Abstract
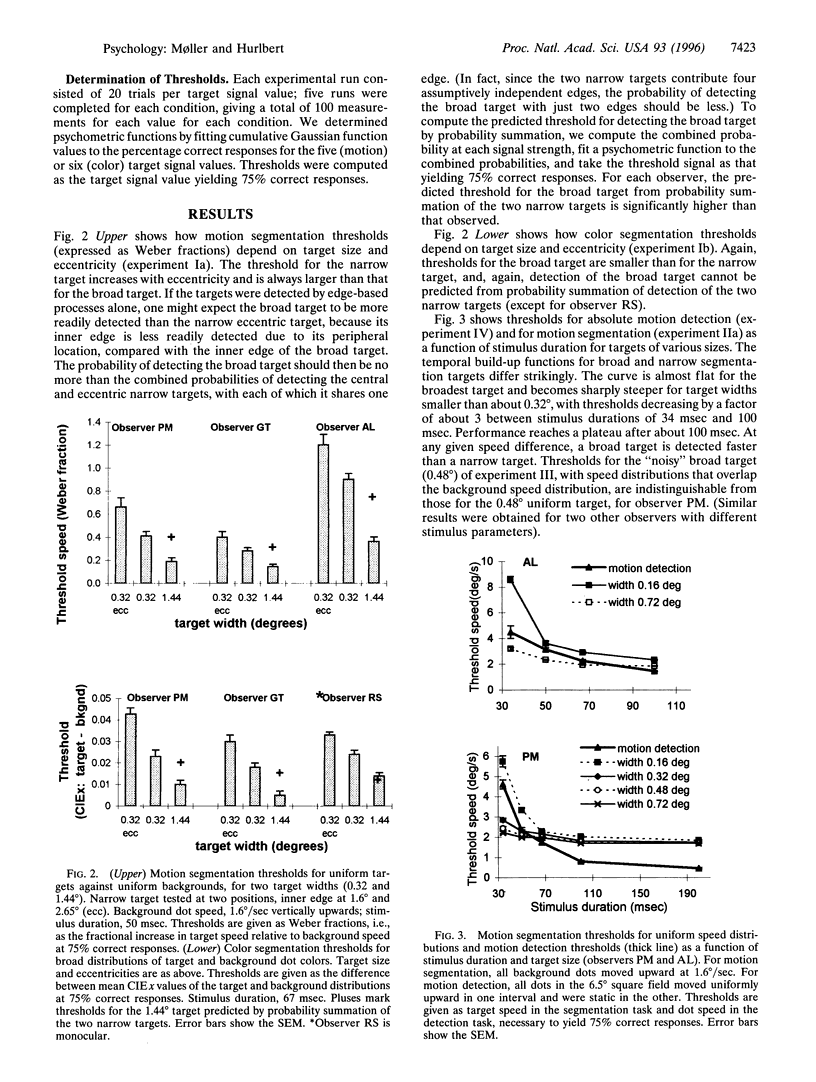
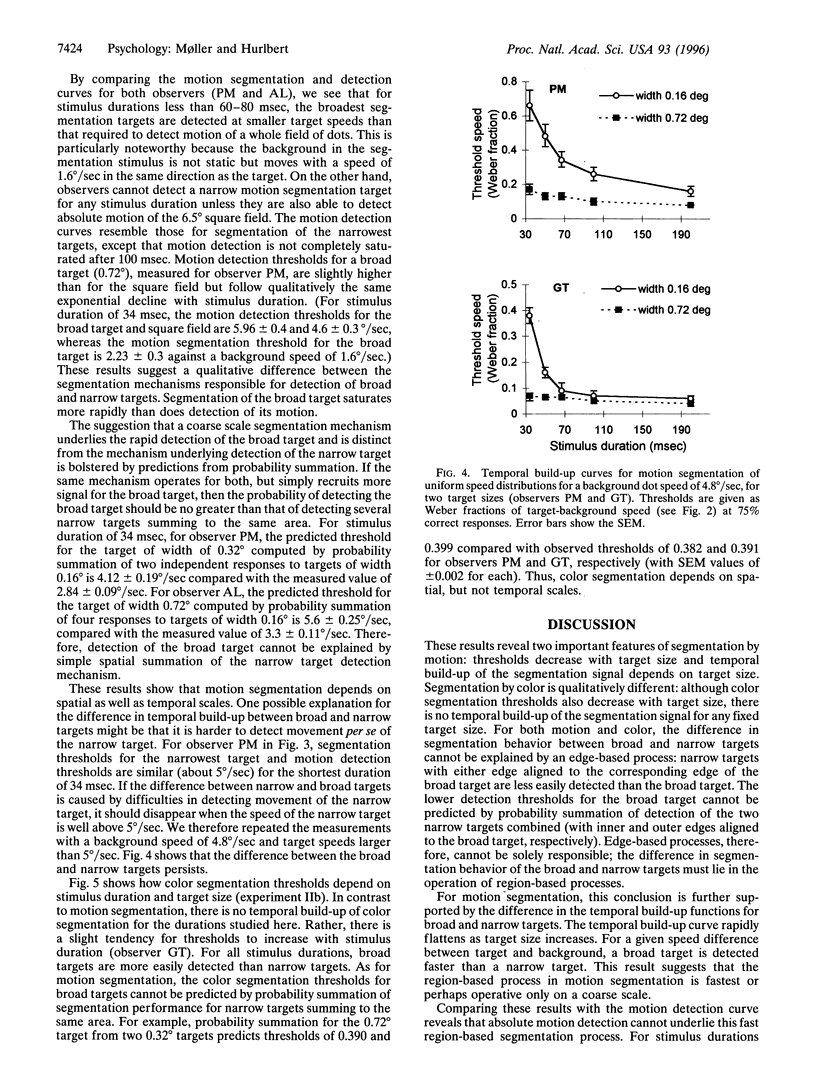
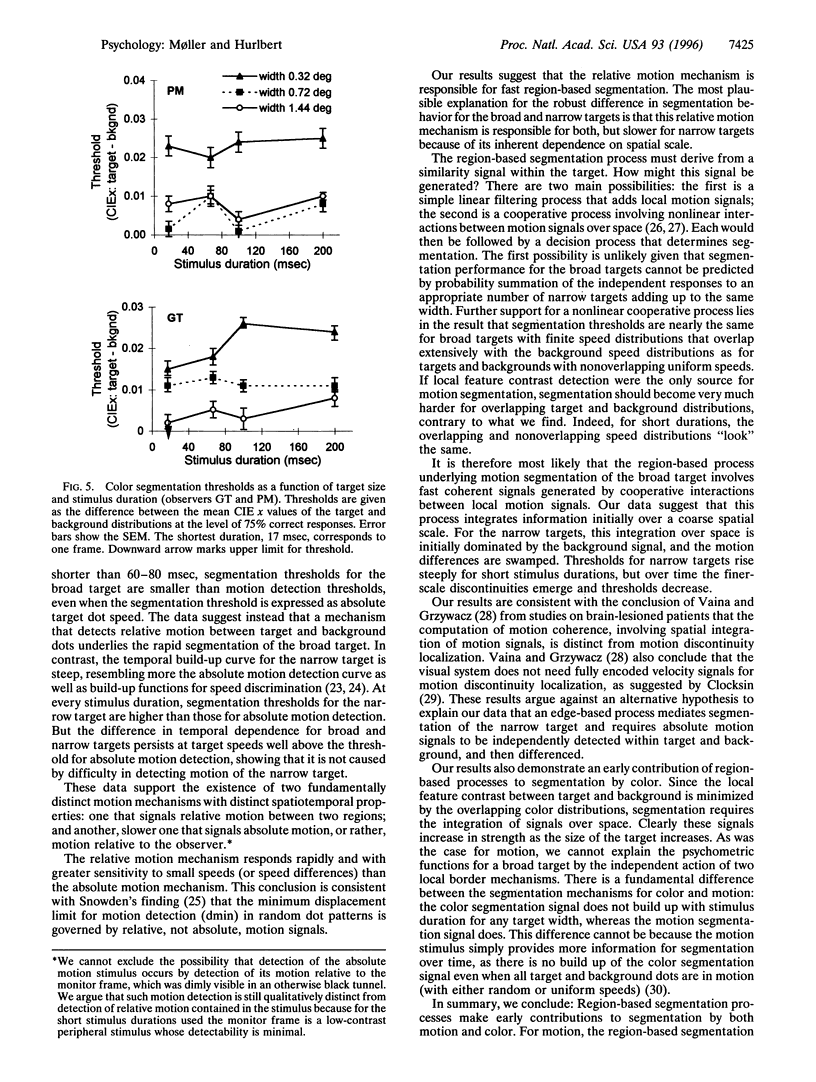
Theories of image segmentation suggest that the human visual system may use two distinct processes to segregate figure from background: a local process that uses local feature contrasts to mark borders of coherent regions and a global process that groups similar features over a larger spatial scale. We performed psychophysical experiments to determine whether and to what extent the global similarity process contributes to image segmentation by motion and color. Our results show that for color, as well as for motion, segmentation occurs first by an integrative process on a coarse spatial scale, demonstrating that for both modalities the global process is faster than one based on local feature contrasts. Segmentation by motion builds up over time, whereas segmentation by color does not, indicating a fundamental difference between the modalities. Our data suggest that segmentation by motion proceeds first via a cooperative linking over space of local motion signals, generating almost immediate perceptual coherence even of physically incoherent signals. This global segmentation process occurs faster than the detection of absolute motion, providing further evidence for the existence of two motion processes with distinct dynamic properties.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker C. L., Jr, Braddick O. J. Does segregation of differently moving areas depend on relative or absolute displacement? Vision Res. 1982;22(7):851–856. doi: 10.1016/0042-6989(82)90019-0. [DOI] [PubMed] [Google Scholar]
- Clocksin W. F. Perception of surface slant and edge labels from optical flow: a computational approach. Perception. 1980;9(3):253–269. doi: 10.1068/p090253. [DOI] [PubMed] [Google Scholar]
- De Bruyn B., Orban G. A. Human velocity and direction discrimination measured with random dot patterns. Vision Res. 1988;28(12):1323–1335. doi: 10.1016/0042-6989(88)90064-8. [DOI] [PubMed] [Google Scholar]
- Ferrera V. P., Nealey T. A., Maunsell J. H. Responses in macaque visual area V4 following inactivation of the parvocellular and magnocellular LGN pathways. J Neurosci. 1994 Apr;14(4):2080–2088. doi: 10.1523/JNEUROSCI.14-04-02080.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamme V. A., van Dijk B. W., Spekreijse H. Contour from motion processing occurs in primary visual cortex. Nature. 1993 Jun 10;363(6429):541–543. doi: 10.1038/363541a0. [DOI] [PubMed] [Google Scholar]
- Livingstone M., Hubel D. Segregation of form, color, movement, and depth: anatomy, physiology, and perception. Science. 1988 May 6;240(4853):740–749. doi: 10.1126/science.3283936. [DOI] [PubMed] [Google Scholar]
- Merigan W. H., Maunsell J. H. How parallel are the primate visual pathways? Annu Rev Neurosci. 1993;16:369–402. doi: 10.1146/annurev.ne.16.030193.002101. [DOI] [PubMed] [Google Scholar]
- Mumford D., Kosslyn S. M., Hillger L. A., Herrnstein R. J. Discriminating figure from ground: the role of edge detection and region growing. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7354–7358. doi: 10.1073/pnas.84.20.7354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothdurft H. C. Common properties of visual segmentation. Ciba Found Symp. 1994;184:245–271. doi: 10.1002/9780470514610.ch13. [DOI] [PubMed] [Google Scholar]
- Nothdurft H. C. The role of features in preattentive vision: comparison of orientation, motion and color cues. Vision Res. 1993 Sep;33(14):1937–1958. doi: 10.1016/0042-6989(93)90020-w. [DOI] [PubMed] [Google Scholar]
- Smith A. T., Snowden R. J., Milne A. B. Is global motion really based on spatial integration of local motion signals? Vision Res. 1994 Sep;34(18):2425–2430. doi: 10.1016/0042-6989(94)90286-0. [DOI] [PubMed] [Google Scholar]
- Snowden R. J., Braddick O. J. The temporal integration and resolution of velocity signals. Vision Res. 1991;31(5):907–914. doi: 10.1016/0042-6989(91)90156-y. [DOI] [PubMed] [Google Scholar]
- Snowden R. J. Sensitivity to relative and absolute motion. Perception. 1992;21(5):563–568. doi: 10.1068/p210563. [DOI] [PubMed] [Google Scholar]
- Van Essen D. C., Anderson C. H., Felleman D. J. Information processing in the primate visual system: an integrated systems perspective. Science. 1992 Jan 24;255(5043):419–423. doi: 10.1126/science.1734518. [DOI] [PubMed] [Google Scholar]
- Watt R. J. Scanning from coarse to fine spatial scales in the human visual system after the onset of a stimulus. J Opt Soc Am A. 1987 Oct;4(10):2006–2021. doi: 10.1364/josaa.4.002006. [DOI] [PubMed] [Google Scholar]
- Williams D., Phillips G., Sekuler R. Hysteresis in the perception of motion direction as evidence for neural cooperativity. Nature. 1986 Nov 20;324(6094):253–255. doi: 10.1038/324253a0. [DOI] [PubMed] [Google Scholar]
- Zeki S., Watson J. D., Lueck C. J., Friston K. J., Kennard C., Frackowiak R. S. A direct demonstration of functional specialization in human visual cortex. J Neurosci. 1991 Mar;11(3):641–649. doi: 10.1523/JNEUROSCI.11-03-00641.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Malsburg C. Binding in models of perception and brain function. Curr Opin Neurobiol. 1995 Aug;5(4):520–526. doi: 10.1016/0959-4388(95)80014-x. [DOI] [PubMed] [Google Scholar]


