment approaches, and future pharmaceutical tactics in the management of CRS in patients with CF.
Results:
Optimal medical and surgical strategies for CF CRS are lacking because of a dearth of well-performed clinical trials. Medical and surgical interventions are supported primarily by level 2 or 3 evidence and are aimed at improving clearance of mucus, infection, and inflammation. A number of novel therapeutics that target the basic defect in the cystic fibrosis transmembrane conductance regulator channel are currently under investigation. Ivacaftor, a corrector of the G551D mutation, was recently approved by the Food and Drug Administration. However, sinonasal outcomes using this and other novel drugs are pending.
Conclusion:
CRS is a lifelong disease in CF patients that can lead to substantial morbidity and decreased quality of life. A multidisciplinary approach will be necessary to develop consistent and evidence-based treatment paradigms.
Keywords: Ataluren, CFTR, chloride secretion, chronic sinusitis, cystic fibrosis, dornase alpha, endoscopic medial maxillectomy, functional endoscopic sinus surgery, ivacaftor, lumacaftor, macrolide, mucociliary clearance, Pseudomonas, PTC124, rhinosinusitis, saline irrigation, sinus surgery, transepithelial ion transport, VX-770, VX-809
Cystic fibrosis (CF) is an autosomal recessive disorder that affects the upper and lower airways as well as the digestive system. It is considered the most lethal autosomal recessive disorder among Caucasians and is estimated to affect 1 in 2000 to 1 in 6000 births.1 Thirty years ago this disease regularly led to death in the first decade of life, usually secondary to pulmonary deterioration from opportunistic bacteria. Advancements in therapy have led to substantial improvements in survival with a current median life expectancy of 36.8 years.2 The underlying genetic basis of the disease is related to dysfunction or deficiency of the CF transmembrane conductance regulator (CFTR), an apical membrane anion (e.g., chloride and bicarbonate) channel present in respiratory and exocrine glandular epithelium.3,4 Early diagnosis of CF is crucial to allow for intervention before lung disease ensues.5
Although chronic rhinosinusitis (CRS) is a serious cause of morbidity and may drive pulmonary disease in patients with CF, it is rarely the cause of mortality related to the disease. Sinonasal symptoms can be severe and refractory despite a myriad of medical and surgical interventions leading to frustration for the patient. Quality of life indicators have shown that sinus disease often mirrors pulmonary function and can be predictive of pulmonary disease, particularly in the pediatric population.6 Additionally, few randomized controlled trials are currently available with regard to efficacy of therapies for CF CRS and many studies are limited by the lack of long-term follow-up.7–9 Evidence-based guidelines are deficient and management paradigms concerning disease interventions have not been standardized for all patients.
The purpose of the current review is to provide an update regarding management of this unique CRS population, present a summary of the best available evidence for therapeutic interventions, and discuss exciting new strategies in drug development.
GENETICS
Over 1600 mutations have been described in the coding sequence of the CFTR gene, messenger RNA splice signals, and other regions.10 Mutations are generally classified into six categories according to the mechanistic basis by which they are believed to cause disease (Table 1). The first three classes (I–III) are generally associated with increased phenotypic severity. Class I mutations result in an absence of CFTR gene synthesis and develop secondary to premature termination codons, nonsense mutations (e.g., G542X mutation, the “X” referring to existence of a premature stop codon), or other out of frame mutations (insertions or deletions).11 CFTR is normally transcribed and translated in class II mutations, but the protein folds incorrectly and is recognized as defective in the endoplasmic reticulum during intracellular trafficking. The protein is degraded before it reaches the site of action at the cell surface. The class II F508del mutation (deletion of a phenylalanine residue at the 508 position) is the most common genetic mutation and accounts for ∼70% of defective alleles.12 Class III mutations consist of full-length CFTR protein present in normal quantities at the cell surface, but disrupted regulation or gating of the chloride transporter leads to a lack of ion channel activity (e.g., G551D mutation).13 Although class III mutations possess minimal to no CFTR-dependent transport, class IV defects represent abnormalities of chloride conductance and may have partial activity in vivo. Such mutations may lead to a CF pulmonary phenotype that is less severe than other forms of the disease (e.g., R117H mutation).14 Class V mutations produce decreased quantities of CFTR transcripts and, thus, fewer functional CFTR channels at the cell surface.11,12 Finally, class VI mutations create defects in the stability of the protein leading to accelerated turnover at the cell surface and insufficient quantities of CFTR under steady-state conditions.15
Table 1.
CF genetic mutations
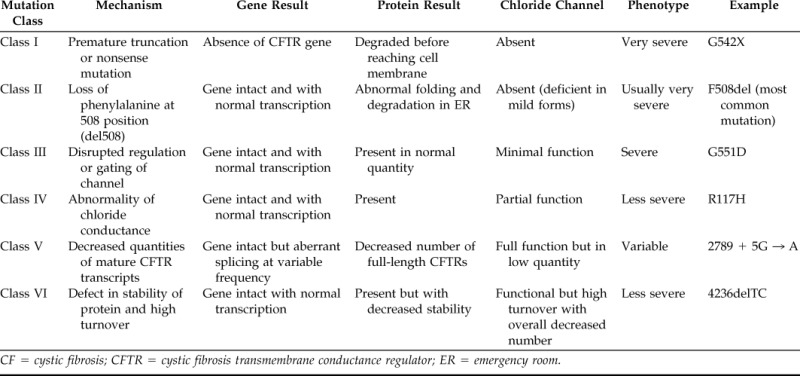
CF = cystic fibrosis; CFTR = cystic fibrosis transmembrane conductance regulator; ER = emergency room.
SPECTRUM OF DISEASE: VARIANCE IN PHENOTYPIC EXPRESSION
Classic CF
Patients with classic CF tend to have class I–III mutations and develop upper and lower airway disease, exocrine pancreatic insufficiency, absence of the vas deferens, and highly elevated sweat chloride concentration, although specific genetic mutations may be associated with increased phenotypic severity (Table 2). For example, the most common mutation F508del homozygosity does not predict disease severity on its own, but this genotype is shown to be an independent risk factor in other manifestations of CF, including reduced mineral bone density and earlier colonization with Pseudomonas aeruginosa.16,17 The diagnosis of classic CF is made when patients exhibit at least one characteristic phenotypic feature, have a family history of CF, or have a positive neonatal screening test. In addition to these requisites, the patients must also have positive testing with either an increased sweat chloride concentration (>60 mmol/L), genetic tests showing two CF causing mutations, or demonstration of an abnormal ancillary test indicative of CFTR abnormality (e.g., nasal epithelial ion transport or intestinal current measurement).18
Table 2.
Summary of therapies

Grades of recommendation: A, consistent level 1 studies; B, consistent level 2 or 3 studies or extrapolations from level 1 studies; C, level 4 studies or extrapolations from level 2 or 3 studies; D, level 5 evidence or troublingly inconsistent or inconclusive studies of any level.
QOL = quality of life; CF = cystic fibrosis; CRS = chronic rhinosinusitis; FESS = functional endoscopic sinus surgery; NPs = nasal polyps.
Atypical CF
Individuals with atypical CF provide a diagnostic challenge as standard diagnostics, including that sweat chloride testing can be normal.19 This group is currently described in the Cystic Fibrosis Foundation consensus document as individuals who show a CF phenotype in at least one organ system and have normal (<40 mmol/L) or borderline (40–60 mmol/L) sweat chloride values.18 These patients tend to have pancreatic exocrine sufficiency in the setting of milder lung or sinonasal disease. They may present with a classic CF phenotype in only a single organ system. This underscores the importance of CF testing in patients with persistent, refractory CRS who do not exhibit classic pulmonary or gastrointestinal CF manifestations. Individuals with nonclassic CF carry two CFTR mutations, at least one of which is usually a “mild/variable” mutation.20
CFTR-Related Diseases
CFTR-related diseases encompass well-known pathological disorders that appear to be influenced by CFTR genotype, including allergic bronchopulmonary aspergillosis, idiopathic bronchiectasis, and CRS. Wang et al.21 performed genetic testing on 147 non-CF patients with severe CRS and discovered a 7% incidence of CFTR mutations compared with the presence of 2% in 123 healthy controls (p = 0.04). Nasal potential difference measurements also documented a slight reduction in CFTR-mediated anion transport in carriers, but not to the levels seen with CF. In another study of 58 children with CRS, seven (12%) carried a single CF mutation, which was much higher than the expected frequency of 4%.22 Although these illnesses appear to be influenced by CFTR dysfunction, they are also influenced by non-CFTR genes and environmental exposures. However, the influence of CFTR indicates that many treatment regimens may be directly applicable to the management of non-CF CRS—specifically, the importance of improving mucus clearance in susceptible individuals.
CYSTIC FIBROSIS CHRONIC RHINOSINUSITIS
Pathophysiology of CRS
The lining of the sinonasal epithelium is comprised of airway surface liquid containing a low viscosity periciliary fluid layer (sol) around the respiratory cilia and a superficial mucus (gel) layer, which function to trap and sweep inhaled particles into the digestive tract through coordinated mucociliary clearance (MCC). Intact MCC is considered the airway's innate defense against disease.23 MCC is grossly impaired in CF because of alterations in the transepithelial passage of anions (chloride and bicarbonate) caused by genetic mutations in the CFTR.24 Disturbances in anion transport result in increased viscosity of mucins that is 30–60 times higher than patients without CF. Tenacious secretions obstruct sinus ostia and create hypoxic conditions with increased edema, secondary ciliary dyskinesia, and subsequent bacterial overgrowth.25,26 Hypoxia has also been shown to affect CFTR transcription and function in epithelium in patients with normal CFTR.27 Patients with classic CF have a high incidence of CRS approaching 100%.25
CF patients also have a high incidence of nasal polyposis associated with CRS (7–48%).28 Nasal polyps (NPs) associated with CF are typically mediated by neutrophilic Th1-mediated inflammation rather than eosinophilic Th2-mediated inflammation seen in atopic and aspirin-sensitive CRS with NPs.29 Claeys et al.30 showed that the Th1 inflammatory mediators IL-8 and myeloperoxidase actually dominate in CF NP compared with eosinophilic cationic protein, eotaxin, and IgE in non–CF NPs. In addition, the antimicrobial peptide (human defensin β2) and pattern recognition receptor Toll-like receptor 2 were significantly increased in CF NPs as well. Other innate defense proteins such as surfactant protein (SP) A, SP-B, and SP-D are also upregulated in CF.31–34 Other molecular differences between CF and non CF polyps include a significantly higher level of lipoxin A4 and slightly elevated cyclooxygenase 2 in CF polyps.35 Onset of bacterial infection and colonization in CF CRS may instigate inflammatory pathways with Toll-like receptors recognizing pathogen-associated molecular patterns with production of these antimicrobial peptides.36–38 The chronic inflammation seen in the sinuses after bacterial contamination also results in goblet cell hyperplasia, squamous metaplasia, and the loss of ciliated cells.26
Mucocele formation is common in CF patients39,40 and its presence in children should be diagnostic of CF unless proven otherwise.41 Decreased paranasal sinus development (hypoplasia) is also a distinguishing characteristic noted in CF patients.42–45 The reasons for this lack of development and the effects of specific CF genotypes on phenotypic expression of sinus development are unclear. One prevailing theory considers that ongoing inflammatory mucosal disease leads to decreased pneumatization similar to poor temporal bone pneumatization in chronic otitis media.46 The general lack of chronic ear disease in CF patients and normal temporal bone development argues against this hypothesis.47 However, genotype does appear to influence paranasal sinus development as individuals homozygous for the F508del mutation have shown a significantly increased frequency of underdeveloped frontal (98%), maxillary (70%), and sphenoid (100%) sinuses when compared with other genetic mutations (69, 8, and 50%, respectively), suggesting CFTR may be a primary contributor to sinus development.45 Studies from the recently developed CF pig model support the premise that CFTR dysfunction as opposed to chronic infection is responsible for decreased sinus pneumatization because pigs lacking intact CFTR have sinus underdevelopment before the development of infection.48
Microbiology
Numerous bacteria are frequently isolated from sinus cultures of CF patients including P. aeruginosa, Staphylococcus aureus, Escherichia coli, Burkholderia cepacia, Acinetobacter species, Stenotrophomonas maltophilia, Haemophilus influenza, Streptococci, and anaerobes.49–52 Muhlebach et al.53 studied lower airway and throat cultures and reported that P. aeruginosa as well S. aureus are the most common bacterial species found in CF patients. There is a higher frequency of Pseudomonas colonization in the lower airways in patients who have CRS with NPs and is more likely to start in the sinuses at a younger age and progress to involve both the upper and the lower airways.54,55 This was confirmed by Godoy et al.56 who showed a significant association between sinus cultures and lower airway cultures from bronchoalveolar lavage. Genotypes of sinus bacteria were also shown to be concordant with the lower airway, indicating the sinuses also may serve as a reservoir for recurrent lung infection,57 increasing the importance of maximizing sinus health. There are increased patterns of antimicrobial resistance secondary to multiple antibiotic exposures and increased prevalence of resistant bacteria within the community, particularly S. aureus. This is problematic because methicillin-resistant S. aureus colonization and infection in the respiratory tract of CF patients is associated with significantly worse overall survival.58
In addition to bacteria, fungi are commonly isolated from CF patients with Candida species being the most prevalent. It is considered a colonizer in pulmonary cultures.59 The use of inhaled steroids in CF patients along with improved culture techniques are likely contributors to increased fungal recovery. Fungus was also retrieved in 33% of patients in a study by Wise et al.,60 with two patients fulfilling the criteria for allergic fungal rhinosinusitis. The implications of these findings are unclear and further studies are required to examine the potential pathogenic role of these fungi.
Clinical Manifestations
Symptoms of CRS, when present, frequently include rhinorrhea, nasal obstruction, mouth breathing, headache, anosmia, and restless sleep.61,62 Other symptoms include facial pain, activity intolerance, halitosis, and voice changes.63–65 When associated with nasal CRS with NPs, the most common complaint is nasal obstruction whereas in patients without NPs, the most common complaint is headache or facial pain.25
Mouth breathing coupled with thick anterior and posterior nasal discharge may be the result of sinonasal polyposis. CF patients may also have facial deformation such as broadening of the nasal bridge, hypertelorism, and proptosis from chronic polyp expansion.26 NPs are frequently seen on rhinoscopy, which are usually multiple and bilateral.66 Rhinoscopy or nasal endoscopy may also show medial bulging of the lateral nasal wall.67 Unfortunately, physical examination findings do not correlate or fluctuate with ongoing changes in clinical status.9
Imaging
Radiographic abnormalities are frequent and often multiple in CF patients. The prevalence and detection of these abnormalities has improved dramatically with the increased use and improvement in CT scans. Findings on CT scan can aid in the diagnosis of CF, particularly among children, including demineralization and medial displacement of the uncinate process with inspissated secretions in the maxillary sinuses. Imaging is often crucial in this patient population because of the loss of important anatomic landmarks in particularly severe cases. Imaging may also be of use during surgical planning but also intraoperatively by using stereotactic imaging in conjunction with an endoscopic view.
Management
Management of CF sinusitis can be a daunting task, but most treatment recommendations dictate conservative management with medical therapy in lieu of primary surgical intervention.68 Few prospective studies exist to address medical management strategy of patients with CF sinusitis. Conservative therapy is favored with the use of sinus irrigations, mucolytics, oral steroids, and oral or i.v. antibiotics, as well as topical antibiotic and steroid therapeutic delivery. Patients who fail medical management or who present with complications such as bone erosion are good candidates for sinus surgery. The need for surgical management of the sinuses in CF patients continues to be controversial. Several studies showing severe CF sinusitis as a risk factor for forced expiratory volume in 1 second (FEV1) decline and thus worsened prognosis in children suggest aggressive treatment may have a role in select patients.69–71
Medical Management
Medical management consists of nasal saline irrigations as well as medications including antibiotics, decongestants, antihistamines, topical and systemic steroids, dornase alfa, and N-acetyl cysteine as well as surfactant lavage.72,73 Evidence regarding several interventions is discussed later.
Nasal Saline Irrigations.
Irrigations with isotonic and hypotonic saline solutions serve to mechanically debride crusting along the sinonasal mucosa while hypertonic saline has the added theoretical benefit of decongestion by osmosis.74 A Cochrane meta-analysis concluded that quality of life in non-CF patients is improved with saline irrigation when compared with nontreatment.72 Unfortunately, no studies are available regarding the use of saline nasal irrigations in the CF CRS population, and thus recommendations for its use are extrapolated from studies in non-CF patients and the benefit is indicated in the pulmonary airways with nebulized hypertonic saline.75,76 The patients usually are instructed to use nasal irrigations using larger-volume, low-pressure irrigation bottles for comfort. A cadaver study showed the effects of irrigation are greatly enhanced after endoscopic sinus surgery (ESS). The squeeze bottle/neti pot devices provided the best saline irrigation delivery to the paranasal sinuses and are probably the best devices to administer topical delivery of antibiotics and steroids as well.77
Topical Steroids.
Topical steroids are particularly effective for patients with allergic rhinitis as well as eosinophilic NPs in adults and children.78 CF NPs are neutrophilic and generally less responsive to steroids, but polyp size reduction and improvement in symptoms has been noted, particularly when using the Mygind's position or upside down positioning.78–80 Hadfield et al.79 performed a randomized controlled trial (46 participants) comparing topical steroid (betamethasone) nasal drops with placebo and reported significant reduction in polyp size. It was noted in a later Cochrane review that the risk of bias was high in this study because >50% of people enrolled did not complete follow-up.81 However, high-dose topical steroid rinses with budesonide have shown no alteration of the hypothalamic–pituitary axis in several studies.82,83 Low-absorption topical steroid irrigations appear to be a reasonable strategy in CF CRS, although further randomized controlled trials are required.
Topical Antibiotics.
In a systematic review on the use of topical antimicrobials delivered in sinonasal irrigation by Lim et al.,84 the authors noted that there was no sufficient evidence to justify their use in CRS patients in general, but a high level of evidence was reported regarding use in the CF CRS population (IIb).85 Topical antibiotics have fewer adverse effects than oral antibiotics and may achieve a higher drug concentration at the target site.26 Topical tobramycin has been shown to be effective in reducing symptoms and reveals improvements in endoscopic scores in sinusitis.84 Because of the increased risk of recurrent CRS exacerbations after surgery, aggressive topical management is generally recommended. The use of topical antibiotics postoperatively has also been associated with reduced recurrence of CF sinus exacerbations86 with another study showing improved control of sinus disease for at least 2 years after surgery.52
Macrolide Antibiotics.
Macrolides with 14 and 15 membered rings (e.g., azithromycin and clarithromycin) down-regulate inflammatory responses and are effective for the treatment of chronic airway inflammatory diseases including diffuse panbronchiolitis, CRS, and CF. Besides clinical improvement in nasal obstruction and nasal secretions,87 macrolides have been shown to decrease production of IL-8 by nasal epithelial cells88 and they correlate with improvement in eosinophilic CRS with NPs.89 Although there are no discrete studies establishing clinical benefit for CRS in the CF population, similar CF-related pathophysiology between the sinonasal and pulmonary airways indicates this medication class is likely a valuable therapeutic addition for CF-associated sinus inflammation.90 Proper dosage and scheduling is controversial, but use for CF-associated pulmonary disease is 500 mg, 3 times/wk and is supported by randomized, controlled trials.91
Oral Antibiotics.
In addition to macrolides, ciprofloxacin has been used prophylactically in patients with CF. Although studies for CF-associated sinus disease are lacking, an analysis of individuals progressing to pulmonary exacerbation who were provided oral antibiotics for an average of 13.4 days were found to have circumvented the need for i.v. antibiotics in up to 80% of the time92
Ibuprofen.
There have been positive results with the use of high-dose ibuprofen on the progression of pulmonary disease in children with CF.93 In a small series of 12 CF patients with NPs treated with high-dose ibuprofen therapy for pulmonary disease, the absence of polyps was noted at some point during treatment. In addition, clinical regression of NPs was noted in five patients during ibuprofen therapy.94 Confirmatory studies are required to evaluate the effectiveness of this drug in CF CRS.
Dornase Alfa.
Dornase alfa is a recombinant human deoxyribonuclease that hydrolyzes DNA polymers, reduces DNA fragment length, and reduces the viscosity of CF purulent secretions.95 Clinically, it has been shown to reduce the risks of pulmonary exacerbations, improve FEV1, and slow the continued rate of decline of lung function in CF patients >5 years old.96–98 Nasal nebulized dornase alfa has also shown clinical efficacy in CF CRS. Cimmino et al.,99 in a double-blind placebo-controlled trial, reported on the use of nebulized dornase alfa in early postoperative CF ESS. There was a significant improvement in the nasal symptoms and endoscopic findings, as well as FEV1. Mainz et al.100 also reported significant improvement in quality of life (as measured by the 20-item Sino-Nasal Outcome Test) in a double-blind placebo-controlled crossover trial with nebulized dornase alfa compared to normal saline.
Novel Therapeutics: Targeting the Basic Defect
New therapeutic strategies for CF that target rescue of CFTR activity have recently been approved for select CF patients, and are in development in other groups of patients. Based on a dramatic advancement in our understanding of the production, processing, and function of the CFTR channel, small molecules identified by high throughput drug screening that restore activity to the mutant CFTR protein have been discovered and developed for clinical use.101–104 The three drugs that have entered clinical testing in CF include ivacaftor (Kalydeco, formerly VX-770; Vertex Pharmaceuticals, Cambridge, MA), lumacaftor (formerly VX-809), and ataluren (formerly PTC124). Ivacaftor, which potentiates mutant CFTR already present in the cell surface, caused significant improvement in lung function and other outcomes in clinical trials for patients with the G551D CFTR mutation and was recently approved by the Food and Drug Administration for use in individuals aged ≥6 years with at least one copy of this mutation.105 In this form of CF, mutant protein is present in normal quantities on the cell surface, but the channel exhibits severely defective gating. Although the effect on the CF sinuses still has not been studied, it is presumed that augmenting CFTR would generate pronounced improvement in the MCC of the sinus cavities, resulting in improved sinus disease outcomes. This will be evaluated in part during a postapproval study in G551D patients currently in progress. The F508del CFTR mutation is a more challenging target, because channels are “misfolded” and degraded in the endoplasmic reticulum before reaching the cell surface. Lumacaftor, another molecule discovered by high throughput screening, “corrects” the processing of the protein to improve delivery of CFTR to the plasma membrane. Clinical trials are currently using VX-770 and VX-809 together in an attempt to improve therapeutic effects and will be advanced to phase 3 testing.106,107 Ataluren is another small molecule currently under investigation in clinical trials.108 This drug induces translational readthrough of nonsense mutations in CFTR in vitro and in vivo and has shown activity in some but not all proof of concept CF trials. Ivacaftor and other CFTR modulators109are being advanced in CF patients with additional CFTR mutations; if successful, these new treatments could also provide relief of CFTR-mediated mucosal abnormalities that drive CF CRS pathogenesis.
Other approaches designed to improve mucociliary transport in CF include targeting other apical ion channels to improve airway surface liquid hydration. Drugs that either inhibit epithelial sodium channels or stimulate alternative chloride pathways such as calcium-activated chloride channels are also under active investigation.110,111
Surgical Management
Surgical Indications.
The low incidence of self-reported symptoms (20%) despite the presence of radiographic and endoscopic sinus disease in the vast majority of CF patients reflects the difficulties in assessing the indications for surgical management. In general, CF patients with persistent symptoms who have failed medical management are often considered appropriate candidates for functional ESS (FESS). Surgical management of the sinuses in CF patients is also thought to improve pulmonary outcomes and is used to justify intervention in asymptomatic individuals. This has been shown in other diseases such as asthma, where Stammberger112 reported a 70% improvement in asthma symptoms and Lund113 reported pulmonary improvement in two-thirds of the postsurgical patients after ESS. However, pulmonary outcomes after surgical intervention for CF sinusitis are mixed. Several studies reported no change in objective outcomes for children and adults such as hospital admissions and pulmonary status, but showed improvements in clinical symptoms and quality of life.64,114 The best data to support surgical intervention in asymptomatic individuals derive from the identification of identical P. aeruginosa clones isolated in the sputum and the bronchoalveolar lavage of CF lung transplant patients before and after their transplant.115 Several studies have reported on the effect of sinus surgery as well as postoperative nasal care as a better means for controlling the sinuses as a reservoir for pulmonary infection.86,116 A retrospective chart review of patients who received lung transplantation and had subsequent FESS found a significant decrease in rehospitalization rates.117 Surgery coupled with daily nasal irrigations led to a significant reduction in the incidence of tracheobronchitis as well as pneumonia and bronchiolitis obliterans syndrome in this population.117
With conflicting evidence regarding surgical indications, it is understandable that the percentage of CF patients requiring surgical management of their disease varies considerably. Virgin et al.118 used the pediatric health information services database to investigate the number patients with CF who had sinus surgery during a 3-year period at the 43 largest pediatric hospitals in the United States. The frequency of FESS in CF patients varied from 3 to 47% among centers with a positive correlation between hospital size, number of CF patients, and percentage of patients that adhered to the Cystic Fibrosis Foundation guidelines.
FESS Outcomes.
Standard FESS includes maxillary antrostomy, anterior and posterior ethmoidectomy, sphenoidotomy, and, depending on the age of the patient and presence of frontal sinuses, a frontal sinusotomy. Multiple studies have been conducted to report on the safety and effectiveness of FESS in CF patients.9,119–123 Complication rate after FESS in CF patients (11.5%) was found to be similar to the rate of non-CF FESS complications (0–17%).121 Khalid et al. reported on the outcomes of sinus surgery in adult patients both with and without CF.114 Although baseline CT and endoscopy scores were significantly worse in CF patients, the overall quality of life improvements as well as the degree of endoscopic improvement was similar between the two groups. The quality of life scales used in this study were the Rhinosinusitis Disability Index and the Chronic Sinusitis Survey. However, overall failure rates requiring revision surgery range from 13 to 89% in the literature.52,61,122,124,125 As a result, many CF patients have had multiple surgeries by the time they reach adulthood.
Often used as a quality indicator, the need for revision surgery can be difficult to assess. In one study, CT findings were a significant predictor for revision sinus surgery.126 Patients with higher Lund-Mckay scores were found to require revision surgery. However, almost all patients with CF have abnormal findings on CT scan,127 including asymptomatic non-CF patients (18–72%),128,129 but not all require surgery. In CRS patients, CT does not correlate well with symptom scores.130,131 McMurphy et al.132 found no significant difference between the preoperative and postoperative Lund-MacKay scores of pediatric CF patients after initial surgery or in subsequent scans despite medical or surgical interventions. Persistence or worsening of radiographic abnormalities after FESS has been shown in several other studies.120,133 Thus, CT imaging changes alone is probably not an appropriate indicator for recurrence/failure or predicting patient perception of disease except in the development of a mucocele or orbital complication.125,134–136
The presence of NP was found to assess the future likelihood of requiring revision FESS in several studies. In a study by Rowe-Jonce and Mackay122 regarding FESS for CF sinusitis with NPs, the reported rate of revision or return to preoperative symptom severity was 50% with 18–24 months of clinical follow-up. Rickert et al.137 found that preoperative grading of NPs in CF patients was predictive of the need for future surgical revisions. In their study with a longer follow-up of 7.3 years, the reported revision rate for patients with severe polyps was significantly higher (58%) compared with patients with no polyps (28%). The time for surgery was also significantly different between the groups tested with patients who had severe polyps requiring surgery in a shorter period of time.
Pediatric FESS Outcomes.
The pediatric patient population is unique because of the anatomic development peculiar to each age group. Treatment algorithms focus on maximizing medical therapy although surgical intervention has shown to improve patients whose disease course is recalcitrant to therapy. Most CF patients who undergo FESS exhibit improved symptom profile; however, radiographic and endoscopic scores are rarely significantly changed postoperatively.138 Similarly, data on pulmonary outcomes is mixed. Although short-term improvement in lung function has been observed in children, long-term effects have not been found to be significant.139 Other retrospective studies on CF patients fail to show improved pulmonary function tests after sinus surgery.139,140 One pediatric study showed significant improvement in pulmonary function tests after FESS up to the first 2 years postoperatively.141 This was not shown in patients with low socioeconomic status. So far, there are no reports of adverse effects of FESS on facial growth in pediatric patients.123
Extensive Surgical Intervention Outcomes.
The maxillary sinus is a chronic refractory problem area in CF patients because the normal MCC pathway is through the natural ostium and against gravity. Because MCC is impaired, accumulation of mucopurulence in the largest of the sinus cavities is commonplace on CT imaging and endoscopic findings despite previous “adequate” maxillary antrostomies. This is one reason CT findings are unchanged after FESS in CF patients because the maxillary sinus reaccumulates mucopurulence quickly despite the use of nasal irrigations. In essence, the sinuses in CF patients are similar to large abscesses that may be drained temporarily but ultimately lack real change within the cavities because the mucosa will never have normal function because of their underlying genetic defect. Thus, the ultimate treatment goals of aggressive surgical intervention are to establish permanent “access” rather than “ventilation” to the sinus cavities, permitting additional medicinal, mechanical, and physical means for the removal of desiccated mucus.
The modified endoscopic medial maxillectomy (MEMM) involves removal of the medial maxillary wall marsupializing the maxillary sinus into the nasal cavity, but without sacrificing the head of the inferior turbinate or lacrimal system (Figs. 1 and 2). Accumulation of secretions becomes less frequent due to the open cavity and elimination of the physiological requirement of drainage through narrow anatomic ostia. In addition, the procedure allows physical debridement of mucus and polypoid edema in the clinic, improved clearance of mucus with nasal saline irrigations, and increased access for topical delivery of therapeutics.
Figure 1.
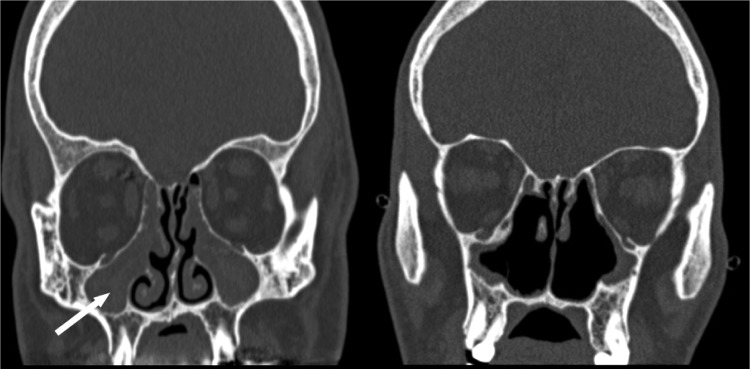
Coronal CT scans showing the preoperative appearance of a patient with cystic fibrosis (CF) after traditional maxillary antrostomies with completely opacified maxillary sinuses (left, white arrow) and postoperative appearance after bilateral modified endoscopic medial maxillectomies and revision sinus surgery (right). The coronal CT image is posterior to the anterior one-third of the inferior turbinate. (Adapted with permission from Ref. 145.)
Figure 2.
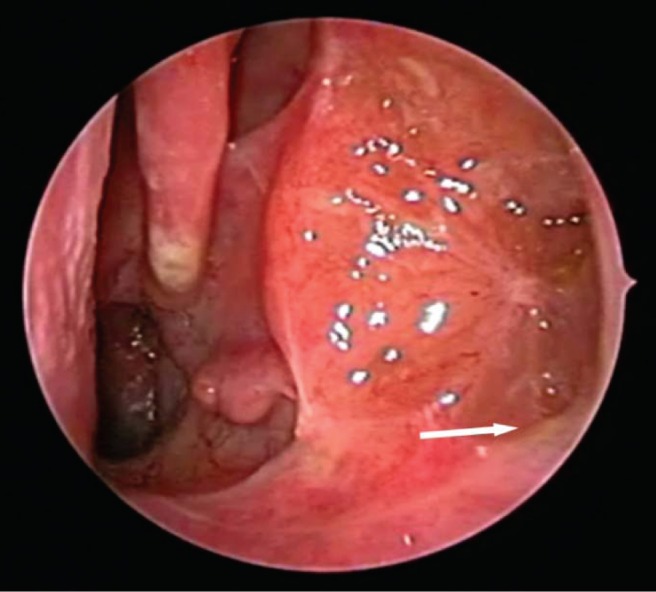
Transnasal endoscopic view of a left maxillary sinus after modified endoscopic medial maxillectomy. A 30° endoscope is inserted past the anterior one-third of the inferior turbinate revealing a well-healed maxillary cavity with no secretions retained in the floor of the sinus (arrow). (Adapted with permission from Ref. 145.)
Multiple studies have been conducted regarding the role of this more aggressive surgery in the management of CF sinusitis. A retrospective study in 2006 was the first investigation regarding the use of MEMM in patients with CF CRS and it showed a low complication rate.142 Shatz143 also reported on a very aggressive surgical approach to the maxillary sinuses in CF children with prior history of FESS. In this study, there was a significant reduction in symptoms and duration of hospitalization as well as FEV1 after bilateral Caldwell-Luc and endoscopic medial maxillectomies in a cohort of 15 pediatric CF patients.143 Cho et al.144 reported results of this technique (referred to as a maxillary mega-antrostomy) and also found it to be safe and effective. In a recent prospective study, FESS and MEMM combined with a comprehensive postoperative medical management regimen (culture-directed antibiotics, oral steroid taper, and topical steroid/antibiotic irrigations) was associated with marked improvement in sinus disease outcomes including a decrease in symptoms (22-item Sino-Nasal Outcome Test) and objective findings (Lund Kennedy scores) at 1 year of clinical follow-up.145 In this study, FEV1 was not significantly changed, but there was significant reduction in the hospital admissions for pulmonary exacerbations in the year postsurgery compared with the year before. Results from these investigations lends support to a more extensive surgical approach in CF sinusitis, but further studies are warranted to determine whether this treatment paradigm will provide long-term symptom improvement and confer advantages in CF pulmonary outcomes.
CONCLUSION
CRS continues to be an important issue in the management of patients with CF, particularly given the improved survival rates associated with this disease. CF is a lifelong disease that requires long-term surveillance, vigilance, and compliance. Quality of life and decreased hospitalization will potentially become quality indicators in the management of this patient group, and a multidisciplinary approach is necessary to develop consistent treatment paradigms for this difficult entity. With improved treatments, MCC may be able to be augmented in the future, reducing symptoms and their contribution to pulmonary progression.
Footnotes
Presented at the North American Allergy and Rhinology Conference, Puerto Rico, February 5, 2012
BA Woodworth received funding from the Flight Attendant's Medical Research Institute Young Clinical Scientist Award (072218) and NIH/NHLBI (1K08HL107142-01), is a consultant for ArthroCare ENT and Gyrus ENT, and he is an inventor on a patent submitted regarding the use of chloride secretagogues for therapy of sinus disease (35 U.S.C. n111(b) and 37 C.F.R n.53 (c)) in the United States Patent and Trademark Office. SM Rowe received funding from R01HL105487-01 and 1R03DK084110-01 and from Vertex Pharmaceuticals to conduct clinical trials in cystic fibrosis and he is an inventor on a patent submitted regarding the use of chloride secretagogues for therapy of sinus disease (35 U.S.C. n111(b) and 37 C.F.R n.53 (c)) in the United States Patent and Trademark Office. The remaining authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med 352:1992–2001, 2005. [DOI] [PubMed] [Google Scholar]
- 2. Cystic Fibrosis Foundation. Patient Registry 2011 Annual Report. Bethesda, MD: Cystic Fibrosis Foundation, pg 6, 2012. [Google Scholar]
- 3. Riordan JR, Rommens JM, Kerem B, et al. Identification of the cystic fibrosis gene: Cloning and characterization of complementary DNA. Science 245:1066–1073, 1989. [DOI] [PubMed] [Google Scholar]
- 4. Collins FS. Cystic fibrosis: Molecular biology and therapeutic implications. Science 256:774–779, 1992. [DOI] [PubMed] [Google Scholar]
- 5. Rosen MJ. Chronic cough due to bronchiectasis: ACCP evidence-based clinical practice guidelines. Chest 129(suppl):122S–131S, 2006. [DOI] [PubMed] [Google Scholar]
- 6. Friedman EM, Stewart M. An assessment of sinus quality of life and pulmonary function in children with cystic fibrosis. Am J Rhinol 20:568–572, 2006. [DOI] [PubMed] [Google Scholar]
- 7. Rosbe KW, Jones DT, Rahbar R, et al. Endoscopic sinus surgery in cystic fibrosis: Do patients benefit from surgery? Int J Pediatr Otorhinolaryngol 61:113–119, 2001. [DOI] [PubMed] [Google Scholar]
- 8. Rickert S, Banuchi VE, Germana JD, et al. Cystic fibrosis and endoscopic sinus surgery: Relationship between nasal polyposis and likelihood of revision endoscopic sinus surgery in patients with cystic fibrosis. Arch Otolaryngol Head Neck Surg 136:988–992, 2010. [DOI] [PubMed] [Google Scholar]
- 9. Keck T, Rozsasi A. Medium-term symptom outcomes after paranasal sinus surgery in children and young adults with cystic fibrosis. Laryngoscope 117:475–479, 2007. [DOI] [PubMed] [Google Scholar]
- 10. Cystic Fibrosis Mutation Database. Available online at www.genet.sickkids.on.ca/cftr/Home.html; accessed August 19, 2012.
- 11. Zielenski J, Tsui LC. Cystic fibrosis: Genotypic and phenotypic variations. Annu Rev Genet 29:777–807, 1995. [DOI] [PubMed] [Google Scholar]
- 12. Welsh MJ, Smith AE. Molecular mechanisms of CFTR chloride channel dysfunction in cystic fibrosis. Cell 73:1251–1254, 1993. [DOI] [PubMed] [Google Scholar]
- 13. Accurso FJ, Rowe SM, Clancy JP, et al. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med 363:1991–2003, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gan KH, Veeze HJ, van den Ouweland AM, et al. A cystic fibrosis mutation associated with mild lung disease. N Engl J Med 333:95–99, 1995. [DOI] [PubMed] [Google Scholar]
- 15. Haardt M, Benharouga M, Lechardeur D, et al. C-terminal truncations destabilize the cystic fibrosis transmembrane conductance regulator without impairing its biogenesis. A novel class of mutation. J Biol Chem 274:21873–21877, 1999. [DOI] [PubMed] [Google Scholar]
- 16. King SJ, Topliss DJ, Kotsimbos T, et al. Reduced bone density in cystic fibrosis: DeltaF508 mutation is an independent risk factor. Eur Respir J 25:54–61, 2005. [DOI] [PubMed] [Google Scholar]
- 17. Maselli JH, Sontag MK, Norris JM, et al. Risk factors for initial acquisition of Pseudomonas aeruginosa in children with cystic fibrosis identified by newborn screening. Pediatr Pulmonol 35:257–262, 2003. [DOI] [PubMed] [Google Scholar]
- 18. Rosenstein BJ, Cutting GR. The diagnosis of cystic fibrosis: A consensus statement. Cystic Fibrosis Foundation Consensus Panel. J Pediatr 132:589–595, 1998. [DOI] [PubMed] [Google Scholar]
- 19. Boyle MP. Nonclassic cystic fibrosis and CFTR-related diseases. Curr Opin Pulm Med 9:498–503, 2003. [DOI] [PubMed] [Google Scholar]
- 20. Rowntree RK, Harris A. The phenotypic consequences of CFTR mutations. Ann Hum Genet 67:471–485, 2003. [DOI] [PubMed] [Google Scholar]
- 21. Wang X, Moylan B, Leopold DA, et al. Mutation in the gene responsible for cystic fibrosis and predisposition to chronic rhinosinusitis in the general population. JAMA 284:1814–1819, 2000. [DOI] [PubMed] [Google Scholar]
- 22. Raman V, Clary R, Siegrist KL, et al. Increased prevalence of mutations in the cystic fibrosis transmembrane conductance regulator in children with chronic rhinosinusitis. Pediatrics 109:E13, 2002. [DOI] [PubMed] [Google Scholar]
- 23. Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest 109:571–577, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Regnis JA, Robinson M, Bailey DL, et al. Mucociliary clearance in patients with cystic fibrosis and in normal subjects. Am J Respir Crit Care Med 150:66–71, 1994. [DOI] [PubMed] [Google Scholar]
- 25. Gentile VG, Isaacson G. Patterns of sinusitis in cystic fibrosis. Laryngoscope 106:1005–1009, 1996. [DOI] [PubMed] [Google Scholar]
- 26. Gysin C, Alothman GA, Papsin BC. Sinonasal disease in cystic fibrosis: Clinical characteristics, diagnosis, and management. Pediatr Pulmonol 30:481–489, 2000. [DOI] [PubMed] [Google Scholar]
- 27. Blount A, Zhang S, Chestnut M, et al. Transepithelial ion transport is suppressed in hypoxic sinonasal epithelium. Laryngoscope 121:1929–1934, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Robertson JM, Friedman EM, Rubin BK. Nasal and sinus disease in cystic fibrosis. Paediatr Respir Rev 9:213–219, 2008. [DOI] [PubMed] [Google Scholar]
- 29. Van Zele T, Claeys S, Gevaert P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy 61:1280–1289, 2006. [DOI] [PubMed] [Google Scholar]
- 30. Claeys S, Van Hoecke H, Holtappels G, et al. Nasal polyps in patients with and without cystic fibrosis: A differentiation by innate markers and inflammatory mediators. Clin Exp Allergy 35:467–472, 2005. [DOI] [PubMed] [Google Scholar]
- 31. Woodworth BA, Lathers D, Neal JG, et al. Immunolocalization of surfactant protein A and D in sinonasal mucosa. Am J Rhinol 20:461–465, 2006. [DOI] [PubMed] [Google Scholar]
- 32. Woodworth BA, Neal JG, Newton D, et al. Surfactant protein A and D in human sinus mucosa: A preliminary report. ORL J Otorhinolaryngol Relat Spec 69:57–60, 2007. [DOI] [PubMed] [Google Scholar]
- 33. Woodworth BA, Wood R, Baatz JE, Schlosser RJ. Sinonasal surfactant protein A1, A2, and D gene expression in cystic fibrosis: A preliminary report. Otolaryngol Head Neck Surg 137:34–38, 2007. [DOI] [PubMed] [Google Scholar]
- 34. Woodworth BA, Wood R, Bhargave G, et al. Surfactant protein B detection and gene expression in chronic rhinosinusitis. Laryngoscope 117:1296–1301, 2007. [DOI] [PubMed] [Google Scholar]
- 35. Rozsasi A, Heinemann A, Keck T. Cyclooxygenase 2 and lipoxin A(4) in nasal polyps in cystic fibrosis. Am J Rhinol Allergy 25:e251–e254. [DOI] [PubMed] [Google Scholar]
- 36. Rampey AM, Lathers DM, Woodworth BA, Schlosser RJ. Immunolocalization of dendritic cells and pattern recognition receptors in chronic rhinosinusitis. Am J Rhinol 21:117–121, 2007. [DOI] [PubMed] [Google Scholar]
- 37. Raoust E, Balloy V, Garcia-Verdugo I, et al. Pseudomonas aeruginosa LPS or flagellin are sufficient to activate TLR-dependent signaling in murine alveolar macrophages and airway epithelial cells. PLoS One 4:e7259, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Laube DM, Yim S, Ryan LK, et al. Antimicrobial peptides in the airway. Curr Top Microbiol Immunol 306:153–182, 2006. [DOI] [PubMed] [Google Scholar]
- 39. Nicollas R, Facon F, Sudre-Levillain I, et al. Pediatric paranasal sinus mucoceles: Etiologic factors, management and outcome. Int J Pediatr Otorhinolaryngol 70:905–908, 2006. [DOI] [PubMed] [Google Scholar]
- 40. Alvarez RJ, Liu NJ, Isaacson G. Pediatric ethmoid mucoceles in cystic fibrosis: Long-term follow-up of reported cases. Ear Nose Throat J 76:538–539, 1997. [PubMed] [Google Scholar]
- 41. Olze H, Matthias C, Degenhardt P. Paediatric paranasal sinus mucoceles. Eur J Pediatr Surg 16:192–196, 2006. [DOI] [PubMed] [Google Scholar]
- 42. Eggesbo HB, Sovik S, Dolvik S, et al. CT characterization of developmental variations of the paranasal sinuses in cystic fibrosis. Acta Radiol 42:482–493, 2001. [DOI] [PubMed] [Google Scholar]
- 43. Eggesbo HB, Sovik S, Dolvik S, et al. Proposal of a CT scoring system of the paranasal sinuses in diagnosing cystic fibrosis. Eur Radiol 13:1451–1460, 2003. [DOI] [PubMed] [Google Scholar]
- 44. Shah RK, Dhingra JK, Carter BL, Rebeiz EE. Paranasal sinus development: A radiographic study. Laryngoscope 113:205–209, 2003. [DOI] [PubMed] [Google Scholar]
- 45. Woodworth BA, Ahn C, Flume PA, Schlosser RJ. The delta F508 mutation in cystic fibrosis and impact on sinus development. Am J Rhinol 21:122–127, 2007. [DOI] [PubMed] [Google Scholar]
- 46. Milczuk HA, Dalley RW, Wessbacher FW, Richardson MA. Nasal and paranasal sinus anomalies in children with chronic sinusitis. Laryngoscope 103:247–252, 1993. [DOI] [PubMed] [Google Scholar]
- 47. Seifert CM, Harvey RJ, Mathews JW, et al. Temporal bone pneumatization and its relationship to paranasal sinus development in cystic fibrosis. Rhinology 48:233–238, 2010. [DOI] [PubMed] [Google Scholar]
- 48. Chang EH, Pezzulo AA, Meyerholz DK, et al. Sinus hypoplasia precedes sinus infection in a porcine model of cystic fibrosis. Laryngoscope 18:1898–1905, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shapiro ED, Milmoe GJ, Wald ER, et al. Bacteriology of the maxillary sinuses in patients with cystic fibrosis. J Infect Dis 146:589–593, 1982. [DOI] [PubMed] [Google Scholar]
- 50. Halvorson DJ, Dupree JR, Porubsky ES. Management of chronic sinusitis in the adult cystic fibrosis patient. Ann Otol Rhinol Laryngol 107:946–952, 1998. [DOI] [PubMed] [Google Scholar]
- 51. Mak GK, Henig NR. Sinus disease in cystic fibrosis. Clin Rev Allergy Immunol 21:51–63, 2001. [DOI] [PubMed] [Google Scholar]
- 52. Moss RB, King VV. Management of sinusitis in cystic fibrosis by endoscopic surgery and serial antimicrobial lavage. Reduction in recurrence requiring surgery. Arch Otolaryngol Head Neck Surg 121:566–572, 1995. [DOI] [PubMed] [Google Scholar]
- 53. Muhlebach MS, Miller MB, Moore C, et al. Are lower airway or throat cultures predictive of sinus bacteriology in cystic fibrosis? Pediatr Pulmonol 41:445–451, 2006. [DOI] [PubMed] [Google Scholar]
- 54. Henriksson G, Westrin KM, Karpati F, et al. Nasal polyps in cystic fibrosis: Clinical endoscopic study with nasal lavage fluid analysis. Chest 121:40–47, 2002. [DOI] [PubMed] [Google Scholar]
- 55. Roby BB, McNamara J, Finkelstein M, Sidman J. Sinus surgery in cystic fibrosis patients: Comparison of sinus and lower airway cultures. Int J Pediatr Otorhinolaryngol 72:1365–1369, 2008. [DOI] [PubMed] [Google Scholar]
- 56. Godoy JM, Godoy AN, Ribalta G, Largo I. Bacterial pattern in chronic sinusitis and cystic fibrosis. Otolaryngol Head Neck Surg 145:673–676, 2011. [DOI] [PubMed] [Google Scholar]
- 57. Mainz JG, Koitschev A. Management of chronic rhinosinusitis in CF. J Cyst Fibros 8(suppl 1):S10–S14, 2009. [DOI] [PubMed] [Google Scholar]
- 58. Dasenbrook EC, Checkley W, Merlo CA, et al. Association between respiratory tract methicillin-resistant Staphylococcus aureus and survival in cystic fibrosis. JAMA 303:2386–2392, 2010. [DOI] [PubMed] [Google Scholar]
- 59. Haase G, Skopnik H, Groten T, et al. Long-term fungal cultures from sputum of patients with cystic fibrosis. Mycoses 34:373–376, 1991. [DOI] [PubMed] [Google Scholar]
- 60. Wise SK, Kingdom TT, McKean L, et al. Presence of fungus in sinus cultures of cystic fibrosis patients. Am J Rhinol 19:47–51, 2005. [PubMed] [Google Scholar]
- 61. Cepero R, Smith RJ, Catlin FI, et al. Cystic fibrosis–An otolaryngologic perspective. Otolaryngol Head Neck Surg 97:356–360, 1987. [DOI] [PubMed] [Google Scholar]
- 62. Naqvi SK, Sotelo C, Murry L, Simakajornboon N. Sleep architecture in children and adolescents with cystic fibrosis and the association with severity of lung disease. Sleep Breath 12:77–83, 2008. [DOI] [PubMed] [Google Scholar]
- 63. Nishioka GJ, Barbero GJ, Konig P, et al. Symptom outcome after functional endoscopic sinus surgery in patients with cystic fibrosis: A prospective study. Otolaryngol Head Neck Surg 113:440–445, 1995. [DOI] [PubMed] [Google Scholar]
- 64. Jones JW, Parsons DS, Cuyler JP. The results of functional endoscopic sinus (FES) surgery on the symptoms of patients with cystic fibrosis. Int J Pediatr Otorhinolaryngol 28:25–32, 1993. [DOI] [PubMed] [Google Scholar]
- 65. Hulka GF. Head and neck manifestations of cystic fibrosis and ciliary dyskinesia. Otolaryngol Clin North Am 33:1333–1341, vii–viii, 2000. [DOI] [PubMed] [Google Scholar]
- 66. Neely JG, Harrison GM, Jerger JF, et al. The otolaryngologic aspects of cystic fibrosis. Trans Am Acad Ophthalmol Otolaryngol 76:313–324, 1972. [PubMed] [Google Scholar]
- 67. Brihaye P, Clement PA, Dab I, Desprechin B. Pathological changes of the lateral nasal wall in patients with cystic fibrosis (mucoviscidosis). Int J Pediatr Otorhinolaryngol 28:141–147, 1994. [DOI] [PubMed] [Google Scholar]
- 68. Loebinger MR, Bilton D, Wilson R. Upper airway 2: Bronchiectasis, cystic fibrosis and sinusitis. Thorax 64:1096–1101, 2009. [DOI] [PubMed] [Google Scholar]
- 69. Amadori A, Antonelli A, Balteri I, et al. Recurrent exacerbations affect FEV(1) decline in adult patients with cystic fibrosis. Respir Med 103:407–413, 2009. [DOI] [PubMed] [Google Scholar]
- 70. Sanders DB, Bittner RC, Rosenfeld M, et al. Pulmonary exacerbations are associated with subsequent FEV1 decline in both adults and children with cystic fibrosis. Pediatr Pulmonolr 46:393–400, 2010. [DOI] [PubMed] [Google Scholar]
- 71. Konstan MW, Morgan WJ, Butler SM, et al. Risk factors for rate of decline in forced expiratory volume in one second in children and adolescents with cystic fibrosis. J Pediatr 151:134–139, 2007. [DOI] [PubMed] [Google Scholar]
- 72. Harvey R, Hannan SA, Badia L, Scadding G. Nasal saline irrigations for the symptoms of chronic rhinosinusitis. Cochrane Database Syst Rev 3:CD006394, 2007. [DOI] [PubMed] [Google Scholar]
- 73. Fokkens WJ, Lund VJ, Mullol J, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology 50:1–12, 2012. [DOI] [PubMed] [Google Scholar]
- 74. Talbot AR, Herr TM, Parsons DS. Mucociliary clearance and buffered hypertonic saline solution. Laryngoscope 107:500–503, 1997. [DOI] [PubMed] [Google Scholar]
- 75. Elkins MR, Bye PT. Inhaled hypertonic saline as a therapy for cystic fibrosis. Curr Opin Pulm Med 12:445–452, 2006. [DOI] [PubMed] [Google Scholar]
- 76. Elkins MR, Robinson M, Rose BR, et al. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Engl J Med 354:229–240, 2006. [DOI] [PubMed] [Google Scholar]
- 77. Harvey RJ, Goddard JC, Wise SK, Schlosser RJ. Effects of endoscopic sinus surgery and delivery device on cadaver sinus irrigation. Otolaryngol Head Neck Surg 139:137–142, 2008. [DOI] [PubMed] [Google Scholar]
- 78. Costantini D, Di Cicco M, Giunta A, Amabile G. Nasal polyposis in cystic fibrosis treated by beclomethasone dipropionate. Acta Univ Carol Med (Praha) 36:220–221, 1990. [PubMed] [Google Scholar]
- 79. Hadfield PJ, Rowe-Jones JM, Mackay IS. A prospective treatment trial of nasal polyps in adults with cystic fibrosis. Rhinology 38:63–65, 2000. [PubMed] [Google Scholar]
- 80. Mainz JG KA. Management of chronic rhinosinusitis in CF. J Cyst Fibros 8(suppl 1):S10–S14, 2009. [DOI] [PubMed] [Google Scholar]
- 81. Beer H, Southern KW, Swift AC. Topical nasal steroids for treating nasal polyposis in people with cystic fibrosis. Cochrane Database Syst Rev 5:CD008253, 2011. [DOI] [PubMed] [Google Scholar]
- 82. Bhalla RK, Payton K, Wright ED. Safety of budesonide in saline sinonasal irrigations in the management of chronic rhinosinusitis with polyposis: Lack of significant adrenal suppression. J Otolaryngol Head Neck Surg 37:821–825, 2008. [PubMed] [Google Scholar]
- 83. Welch KC, Thaler ER, Doghramji LL, et al. The effects of serum and urinary cortisol levels of topical intranasal irrigations with budesonide added to saline in patients with recurrent polyposis after endoscopic sinus surgery. Am J Rhinol Allerg 24:26–28, 2010. [DOI] [PubMed] [Google Scholar]
- 84. Lim M, Citardi MJ, Leong JL. Topical antimicrobials in the management of chronic rhinosinusitis: A systematic review. Am J Rhinol 22:381–389, 2008. [DOI] [PubMed] [Google Scholar]
- 85. Vaughan WC, Carvalho G. Use of nebulized antibiotics for acute infections in chronic sinusitis. Otolaryngol Head Neck Surg 127:558–568, 2002. [DOI] [PubMed] [Google Scholar]
- 86. Davidson TM, Murphy C, Mitchell M, et al. Management of chronic sinusitis in cystic fibrosis. Laryngoscope 105:354–358, 1995. [DOI] [PubMed] [Google Scholar]
- 87. Majima Y. Clinical implications of the immunomodulatory effects of macrolides on sinusitis. Am J Med 117(suppl 9A):20S–25S, 2004. [DOI] [PubMed] [Google Scholar]
- 88. Suzuki H, Shimomura A, Ikeda K, et al. Inhibitory effect of macrolides on interleukin-8 secretion from cultured human nasal epithelial cells. Laryngoscope 107:1661–1666, 1997. [DOI] [PubMed] [Google Scholar]
- 89. Yamada T, Fujieda S, Mori S, et al. Macrolide treatment decreased the size of nasal polyps and IL-8 levels in nasal lavage. Am J Rhinol 14:143–148, 2000. [DOI] [PubMed] [Google Scholar]
- 90. Jaffe A, Francis J, Rosenthal M, Bush A. Long-term azithromycin may improve lung function in children with cystic fibrosis. Lancet 7 351:420, 1998. [DOI] [PubMed] [Google Scholar]
- 91. Saiman L, Marshall BC, Mayer-Hamblett N, et al. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: A randomized controlled trial. JAMA 290:1749–1756, 2003. [DOI] [PubMed] [Google Scholar]
- 92. Briggs EC, Nguyen T, Wall MA, MacDonald KD. Oral antimicrobial use in outpatient cystic fibrosis pulmonary exacerbation management: a single-center experience. Clin Respir J 6:56–64, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Konstan MW, Schluchter MD, Xue W, Davis PB. Clinical use of Ibuprofen is associated with slower FEV1 decline in children with cystic fibrosis. Am J Respir Crit Care Med 1 176:1084–1089, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lindstrom DR, Conley SF, Splaingard ML, Gershan WM. Ibuprofen therapy and nasal polyposis in cystic fibrosis patients. J Otolaryngol 36:309–314, 2007. [DOI] [PubMed] [Google Scholar]
- 95. Shak S, Capon DJ, Hellmiss R, et al. Recombinant human DNase I reduces the viscosity of cystic fibrosis sputum. Proc Natl Acad Sci U S A 87:9188–9192, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Fuchs HJ, Borowitz DS, Christiansen DH, et al. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. The Pulmozyme Study Group. N Engl J Med 331:637–642, 1994. [DOI] [PubMed] [Google Scholar]
- 97. Quan JM, Tiddens HA, Sy JP, et al. A two-year randomized, placebo-controlled trial of dornase alfa in young patients with cystic fibrosis with mild lung function abnormalities. J Pediatr 139:813–820, 2001. [DOI] [PubMed] [Google Scholar]
- 98. Harms HK, Matouk E, Tournier G, et al. Multicenter, open-label study of recombinant human DNase in cystic fibrosis patients with moderate lung disease. DNase International Study Group. Pediatr Pulmonol 26:155–161, 1998. [DOI] [PubMed] [Google Scholar]
- 99. Cimmino M, Nardone M, Cavaliere M, et al. Dornase alfa as postoperative therapy in cystic fibrosis sinonasal disease. Arch Otolaryngol Head Neck Surg 131:1097–1101, 2005. [DOI] [PubMed] [Google Scholar]
- 100. Mainz JG, Schiller I, Ritschel C, et al. Sinonasal inhalation of dornase alfa in CF: A double-blind placebo-controlled cross-over pilot trial. Auris Nasus Larynx 38:220–227, 2011. [DOI] [PubMed] [Google Scholar]
- 101. Rowe SM, Accurso F, Clancy JP. Detection of cystic fibrosis transmembrane conductance regulator activity in early-phase clinical trials. Proc Am Thorac Soc 4:387–398, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Rowe SM, Clancy JP, Sorscher EJ. A breath of fresh air. Sci Am 305:68–73, 2011. [DOI] [PubMed] [Google Scholar]
- 103. Rowe SM, Pyle LC, Jurkevante A, et al. DeltaF508 CFTR processing correction and activity in polarized airway and non-airway cell monolayers. Pulm Pharmacol Ther 23:268–278, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Rowe SM, Varga K, Rab A, et al. Restoration of W1282X CFTR activity by enhanced expression. Am J Respir Cell Mol Biol 37:347–356, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Accurso FJ, Rowe SM, Clancy JP, et al. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med 363:1991–2003, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kim Chiaw P, Eckford PD, Bear CE. Insights into the mechanisms underlying CFTR channel activity, the molecular basis for cystic fibrosis and strategies for therapy. Essays Biochem 50:233–248, 2011. [DOI] [PubMed] [Google Scholar]
- 107. Pettit RS. Cystic fibrosis transmembrane conductance regulator-modifying medications: The future of cystic fibrosis treatment. Ann Pharmacother 46:1065–1075, 2012. [DOI] [PubMed] [Google Scholar]
- 108. Peltz SW, Welch EM, Jacobson A, et al. Nonsense suppression activity of PTC124 (ataluren). Proc Natl Acad Sci U S A 106:E64, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Zhang S, Smith N, Schuster D, et al. Quercetin increases cystic fibrosis transmembrane conductance regulator-mediated chloride transport and ciliary beat frequency: Therapeutic implications for chronic rhinosinusitis. Am J Rhinol Allergy 25:307–312, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Jones AM, Helm JM. Emerging treatments in cystic fibrosis. Drugs 69:1903–1910, 2009. [DOI] [PubMed] [Google Scholar]
- 111. Kellerman D, Rossi Mospan A, Engels J, et al. Denufosol: A review of studies with inhaled P2Y(2) agonists that led to Phase 3. Pulm Pharmacol Ther 21:600–607, 2008. [DOI] [PubMed] [Google Scholar]
- 112. Stammberger H. Endoscopic endonasal surgery–Concepts in treatment of recurring rhinosinusitis. Part II. Surgical technique. Otolaryngol Head Neck Surg 94:147–156, 1986. [DOI] [PubMed] [Google Scholar]
- 113. Lund VJ, Holmstrom M, Scadding GK. Functional endoscopic sinus surgery in the management of chronic rhinosinusitis. An objective assessment. J Laryngol Otol 105:832–835, 1991. [DOI] [PubMed] [Google Scholar]
- 114. Khalid AN, Mace J, Smith TL. Outcomes of sinus surgery in adults with cystic fibrosis. Otolaryngol Head Neck Surg 141:358–363, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Mainz JG, Hentschel J, Schien C, et al. Sinonasal persistence of Pseudomonas aeruginosa after lung transplantation. J Cyst Fibrosr 11:158–161, 2011. [DOI] [PubMed] [Google Scholar]
- 116. Lewiston N, King V, Umetsu D, et al. Cystic fibrosis patients who have undergone heart-lung transplantation benefit from maxillary sinus antrostomy and repeated sinus lavage. Transplant Proc 23:1207–1208, 1991. [PubMed] [Google Scholar]
- 117. Holzmann D, Speich R, Kaufmann T, et al. Effects of sinus surgery in patients with cystic fibrosis after lung transplantation: A 10-year experience. Transplantation 77:134–136, 2004. [DOI] [PubMed] [Google Scholar]
- 118. Virgin FW, Huang L, Roberson D, Sawicki G. Inter-hospital variation in the frequency of sinus surgery in pediatric patients with cystic fibrosis. Pediatr Pulmonol Suppl 47:358, 2012. [DOI] [PubMed] [Google Scholar]
- 119. Schulte DL, Kasperbauer JL. Safety of paranasal sinus surgery in patients with cystic fibrosis. Laryngoscope 108:1813–1815, 1998. [DOI] [PubMed] [Google Scholar]
- 120. Cuyler JP. Follow-up of endoscopic sinus surgery on children with cystic fibrosis. Arch Otolaryngol Head Neck Surg 118:505–506, 1992. [DOI] [PubMed] [Google Scholar]
- 121. Albritton FD, Kingdom TT. Endoscopic sinus surgery in patients with cystic fibrosis: An analysis of complications. Am J Rhinol 14:379–385, 2000. [DOI] [PubMed] [Google Scholar]
- 122. Rowe-Jones JM, Mackay IS. Endoscopic sinus surgery in the treatment of cystic fibrosis with nasal polyposis. Laryngoscope 106:1540–1544, 1996. [DOI] [PubMed] [Google Scholar]
- 123. Van Peteghem A, Clement PA. Influence of extensive functional endoscopic sinus surgery (FESS) on facial growth in children with cystic fibrosis. Comparison of 10 cephalometric parameters of the midface for three study groups. Int J Pediatr Otorhinolaryngol 70:1407–1413, 2006. [DOI] [PubMed] [Google Scholar]
- 124. Jaffe BF, Strome M, Khaw KT, Shwachman H. Nasal polypectomy and sinus surgery for cystic fibrosis–A 10 year review. Otolaryngol Clin North Am 10:81–90, 1977. [PubMed] [Google Scholar]
- 125. Yung MW, Gould J, Upton GJ. Nasal polyposis in children with cystic fibrosis: A long-term follow-up study. Ann Otol Rhinol Laryngol 111:1081–1086, 2002. [DOI] [PubMed] [Google Scholar]
- 126. Becker SS, de Alarcon A, Bomeli SR, et al. Risk factors for recurrent sinus surgery in cystic fibrosis: Review of a decade of experience. Am J Rhinol 21:478–482, 2007. [DOI] [PubMed] [Google Scholar]
- 127. April MM, Zinreich SJ, Baroody FM, Naclerio RM. Coronal CT scan abnormalities in children with chronic sinusitis. Laryngoscope 103:985–990, 1993. [DOI] [PubMed] [Google Scholar]
- 128. Lesserson JA, Kieserman SP, Finn DG. The radiographic incidence of chronic sinus disease in the pediatric population. Laryngoscope 104:159–166, 1994. [DOI] [PubMed] [Google Scholar]
- 129. Diament MJ, Senac MO, Jr, Gilsanz V, et al. Prevalence of incidental paranasal sinuses opacification in pediatric patients: A CT study. J Comput Assist Tomogr 11:426–431, 1987. [DOI] [PubMed] [Google Scholar]
- 130. Smith TL, Mendolia-Loffredo S, Loehrl TA, et al. Predictive factors and outcomes in endoscopic sinus surgery for chronic rhinosinusitis. Laryngoscope 115:2199–2205, 2005. [DOI] [PubMed] [Google Scholar]
- 131. Stewart MG, Donovan DT, Parke RB, Jr, Bautista MH. Does the severity of sinus computed tomography findings predict outcome in chronic sinusitis? Otolaryngol Head Neck Surg 123:81–84, 2000. [DOI] [PubMed] [Google Scholar]
- 132. McMurphy AB, Morriss C, Roberts DB, Friedman EM. The usefulness of computed tomography scans in cystic fibrosis patients with chronic sinusitis. Am J Rhinol 21:706–710, 2007. [DOI] [PubMed] [Google Scholar]
- 133. Eggesbo HB, Sovik S, Dolvik S, Kolmannskog F. CT characterization of inflammatory paranasal sinus disease in cystic fibrosis. Acta Radiol 43:21–28, 2002. [DOI] [PubMed] [Google Scholar]
- 134. Tandon R, Derkay C. Contemporary management of rhinosinusitis and cystic fibrosis. Curr Opin Otolaryngol Head Neck Surg 11:41–44, 2003. [DOI] [PubMed] [Google Scholar]
- 135. Nishioka GJ, Cook PR. Paranasal sinus disease in patients with cystic fibrosis. Otolaryngol Clin North Am 29:193–205, 1996. [PubMed] [Google Scholar]
- 136. Krzeski A, Kapiszewska-Dzedzej D, Jakubczyk I, et al. Extent of pathological changes in the paranasal sinuses of patients with cystic fibrosis: CT analysis. Am J Rhinol 15:207–210, 2001. [DOI] [PubMed] [Google Scholar]
- 137. Rickert S, Banuchi VE, Germana JD, et al. Cystic fibrosis and endoscopic sinus surgery: Relationship between nasal polyposis and likelihood of revision endoscopic sinus surgery in patients with cystic fibrosis. Arch Otolaryngol Head Neck Surg 136:988–992, 2010. [DOI] [PubMed] [Google Scholar]
- 138. Rosbe KW, Jones DT, Rahbar R, et al. Endoscopic sinus surgery in cystic fibrosis: Do patients benefit from surgery? Int J Pediatr Otorhinolaryngol 1 61:113–119, 2001. [DOI] [PubMed] [Google Scholar]
- 139. Jarrett WA, Militsakh O, Anstad M, Manaligod J. Endoscopic sinus surgery in cystic fibrosis: effects on pulmonary function and ideal body weight. Ear Nose Throat J 83:118–121, 2004. [PubMed] [Google Scholar]
- 140. Madonna D, Isaacson G, Rosenfeld RM, Panitch H. Effect of sinus surgery on pulmonary function in patients with cystic fibrosis. Laryngoscope 107:328–331, 1997. [DOI] [PubMed] [Google Scholar]
- 141. Kovell LC, Wang J, Ishman SL, et al. Cystic fibrosis and sinusitis in children: Outcomes and socioeconomic status. Otolaryngol Head Neck Surg 145:146–153, 2011. [DOI] [PubMed] [Google Scholar]
- 142. Woodworth BA, Parker RO, Schlosser RJ. Modified endoscopic medial maxillectomy for chronic maxillary sinusitis. Am J Rhinol 20:317–319, 2006. [DOI] [PubMed] [Google Scholar]
- 143. Shatz A. Management of recurrent sinus disease in children with cystic fibrosis: A combined approach. Otolaryngol Head Neck Surg 135:248–252, 2006. [DOI] [PubMed] [Google Scholar]
- 144. Cho DY, Hwang PH. Results of endoscopic maxillary mega-antrostomy in recalcitrant maxillary sinusitis. Am J Rhinol 22:658–662, 2008. [DOI] [PubMed] [Google Scholar]
- 145. Virgin F, Rowe SM, Wade MB, et al. Extensive surgical and comprehensive postoperative medical management for severe, recalcitrant cystic fibrosis chronic rhinosinusitis. Am J Rhinol Allergy 26:70–75, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]


