Abstract
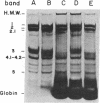
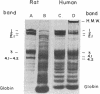
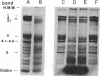
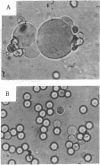
Rat, but not human, erythrocytes undergo fusion promoted by the membrane-mobility agent 2-(2-methoxyethoxy)-ethyl cis-8-(2-octylcyclopropyl)octanoate (A2C). The difference in behavior is correlated with rat erythrocyte membrane protein degradation caused by Ca2+-activated proteases. The human erythrocyte is deficient in such protease activity. Membrane protein degradation is a necessary, but not sufficient, requirement for membrane fusion. Membrane protein degradation probably releases membrane components from certain constraints. In addition, the motion of membrane components precedes fusion and must be promoted by reagents such as A2C, leading to the creation of fusion-potent lipid areas. This sequence of chemical and physical events occurs in other fusion processes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahkong Q. F., Botham G. M., Woodward A. W., Lucy J. A. Calcium-activated thiol-proteinase activity in the fusion of rat erythrocytes induced by benzyl alcohol. Biochem J. 1980 Dec 15;192(3):829–836. doi: 10.1042/bj1920829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahkong Q. F., Fisher D., Tampion W., Lucy J. A. The fusion of erythrocytes by fatty acids, esters, retinol and alpha-tocopherol. Biochem J. 1973 Sep;136(1):147–155. doi: 10.1042/bj1360147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J. The many forms and functions of cellular proteinases. Fed Proc. 1980 Jan;39(1):9–14. [PubMed] [Google Scholar]
- Bennett V. The molecular basis for membrane - cytoskeleton association in human erythrocytes. J Cell Biochem. 1982;18(1):49–65. doi: 10.1002/jcb.1982.240180106. [DOI] [PubMed] [Google Scholar]
- Beutler E., West C., Blume K. G. The removal of leukocytes and platelets from whole blood. J Lab Clin Med. 1976 Aug;88(2):328–333. [PubMed] [Google Scholar]
- Carraway K. L., Triplett R. B., Anderson D. R. Calcium-promoted aggregation of erythrocyte membrane proteins. Biochim Biophys Acta. 1975 Feb 27;379(2):571–581. doi: 10.1016/0005-2795(75)90163-4. [DOI] [PubMed] [Google Scholar]
- Couch C. B., Strittmatter W. J. Rat myoblast fusion requires metalloendoprotease activity. Cell. 1983 Jan;32(1):257–265. doi: 10.1016/0092-8674(83)90516-0. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Golan D. E., Veatch W. Lateral mobility of band 3 in the human erythrocyte membrane studied by fluorescence photobleaching recovery: evidence for control by cytoskeletal interactions. Proc Natl Acad Sci U S A. 1980 May;77(5):2537–2541. doi: 10.1073/pnas.77.5.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golovtchenko-Matsumoto A. M., Matsumoto I., Osawa T. Degradation of band-3 glycoprotein in vitro by a protease isolated from human erythrocyte membrane. Eur J Biochem. 1982 Jan;121(2):463–467. doi: 10.1111/j.1432-1033.1982.tb05810.x. [DOI] [PubMed] [Google Scholar]
- Haest C. W. Interactions between membrane skeleton proteins and the intrinsic domain of the erythrocyte membrane. Biochim Biophys Acta. 1982 Dec;694(4):331–352. doi: 10.1016/0304-4157(82)90001-6. [DOI] [PubMed] [Google Scholar]
- Hallett M. B., Fuchs P., Campbell A. K. Sendai virus causes a rise in intracellular free Ca2+ before cell fusion. Biochem J. 1982 Sep 15;206(3):671–674. doi: 10.1042/bj2060671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann J. X., Galla J. D., Emma D. A., Kao K. N., Gamborg O. L. The fusion of erythrocytes by treatment with proteolytic enzymes and polyethylene glycol. Can J Genet Cytol. 1976 Sep;18(3):503–512. doi: 10.1139/g76-062. [DOI] [PubMed] [Google Scholar]
- Kim J., Okada Y. Morphological changes in Ehrlich ascites tumor cells during the cell fusion reaction with HVJ (Sendai virus). II. Cluster formation of intramembrane particles in the early stage of cell fusion. Exp Cell Res. 1981 Mar;132(1):125–136. doi: 10.1016/0014-4827(81)90089-6. [DOI] [PubMed] [Google Scholar]
- King L. E., Jr, Morrison M. Calcium effects on human erythrocyte membrane proteins. Biochim Biophys Acta. 1977 Nov 15;471(1):162–168. doi: 10.1016/0005-2736(77)90404-7. [DOI] [PubMed] [Google Scholar]
- Kosower E. M., Kosower N. S., Faltin Z., Diver A., Saltoun G., Frensdorff A. Membrane mobility agents. A new class of biologically active molecules. Biochim Biophys Acta. 1974 Sep 6;363(2):261–266. doi: 10.1016/0005-2736(74)90065-0. [DOI] [PubMed] [Google Scholar]
- Kosower E. M., Kosower N. S., Wegman P. Membrane mobility agents. IV. The mechanism of particle-cell and cell-cell fusion. Biochim Biophys Acta. 1977 Dec 1;471(2):311–329. doi: 10.1016/0005-2736(77)90259-0. [DOI] [PubMed] [Google Scholar]
- Kosower N. S., Faltin Z., Kosower E. M. Cell membrane receptor classes delimited through cap formation either with diamide or with membrane mobility agent, A2C. J Immunol Methods. 1981;41(2):215–223. doi: 10.1016/0022-1759(81)90244-1. [DOI] [PubMed] [Google Scholar]
- Kosower N. S., Kosower E. M., Lustig S., Pluznik D. H. F20C, a new fluorescent membrane probe, moves more slowly in malignant and mitogen-transformed cell membranes than in normal cell membranes. Biochim Biophys Acta. 1978 Feb 2;507(1):128–136. doi: 10.1016/0005-2736(78)90380-2. [DOI] [PubMed] [Google Scholar]
- Kosower N. S., Kosower E. M., Wegman P. Membrane mobility agents. II. Active promoters of cell fusion. Biochim Biophys Acta. 1975 Sep 2;401(3):530–534. doi: 10.1016/0005-2736(75)90250-3. [DOI] [PubMed] [Google Scholar]
- Kosower N. S., Wegman P. O., Neiman T., Kosower E. M. Membrane mobility agents. V. Genetic variability in the fusibility of hen red cells. Exp Cell Res. 1978 Oct 15;116(2):454–457. doi: 10.1016/0014-4827(78)90469-x. [DOI] [PubMed] [Google Scholar]
- Kosower N. S., Zipser Y., Kosower E. M. Membrane mobility agents: alteration of human red blood cell membrane properties. Arch Biochem Biophys. 1980 Aug;203(1):325–331. doi: 10.1016/0003-9861(80)90183-6. [DOI] [PubMed] [Google Scholar]
- Liu S. C., Fairbanks G., Palek J. Spontaneous, reversible protein cross-linking in the human erythrocyte membrane. Temperature and pH dependence. Biochemistry. 1977 Sep 6;16(18):4066–4074. doi: 10.1021/bi00637a020. [DOI] [PubMed] [Google Scholar]
- Lorand L., Bjerrum O. J., Hawkins M., Lowe-Krentz L., Siefring G. E., Jr Degradation of transmembrane proteins in Ca2+-enriched human erythrocytes. An immunochemical study. J Biol Chem. 1983 Apr 25;258(8):5300–5305. [PubMed] [Google Scholar]
- Lorand L., Siefring G. E., Jr, Lowe-Krentz L. Formation of gamma-glutamyl-epsilon-lysine bridges between membrane proteins by a Ca2+-regulated enzyme in intact erythrocytes. J Supramol Struct. 1978;9(3):427–440. doi: 10.1002/jss.400090313. [DOI] [PubMed] [Google Scholar]
- Lorand L., Weissmann L. B., Epel D. L., Bruner-Lorand J. Role of the intrinsic transglutaminase in the Ca2+-mediated crosslinking of erythrocyte proteins. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4479–4481. doi: 10.1073/pnas.73.12.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui C. W., Meizel S. Further evidence in support of a role for hamster sperm hydrolytic enzymes in the acrosome reaction. J Exp Zool. 1979 Feb;207(2):173–185. doi: 10.1002/jez.1402070202. [DOI] [PubMed] [Google Scholar]
- Lustig S., Kosower N. S., Pluznik D. H., Kosower E. M. Inhibition of cytokinesis in Friend leukemia cells by membrane mobility agents. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2884–2888. doi: 10.1073/pnas.74.7.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig S., Pluznik D. H., Kosower N. S., Kosower E. M. Membrane mobility agent alters the consequences of lectin-cell interaction in a malignant cell membrane. Biochim Biophys Acta. 1975 Sep 2;401(3):458–467. doi: 10.1016/0005-2736(75)90243-6. [DOI] [PubMed] [Google Scholar]
- Lux S. E. Spectrin-actin membrane skeleton of normal and abnormal red blood cells. Semin Hematol. 1979 Jan;16(1):21–51. [PubMed] [Google Scholar]
- MORRISON W. L., NEURATH H. Proteolytic enzymes of the formed elements of human blood. I. Erythrocytes. J Biol Chem. 1953 Jan;200(1):39–51. [PubMed] [Google Scholar]
- Maeda T., Eldridge C., Toyama S., Ohnishi S. I., Elson E. L., Webb W. W. Membrane receptor mobility changes by Sendai virus. Exp Cell Res. 1979 Oct 15;123(2):333–343. doi: 10.1016/0014-4827(79)90475-0. [DOI] [PubMed] [Google Scholar]
- Moore G. L., Kocholaty W. F., Cooper D. A., Gray J. L., Robinson S. L. A proteinase from human erythrocyte membranes. Biochim Biophys Acta. 1970 Jul 15;212(1):126–133. doi: 10.1016/0005-2744(70)90185-3. [DOI] [PubMed] [Google Scholar]
- OKADA Y. Analysis of giant polynuclear cell formation caused by HVJ virus from Ehrlich's ascites tumor cells. I. Microscopic observation of giant polynuclear cell formation. Exp Cell Res. 1962 Feb;26:98–107. doi: 10.1016/0014-4827(62)90205-7. [DOI] [PubMed] [Google Scholar]
- Pant H. C., Gallant P. E., Gould R., Gainer H. Distribution of calcium-activated protease activity and endogenous substrates in the squid nervous system. J Neurosci. 1982 Nov;2(11):1578–1587. doi: 10.1523/JNEUROSCI.02-11-01578.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontremoli S., Salamino F., Sparatore B., Melloni E., Morelli A., Benatti U., De Flora A. Isolation and partial characterization of three acidic proteinases in erythrocyte membranes. Biochem J. 1979 Sep 1;181(3):559–568. doi: 10.1042/bj1810559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi K., Asano A. Participation of spectrin in Sendai virus-induced fusion of human erythrocyte ghosts. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1740–1744. doi: 10.1073/pnas.75.4.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohet S. B. Reconstitution of spectrin-deficient, spherocytic mouse erythrocyte membranes. J Clin Invest. 1979 Aug;64(2):483–494. doi: 10.1172/JCI109486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel D. L., Goodman S. R., Branton D. The effect of endogenous proteases on the spectrin binding proteins of human erythrocytes. Biochim Biophys Acta. 1980 Jun 6;598(3):517–527. doi: 10.1016/0005-2736(80)90032-2. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Kant J. A. Preparation of impermeable ghosts and inside-out vesicles from human erythrocyte membranes. Methods Enzymol. 1974;31:172–180. doi: 10.1016/0076-6879(74)31019-1. [DOI] [PubMed] [Google Scholar]
- Tarone G., Hamasaki N., Fukuda M., Marchesi V. T. Proteolytic degradation of human erythrocyte band 3 by membrane-associated protease activity. J Membr Biol. 1979 Jun 29;48(1):1–12. doi: 10.1007/BF01869253. [DOI] [PubMed] [Google Scholar]
- Tavassoli M., Kosower N. S., Halverson C., Aoki M., Kosower E. M. Membrane fusion induced by the membrane mobility agent, A2C. Differentiation between fusible and non-fusible cells. Transfer of fusibility. Biochim Biophys Acta. 1980 Oct 2;601(3):544–558. doi: 10.1016/0005-2736(80)90557-x. [DOI] [PubMed] [Google Scholar]
- Tökés Z. A., Chambers S. M. Proteolytic activity associated with human erythrocyte membranes. Self-digestion of isolated human erythrocyte membranes. Biochim Biophys Acta. 1975 May 6;389(2):325–338. doi: 10.1016/0005-2736(75)90325-9. [DOI] [PubMed] [Google Scholar]
- Waxman L. Calcium-activated proteases in mammalian tissues. Methods Enzymol. 1981;80(Pt 100):664–680. doi: 10.1016/s0076-6879(81)80051-1. [DOI] [PubMed] [Google Scholar]