Abstract
The Lyn tyrosine kinase regulates inhibitory signaling in B and myeloid cells – loss of Lyn results in a lupus-like autoimmune disease with hyperactive B cells and myeloproliferation. We have characterized the relative contribution of Lyn-regulated signaling pathways in B cells specifically to the development of autoimmunity by crossing the novel lynf/f animals with mice carrying the Cre recombinase under the control of the Cd79a promoter, resulting in deletion of Lyn in B cells. The specific deletion of Lyn in B cells is sufficient for the development of immune complex-mediated glomerulonephritis. The B cell-specific Lyn-deficient mice have no defects in early bone marrow B cell development but have reduced numbers of mature B cells with poor germinal centers, and increased numbers of plasma and B1a cells, similar to the lyn−/− animals. Within 8 months of life, B cell-specific Lyn mutant mice develop high titers of IgG anti-sm/RNP and anti-dsDNA autoantibodies, which deposit in their kidneys resulting in glomerulonephritis. B cell-specific Lyn mutant mice also develop myeloproliferation, similar to the lyn−/− animals. The additional deletion of MyD88 in B cells, achieved by crossing lynf/fCd79a-cre mice with myd88f/f animals, reversed the autoimmune phenotype observed in B cell-specific Lyn-deficient mice by blocking production of class-switched pathogenic IgG autoantibodies. Our results demonstrate that B cell intrinsic Lyn-dependent signaling pathways regulate B cell homeostasis and activation, which in concert with B cell-specific MyD88 signaling pathways can drive the development of autoimmune disease.
INTRODUCTION
Lyn is a Src-family tyrosine kinase (SFK) expressed by hematopoietic cells. It has unique regulatory properties, as it triggers both activation and inhibitory signals (1, 2). In B lymphocytes, Lyn functions at the initial step of B cell receptor (BCR) signaling by phosphorylating tyrosines in the immunoreceptor tyrosine-based activation motifs (ITAM) of the Igα/Igβ (CD79a/CD79b) BCR subunits, initiating signaling events that lead to B cell proliferation and antibody production. However, Lyn is not uniquely required for the initiation of BCR signaling, as the SFK members Fyn and Blk compensate for its deficiency (3). By contrast, Lyn has the sole capability to engage feedback inhibitory pathways by phosphorylating the immunoreceptor tyrosine-based inhibitory motifs (ITIM) of the sialic acid-binding protein CD22 and the inhibitory Fc receptor for IgG FcγRIIb (4). Phosphorylation of these ITIM-containing receptors by Lyn leads to the recruitment to the membrane of the SH2-domain-containing inositol phosphatase (SHIP-1) and SH2-domain-containing tyrosine phosphatase (SHP-1) that inhibit downstream BCR signaling. The function of Lyn in inhibitory signaling is dominant over its role in B cell activation. Hence, Lyn-deficiency in B cells leads to enhanced BCR signaling characterized by increased calcium flux, increased activation of the MAPK pathway and hyper-proliferative responses following BCR crosslinking (5, 6).
Systemic lupus erythematosus (SLE) is a complex autoimmune disease triggered by genetic and environmental factors. It is characterized by a loss of tolerance to nuclear antigens leading to the production of autoreactive antibodies, which deposit in tissues as immune complexes causing inflammation and end organ damage. In humans, polymorphisms in the LYN gene have been correlated with lupus disease and a reduction in LYN expression in B cells has been found in patients with SLE (7, 8). Lyn−/− mice develop an autoimmune inflammatory disease that resembles human lupus. The Lyn-deficient mice have elevated numbers of plasma cells that produce high levels of autoreactive antibodies (anti-double stranded (ds) DNA, anti-single stranded (ss) RNA) leading to severe glomerulonephritis (9-12). Additionally, the lyn−/− mice manifest considerable reduction in the numbers of mature follicular and immature transitional (T1, T2 and T3) B cells in lymphoid organs but equal numbers of newly formed immature B cells in the bone marrow (10, 13-15). The reduction in mature B cells in lyn−/− mice is thought to be due to defects in survival (increased Bim levels) rather than defects in developmental maturation (6). Interestingly, the exaggerated BCR signaling associated with Lyn-deficiency is predominant in transitional T3 and mature follicular B cell populations, but only moderate in immature T1 and T2 cells. Lyn−/− mature follicular B cells display enhanced basal calcium signaling and ERK activation upon BCR engagement (16). In response, Lyn-deficient transitional B cells and mature follicular B cells exhibit increased expression of CD69, MHCII and CXCR5 (6). Lyn−/− mice also present defects in germinal center (GC) formation with decreased numbers of GC B cells but accumulation of splenic plasmablasts and autoantibody-producing plasma cells (9-12).
The autoimmune phenotype of lyn−/− mice has been mainly attributed to alterations of B cell function. However, recent findings from our lab have shown that lyn−/− macrophages and dendritic cells (DCs) also play an important role in the pathogenesis of lupus-like disease in the lyn−/− model. Indeed, deletion of Lyn in myeloid cells (using mixed bone marrow chimeric mice) leads to myeloid expansion and the development of an autoimmune phenotype similar to, although not as severe as, the disease in total lyn−/− animals (17). In addition, the specific deletion of Lyn in DCs is sufficient for the spontaneous activation of B and T cells and the subsequent development of autoantibodies and severe nephritis in mice (18). Therefore, it remains unclear whether the altered signaling in B cells lacking Lyn kinase can drive disease in the context of wild type myeloid or dendritic cells. These studies raise the question as to the relative contributions of different immune cell types to autoimmune pathogenesis in the lyn−/− model.
To directly assess whether dysregulated Lyn-deficient B cells initiate or drive autoimmunity, we took advantage of the novel lynflox/flox transgenic mouse (lynf/f) (18), which we crossed with mice carrying the Cre recombinase under the control of the CD79a/mb1 promoter (Cd79a-cre) (19), resulting in deletion of Lyn in B cells. Here we show that the specific deletion of Lyn in B cells alone is sufficient for the spontaneous activation of B cells, the development of autoreactive antibodies and glomerulonephritis in mice. B cell-specific Lyn-deficient mice exhibited an increase in plasma cell formation as well as a reduction in splenic germinal centers. The additional deletion of MyD88 in B cells reversed the autoimmune phenotype observed in B cell-specific Lyn-deficient mice by blocking the development of pathogenic IgG autoantibodies. Thus we demonstrate that the absence of Lyn inhibitory signaling in B cells alone is sufficient to drive autoimmunity.
MATERIALS AND METHODS
Mice
Lyn−/−, lynflox/flox (lynf/f), myd88flox/flox (myd88f/f) and Cd79a-cre mice were previously described (18-20)}. All animals were backcrossed at least 9 generations onto the C57BL/6 background, and kept in a specific pathogen-free facility at UCSF. Animal experiments were done in compliance with the Animal Welfare Act and Regulations, the NIH Guide for the Care and Use of Laboratory Animals, the Public Health Service Policy on the Humane Care and Use of Laboratory Animals, and UCSF Policies and Guidelines.
All experiments were done comparing lynf/f Cd79a-cre+/+, lyn+/+ Cd79a-creTg/+ and C57BL/6 animals to lynf/f Cd79a-creTg/+ (lynf/f Cd79a-cre thereafter) and lyn−/−. The groups lynf/f Cd79a-cre+/+, lyn+/+ Cd79a-creTg/+ and C57BL/6 showed similar results (data not shown). For clarity, the data from these three groups were pooled and defined as the control group.
Flow cytometry
Single cell suspensions were prepared from spleens as previously described (17), blocked with anti-CD16/32 (clone 2.4G2) and murine IgG (Sigma-Aldrich), and stained with the following FITC-, RPE-, APC-, APC-Cy7 or APC-AlexaFluor 780-, PE-Cy7- or Biotin-conjugated antibodies from BD PharMingen: CD11c (clone HL3), CD19 (1D3), CD21 (7G6), CD95/Fas (Jo2), CD117/cKit (2B8), CD138 (281-2), GL7 (GL7), Gr-1 (RB6-8C5), IgDb (Igh-5b), Ly6C (AL-21) and MHCII (I-Ab, AF6-120.1); from eBiosciences: B220 (RA3-6B2), CD4 (RM4-5), CD5 (53-7.3), CD8α (53-6.7), CD11b (M1/70), CD23 (B3B4), CD25 ( PC61.5), CD44 (IM7), CD62L (MEL-14), CD86 (GL1), CD93 (AA4.1), IgM (II/41) and TCRβ (H57-597); from Abcam: 7/4; from Serotec: F4/80; from Jackson ImmunoResearch Laboratories: IgM Fab. The rabbit polyclonal antibody anti-mouse Lyn was previously described (17). The gating strategies used to identify the different myeloid, dendritic and T cell populations by FACS have been previously reported (18). The gating strategies used to identify B cell populations are shown in Fig. S2. DAPI (0.1 μg/ml; Sigma-Aldrich) staining was used to exclude dead cells.
Flow cytometry data were collected on a Fortessa flow cytometer (Becton Dickinson) from the Flow Cytometry Center at UCSF, and analyzed using FlowJo software (TreeStar).
Analysis of calcium flux
For measurements of intracellular-free calcium levels, splenocytes were loaded with Indo-1 AM (Invitrogen) and then labeled for CD93, CD23, IgM Fab and B220. Cells were resuspended in RPMI 1640 supplemented with 1% BSA and 20 mM HEPES and warmed to 37°C for 3 min, and analysis was initiated with flow cytometry. After the baseline was established for 30 – 45 seconds, cells were stimulated with 15 μg/ml goat anti-mouse IgM F(ab’)2 (Jackson ImmunoResearch Laboratories). The median intracellular calcium concentration as indicated by the ratio of fluorescence 405 nm emission to 530 nm emission was measured over time by flow cytometry. Propidium iodide staining was used to exclude dead cells.
Histology
Kidneys were processed and stained with hematoxylin & eosin (H&E) by the UCSF Pathology core. The presence of nephritis was determined on H&E-stained sections. The severities of the disease in the glomerular and interstitial compartments were arbitrarily graded as 0 (absent), 1 (mild), 2 (moderate) or 3 (severe). Morphological analysis of glomerulonephritis involved assessment of glomerular hypercellularity, glomerular size, and presence of glomerular sclerosis. Morphological analysis of interstitial nephritis involved the assessment of infiltration of mononuclear cells and loss of normal architecture. Kidneys were snap frozen in OCT and five-micron sections were stained for C3or IgG as previously described (17).
Quantification of serum immunoglobulins, autoantibodies and cytokines
Serum levels of BAFF, total IgG and IgM, anti-dsDNA IgG and IgM, and anti sm/RNP IgG and IgM were determined by ELISA as previously described (17). Serum levels of specific IgG isotypes against dsDNA and sm/RNP were determined by ELISA using Southern Biotech reagents. Cytokine levels in serum were quantified using Milliplex®MAP mouse cytokine/chemokine kit from Millipore, following the manufacturer’s instructions.
Statistical analyses
Statistical analyses were performed using GraphPad Prism. Statistical differences between three groups or more were calculated with a one-way ANOVA test and a Bonferroni’s multiple comparison post-test. Only significant differences (P ≤ 0.05) are indicated.
RESULTS
Generation of B cell-specific Lyn-deficient mice
To investigate the specific role of Lyn in B cells we crossed mice carrying the lynf/f allele with Cd79a-cre transgenic mice (18, 19). The efficiency of deletion of the lynf/f allele by Cre recombination under the control of the Cd79a promoter was determined by intracellular detection of Lyn followed by flow cytometry using peripheral blood leukocytes from 2 month-old mice (Fig. S1). In the control mice, Lyn was expressed in B and myeloid cells, but not T cells. Lyn expression in the B cells of lynf/f Cd79a-cre+ mice (B-lyn−/− mice hereafter) was reduced to the background levels seen in the total lyn−/− animals. We found no reduction in Lyn expression in peripheral blood myeloid cells in B-lyn−/− mice, demonstrating the specificity of Lyn deletion in B cells of B-lyn−/− mice.
Lyn inhibitory signaling in B cells is required to maintain B cell homeostasis and activation
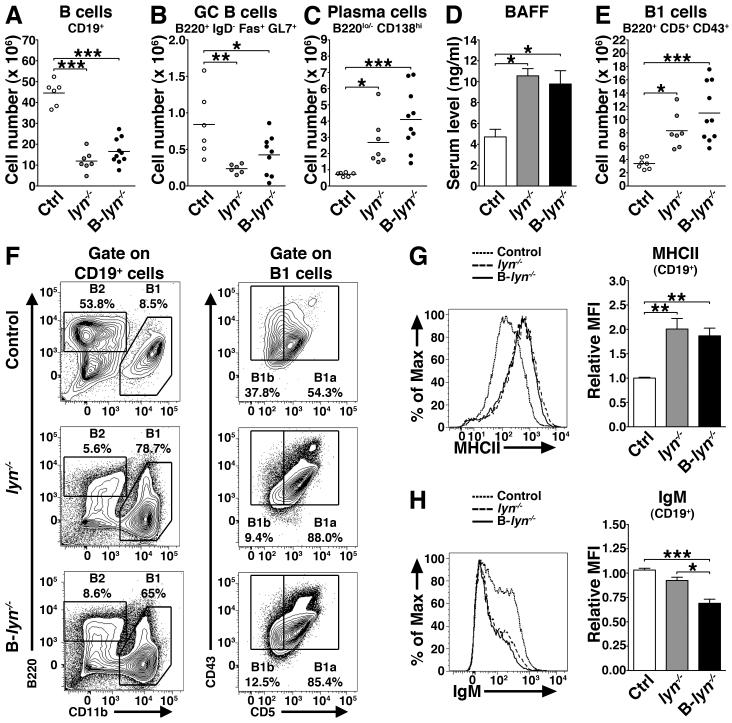
Previous studies have reported that lyn−/− mice exhibit alterations in their B cell compartment that correlate with their autoimmune phenotype (10-12, 16). We tested whether deletion of Lyn in B cells alone was sufficient to affect the B cell lineage. To this end we analyzed by flow cytometry the expression of cell surface markers indicative of developmental and activation status of B cells in the spleens of B-lyn−/− mice, compared to lyn−/− and control mice (gating strategies are shown in Fig. S2). Similar to lyn−/−, aged B-lyn−/− animals had significantly decreased numbers of CD19+ and GC B cells, with increased numbers of antibody-producing CD19lo/− CD138hi plasma cells and B1 cells in the spleens (Fig. 1 A-C and 1E). In the peritoneal cavity, B1 cells were expanded in number (Control 1.82×105 ± 0.66; lyn−/− 6.7×105 ± 0.75, *** vs. control; B-lyn−/− 17.29×105 ± 0.29, *** vs. control, * vs. lyn−/−), with a reciprocal reduction in peritoneal B2 cells (Fig. 1F). The B1 cells in the spleen and peritoneal cavity were CD5+ and therefore were B1a cells (Fig. 1E and F). Interestingly B-lyn−/− mice exhibited high level of circulating BAFF, similar to that observed in lyn−/− animals (Fig. 1D), which might contribute to the survival, activation and differentiation of plasma and B1 cells in B-lyn−/− and lyn−/− mice. We next characterized by flow cytometry the expression of cell surface MHCII and IgM on the splenic B cells of 8 month-old B-lyn−/− mice, compared to lyn−/− and control mice. We found that, like lyn−/−, expression of MHCII was significantly elevated in B-lyn−/− B cells, while IgM cell surface levels were significantly downregulated (Fig. 1G and H). These observations likely reflect an enhanced degree of BCR signaling in vivo in both lyn−/− and B-lyn−/− mice (21).
Figure 1. Lyn inhibitory signal in B cells controls B cell homeostasis.
(A-C) Absolute numbers of total B cells (A), GC B cells (B) and plasma cells (C) in the spleens of 8 month-old control, lyn−/− and B-lyn−/− mice. Data represent mean of independent experiments. Each dot represents an individual mouse.
(D) BAFF levels in the serum of control, lyn−/− and B-lyn−/− mice (8 month-old) were determined by ELISA. Bars represent mean ± SEM from 6 – 10 mice per group.
(E) Absolute numbers of B1 cells in the spleens of 8 month-old control, lyn−/− and B-lyn−/− mice. Data represent mean of independent experiments. Each dot represents an individual mouse.
(F) Representative FACS contours showing the percentages of B1 and B2 cells in the peritoneal cavity of 8 month-old control, lyn−/− and B-lyn−/− mice. B1 cells were determined as CD19+ CD11b+ B220lo/− CD43+ cells and further defined as B1a (CD5+) or B1b (CD5−) subsets. B2 cells were determined as CD19+ B220+ CD11b− cells.
(G) Representative FACS histogram showing the expression level of MHCII by splenic B cells from 8 month-old mice (left panel). MFI (Relative to control) of MHCII expressed by B cells in the spleens of 8 month-old mice (right panel). Bars represent mean ± SEM of independent experiments from 6-10 mice per group.
(H) Representative FACS histogram showing the expression level of IgM by splenic B cells from 8 month-old mice (left panel). MFI (relative to control) of IgM expressed by B cells in the spleens of 8 month-old mice (right panel). Bars represent mean ± SEM of independent experiments from 6-10 mice per group.
(A-H) * P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001 (One-way ANOVA).
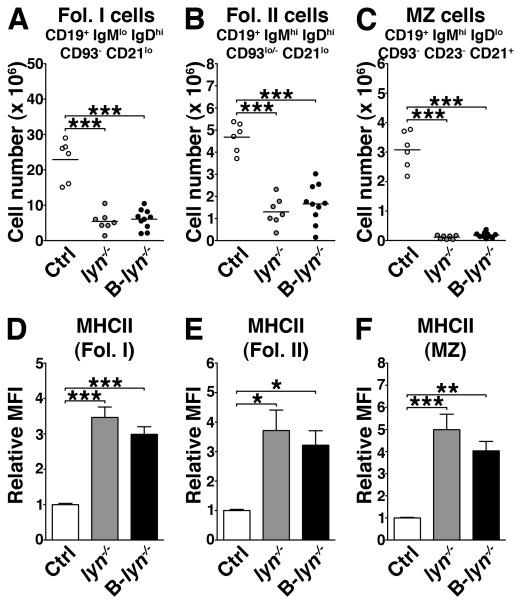
Lyn−/− mice have a significant reduction in mature peripheral B cells due compromised survival as cells pass from transitional stage 1 (T1) to T2 and T2/T3 to mature stages (15, 16). We analyzed by flow cytometry the effect of B cell-specific deletion of Lyn on B cell subpopulations in the spleens of 8 month-old control, lyn−/− and B-lyn−/− mice. We observed a significant decrease in the numbers of follicular (Fol. I and Fol. II) and marginal zone (MZ) B cells, in both lyn−/− and B-lyn−/− animals (Fig. 2 A-C). The remaining mature B cells in both strains had significantly increased MHCII expression levels, suggesting that these cells were activated (Fig. 2 D-F).
Figure 2. Lyn is required for the development of mature B cells.
(A-C) Absolute numbers of follicular I (Fol. I, A), follicular II (Fol. II, B) and marginal zone (MZ) B cells (C) in the spleens of 8 month-old control, lyn−/− and B-lyn−/− mice. Data represent mean of independent experiments. Each dot represents an individual mouse.
(D-F) MFI (relative to control) of MHCII expressed by Fol. I (D), Fol. II (E) and MZ (F) B cells in the spleens of 8 month-old mice. Bars represent mean ± SEM of independent experiments from 6-10 mice per group.
(A-F) * P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001 (One-way ANOVA).
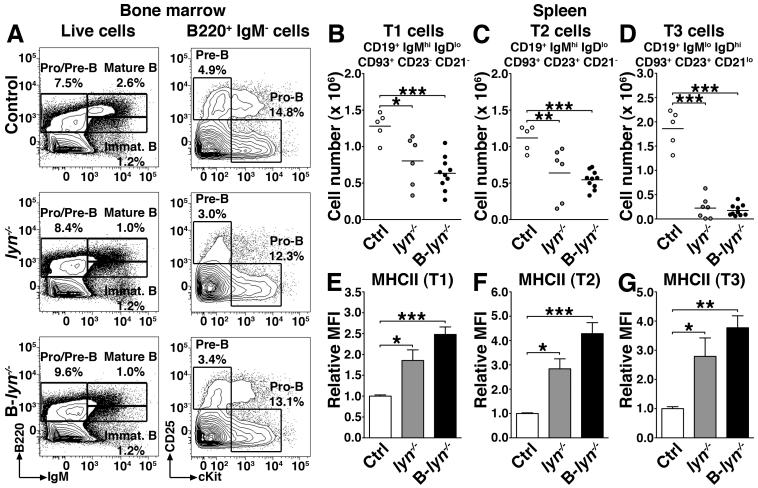
To more extensively characterize the effects of Lyn deletion on B cell development we examined the bone marrow compartment of 2 month-old lyn−/− and B-lyn−/− mice. Both total lyn−/−and B-lyn−/− mice exhibited normal percentages of Pre-B, Pro-B and immature B cells in the bone marrow (Fig. 3A), but showed significantly decreased numbers of mature recirculating B cells as it has been previously observed (6, 10, 22). By contrast, splenic B cell development was equivalently reduced in both lyn−/− and B-lyn−/− mice; both strains manifested progressively reduced numbers of splenic transitional B cells, with T3 cells being the lowest (Fig. 3 B-D). The remaining splenic transitional B cell types all had significantly elevated MHCII expression (Fig. 3 E-G). These results demonstrate that a Lyn-dependent mechanism, intrinsic to B cells, controls B cell homeostasis and activation.
Figure 3. B cell intrinsic loss of Lyn leads to reduction in splenic T1, T2 and T3 cells.
(A) Representative contour plots showing the percentages of Pro/Pre-B and mature/immature B cells in the bone marrow of 2 month-old control, lyn−/− and B-lyn−/− mice.
(B-D) Absolute numbers of transitional T1 (B), T2 (C) and T3 (D) B cells in the spleens of 2 month-old control, lyn−/− and B-lyn−/− mice. Data represent mean of independent experiments. Each dot represents an individual mouse.
(E-G) MFI (relative to control) of MHCII expressed by transitional T1 (E), T2 (F) and T3 (G) B cells in the spleens of 2 month-old mice. Bars represent mean ± SEM of independent experiments from 5-10 mice per group.
(B-G) * P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001 (One-way ANOVA).
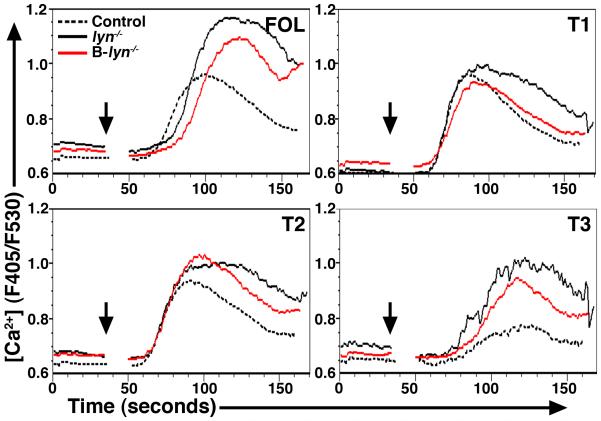
B cells isolated from total lyn−/− mice have enhanced signaling responses to BCR engagement (14, 16, 23). We asked whether the B cells in B-lyn−/− mice manifested a similar phenotype by measuring intracellular-free calcium in splenic B cells upon stimulation with 15 μg/ml of anti-IgM F(ab’)2 (Fig. 4). Similar to lyn−/−, B-lyn−/− follicular and T3 cells manifested increased calcium flux responses following BCR crosslinking. Lyn-deficiency had a minor effect on calcium responses in T1 and T2 B cells in both strains of mice. These results confirm that Lyn expressed by mature follicular and T3 cells restrains calcium signaling upon BCR engagement.
Figure 4. Lyn signal in follicular and T3 B cells restrains calcium signaling upon BCR engagement.
Calcium release in control, lyn−/− and B-lyn−/− T1, T2, T3 and follicular splenic B cells after stimulation with 15 μg/ml anti-IgM F(ab’)2. Histograms show median intracellular Ca2+ concentration as measured by fluorescence ratio (F405/F530). Data are representative of three independent experiments.
Selective deletion of lyn in B cell leads to T cell activation and myeloid cell expansion
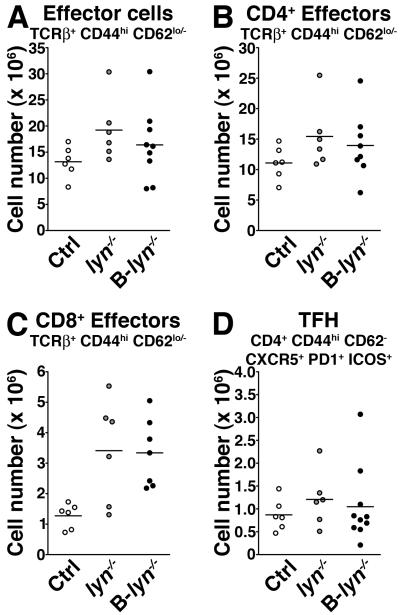
T cells do not express Lyn, but it has been shown that aged lyn−/− mice have increased numbers of activated T cells, which fuels the autoimmune disease via a feedback loop between T and myeloid cells (17, 24, 25). Specific deletion of Lyn in B cells led to a non-significant trend towards increased numbers of effector T cell populations in the spleen (Fig. 5 A-C). Consistent with the lack of GC formation in lyn−/− and B-lyn−/− mice, there was no change in the numbers of ICOS+ CXCR5+ PD1+ T follicular helper cells (among the CD4+ CD44hi CD62L− T cell population, Fig. 5D).
Figure 5. Selective deletion of Lyn in B cells leads to mild T cell activation.
Absolute numbers of total (A), CD4+ (B), CD8+ (C) effector and T follicular helper cells (D) in the spleens of 8 month-old control, lyn−/− and B-lyn−/− mice. Data represent mean of independent experiments. Each dot represents an individual mouse. (A-D) Not statistically significant (One-way ANOVA).
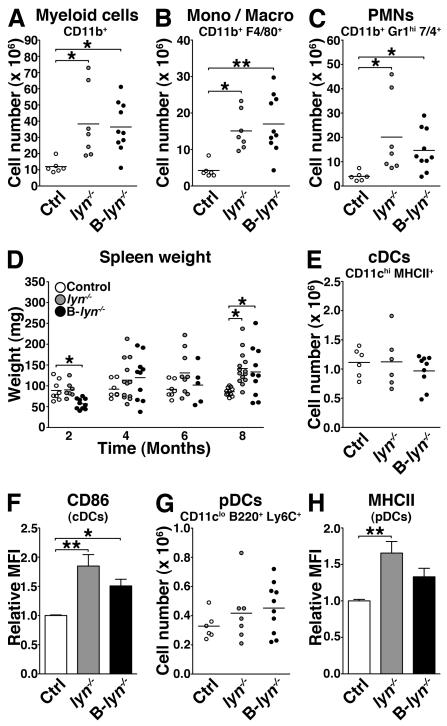
Although B-lyn−/− myeloid cells express Lyn, we observed a significant increase in the numbers of CD11b+ cells in both 8 month-old B-lyn−/− and lyn−/− spleens (Fig. 6A), due to increases in both the monocyte/macrophage (CD11b+ F4/80+) and granulocyte (CD11b+ 7/4+ Gr-1hi) subsets (Fig. 6 B and C). The myeloid expansion observed in the B-lyn−/− mice resulted in significant splenomegaly and altered architecture of the lymphoid tissues at 8 months of age, comparable to that observed in the lyn−/− mice (Fig. 6D and Fig. S3).
Figure 6. Specific deletion of Lyn in B cells leads to myeloproliferative phenotype comparable to lyn−/−.
(A-C) Absolute numbers of total myeloid cells (A), monocytes/macrophages (B) and granulocytes (C) in the spleens of 8 month-old control, lyn−/− and B-lyn−/− mice.
(D) Weights of spleens harvested from control, lyn−/− and B-lyn−/− mice were recorded at 2, 4, 6 and 8 months of age.
(E) Absolute numbers of conventional DCs in the spleens of 8 month-old control, lyn−/− and B-lyn−/− mice.
(F) MFI (relative to control) of CD86 expressed by conventional DCs in the spleens of 8 month-old mice. Bars represent mean ± SEM of independent experiments from 6-10 mice per group.
(G) Absolute numbers of plasmacytoid DCs in the spleens of 8 month-old control, lyn−/− and B-lyn−/− mice.
(H) MFI (relative to control) of MHCII expressed by plasmacytoid DCs in the spleens of 8 month-old mice. Bars represent mean ± SEM of independent experiments from 6-10 mice per group.
(A-E, G) Data represent mean of independent experiments. Each dot represents an individual mouse. (A-H) * P ≤ 0.05, ** P ≤ 0.01 (One-way ANOVA).
In lyn−/− mice, conventional DCs (cDCs) and plasmacytoid DCs (pDCs) express high levels of the costimulatory molecules CD86 and MHCII respectively, indicative of their activated state. In the spleens of 8 month-old B-lyn−/− mice, the numbers of cDCs and pDCs remained unchanged (Fig. 6 E and G). However, the Lyn-sufficient cDCs from B-lyn−/− mice expressed high levels of CD86 (Fig. 6F). Hence, deletion of Lyn in B cells alone is sufficient to induce expansion of the myeloid cell compartments and modest activation of DCs.
Specific deletion of lyn in B cells results in the development of autoimmunity
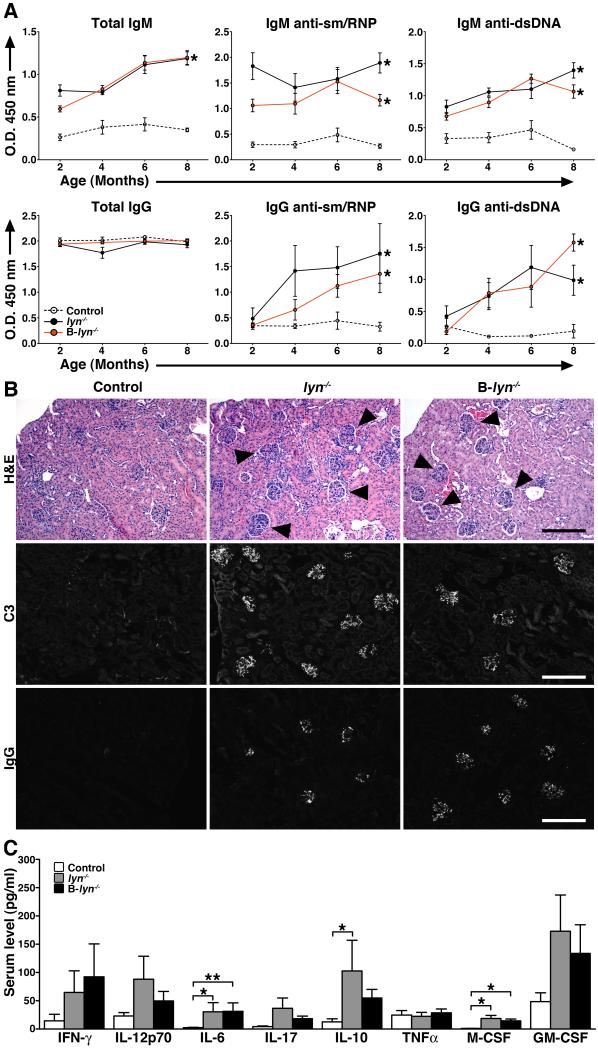
To provide a conclusive proof that Lyn-deficiency in B cells alone can lead to lupus-like autoimmune disease, B-lyn−/− mice were examined for the presence of circulating autoantibodies and tissue immune complexes. Consistent with the presence of exaggerated numbers of plasma cells in the spleens, B-lyn−/− mice showed high levels of IgM- and IgG-isotype anti-sm/RNP and anti-dsDNA autoreactive antibodies in the serum, at levels similar to those found in lyn−/− animals (Fig. 7A). As a result, B-lyn−/− mice showed enlarged kidney glomeruli, with both IgG and C3 deposition, as well as increased leukocyte infiltration representative of glomerulonephritis (Fig. 7B). The degrees of IgG and C3 deposition, as well glomerulonephritis, were similar in lyn−/− versus B-lyn−/− animals (Table 1). Hence, specific deletion of Lyn in B cells alone is sufficient to cause development of immune complex-mediated nephritis in mice.
Figure 7. Specific deletion of Lyn in B cells is sufficient for the development of lupus-like autoimmunity but not inflammatory disease.
(A) Detection of autoreactive IgM- (upper panel) and IgG-isotype (lower panel) antibodies in the serum of 2-, 4-, 6- and 8- month-old control, lyn−/− and B-lyn−/− mice. Data represent mean ± SEM from 6 – 10 mice per group.
(B) H&E, C3 and IgG staining of kidney sections from 8 month-old control, lyn−/− and B-lyn−/−mice. Arrowheads indicate enlarged glomeruli. Representative pictures are shown. Scale bars represent 50 μm.
(C) Serum cytokine levels comparing control, lyn−/− and B-lyn−/− mice (6-8 month-old) were determined by Luminex assay. Bars represent mean ± SEM from 6 – 14 mice per group. (A and C), * P ≤ 0.05, ** P ≤ 0.01, ** P ≤ 0.001 (One-way ANOVA).
Table I.
Histological scoringa
| Nephritis | C3 | IgG | |
|---|---|---|---|
| Control | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.0 ± 0.0 |
| lyn−/− | 1.40 ± 0.20 ** vs. control |
1.40 ± 0.89 * vs. control |
1.40 ± 0.40 |
| B-lyn−/− | 1.50 ± 0.19 *** vs. control |
0.63 ± 0.26 | 1.12 ± 0.35 |
| B-lyn−/− myd88−/− | 0.40 ± 0.24 * vs. lyn−/− ** vs. B-lyn−/− |
0.17 ± 0.17 * vs. lyn−/− |
0.33 ± 0.21 |
Kidney inflammation was scored in a blinded fashion as described in Materials and Methods. Briefly, the presence and severities of the disease in the glomerular and interstitial compartments were arbitrarily graded as 0 (absent), 1 (mild), 2 (moderate) or 3 (severe). Data are averaged from at least 5 microscopic fields per specimen, with 5-7 mice (8 month-old) per group.
P ≤ 0.05 versus control (One-way ANOVA).
Cytokines play an important role in the pathogenesis of SLE by stimulating immune cell activation leading to organ damage. We measured the concentration of cytokines in the sera of 6 month-old B-lyn−/−, lyn−/− and control mice by Luminex bead array (Fig. 7C). As previously observed, the lyn−/− mice had elevated levels of numerous cytokines such as IFN-γ, IL-6, IL-10, IL-17, IL12-p40, M-CSF and GM-CSF (26). To a lesser extent, similar changes were also observed in the B-lyn−/− animals (Fig. 7C). However, neither lyn−/− nor B-lyn−/− mice showed signs of exaggerated tissue inflammation other than in the kidney. Thus we demonstrate that the absence of Lyn in B cells alone is sufficient to induce the development of glomerulonephritis in mice, resembling the process seen in the global lyn−/− strain.
Lack of MyD88 in Lyn-deficient B cells prevents the development of pathogenic class-switched IgG autoantibodies
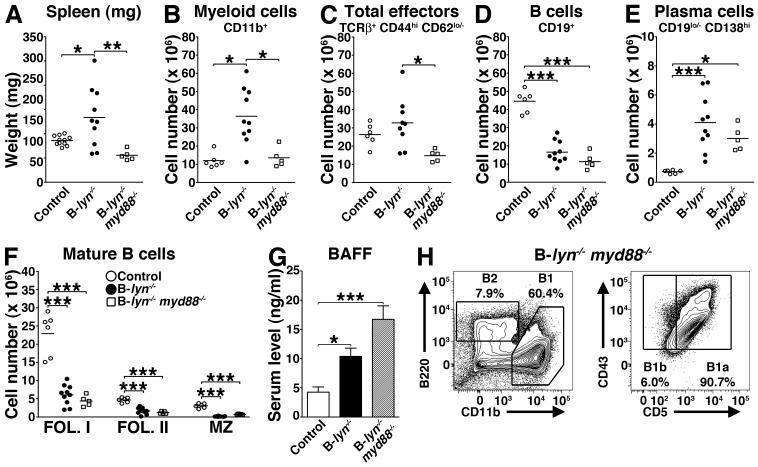
MyD88-dependent Toll-like receptor (TLR) signals in B cells or DCs regulate the degree of IgG responses during immunization (27). We investigated whether MyD88 signals in B cells also control the anti-self antibody response in B-lyn−/− animals that leads to the lupus-like disease in these mice. To this end we generated mice bearing deletion of both MyD88 and Lyn in B cells (20). Double mutant lynf/f myd88f/f Cd79a-cre+ mice (B-lyn−/− myd88−/− mice hereafter) were euthanized at 8 months of age for analysis. MyD88 deletion in B cells of B-lyn−/− mice was sufficient to reverse the splenomegaly, myeloid expansion as well as reduce the T cell activation present in B-lyn−/− mice (Fig. 8 A-C). Indeed, the weights of B-lyn−/− myd88−/− spleens were decreased compared to B-lyn−/− mice, similar to those observed in control mice (Fig. 8A). Analysis of splenocytes by flow cytometry revealed that the numbers of myeloid and total effector T cells in B-lyn−/− myd88−/− mice were significantly diminished compared to B-lyn−/− mice, reaching the baseline numbers of control mice (Fig. 8 B and C). However, deletion of MyD88 in Lyn-deficient B cells did not rescue the defect in B cell homeostasis observed in Lyn-deficient B cells. B-lyn−/− myd88−/− mice exhibited decreased numbers of total and mature B cells, but increased numbers of plasma cells, similar to the B-lyn−/− animals (Fig. 8 D-F). Interestingly, B-lyn−/−myd88−/− mice still showed high levels of serum BAFF and increased numbers of B1 cells in the peritoneal cavity as observed in the B-lyn−/− and lyn−/− mice (10×105 ± 1.8 B1 cells, *** vs. control, ** vs. lyn−/−). Our observations suggest that elevated BAFF levels might be responsible for the expansion of the B1 compartment (Fig. 8 G and H).
Figure 8. Deletion of MyD88 in Lyn-deficient B cells rescues the myeloproliferative phenotype but not the defect in B cell homeostasis observed in Lyn-deficient B cells.
(A) Weights of spleens harvested from 8 month-old control, B-lyn−/− and B-lyn−/− myd88−/− mice. (B-F) Absolute numbers of myeloid (B), total effector (C), B (D), plasma (E) and mature B cells (F) in the spleens of 8 month-old control, B-lyn−/− and B-lyn−/− myd88−/− mice. (A-F) Data from control and B-lyn−/− groups are the same as represented in Figs. 1, 2, 6 and 7 to perform comparisons to the B-lyn−/− myd88−/− mice. Data represent mean of independent experiments. Each dot represents an individual mouse. * P ≤ 0.05, ** P ≤ 0.01, ** P ≤ 0.001 (One-way ANOVA).
(G) BAFF levels in the serum of control, B-lyn−/− and B-lyn−/− myd88−/− mice (8 month-old) were determined by ELISA. Bars represent mean ± SEM from 6 – 10 mice per group.
(H) Representative FACS contours showing the percentages of B1 and B2 cells in the peritoneal cavity of 8 month-old B-lyn−/− myd88−/− mice. B1 cells were determined as CD19+ CD11b+ B220lo/− CD43+ cells and further defined as B1a (CD5+) or B1b (CD5−) subsets. B2 cells were determined as CD19+ B220+ CD11b− cells.
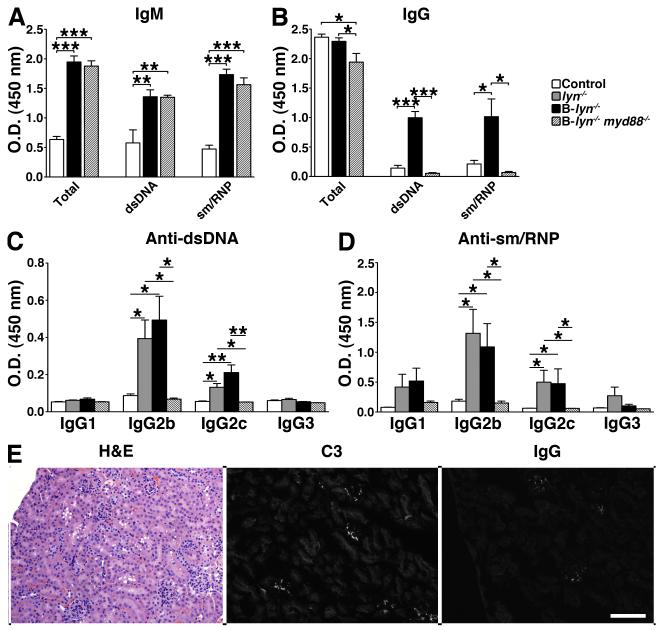
We then asked whether double mutant mice developed autoimmunity by examining their serum levels of autoreactive antibodies and their kidneys for signs of lupus-like disease. Although they showed similar levels of IgM antibodies, B-lyn−/− myd88−/− mice failed to develop pathogenic anti-dsDNA or anti-sm/RNP IgG antibodies compared to B-lyn−/− mice (Fig. 9 A and B). We further investigated the specific IgG subclasses of the autoantibodies in the serum of these mice. In the serum of lyn−/− and B-lyn−/− mice, IgG autoantibodies (anti-dsDNA and anti-sm/RNP) were mainly of the IgG2b and 2c subclasses, which are highly pathogenic (Fig. 9 C and D). MyD88-deficiency in B-lyn−/− mice resulted in the abrogation of the IgG2b and 2c anti-dsDNA and anti-sm/RNP autoreactive antibodies (Fig. 9 C and D). In agreement, kidney glomeruli from B-lyn−/− myd88−/− mice were normal in size and cellularity, and showed little or no evidence of leukocyte infiltration, C3 or IgG deposition (Fig. 9E). Thus, the loss of MyD88-dependent signals in Lyn-deficient B cells prevented class switching to pathogenic IgG autoantibodies, reversing the autoimmune phenotype observed in B-lyn−/− mice.
Figure 9. Specific deletion of Lyn and MyD88 in B cells prevents the development of pathogenic class-switched autoantibodies and autoimmunity.
(A-D) Detection of autoreactive IgM-isotype (A), IgG-isotype (B) and pathogenic class-switched IgG anti-dsDNA (C) or anti-sm/RNP (D) antibodies in the serum of 8- month-old control, B-lyn−/− and B-lyn−/− myd88−/− mice. Bars represent mean ± SEM from 6 mice per group. * P ≤ 0.05, ** P ≤ 0.01, ** P ≤ 0.001 (One-way ANOVA).
(E) H&E, C3 and IgG staining of kidney sections from 8 month-old B-lyn−/− myd88−/− mice. Representative pictures are shown. Scale bars represent 50 μm.
DISCUSSION
There is growing recognition that complex interactions between B, T and dendritic cells underlie the pathogenesis of autoimmune diseases such as systemic lupus erythematosus (SLE) (28, 29). Clearly, this is the case in the Lyn kinase-deficient mouse model. While many studies have focused on the role of this kinase as a negative regulator in B lymphocytes, it is also a major regulator of innate immune cells, including DCs, neutrophils and mast cells (2). To determine whether loss of Lyn-mediated inhibitory signaling in B cells alone is sufficient to initiate lupus-like autoimmunity, we have used the newly generated lynf/f mouse to delete Lyn specifically in B cells (18). The B-lyn−/− mice displayed alterations in B cell homeostasis (low follicular, MZ and GC cells) and signaling (elevated calcium responses) as seen in the global lyn−/−, confirming that these defects are due to B cell intrinsic alterations. Importantly, the B-lyn−/− animals developed autoantibodies leading to glomerulonephritis, accompanied by myeloproliferation, T cell activation, similar to what was seen in the global lyn−/− strain. The disease process in the B-lyn−/− mutants was dependent on MyD88-mediated signals in B cells, as deletion of myd88 in the B cells of B-lyn−/− mice (i.e. double conditional mutant mice lacking both Lyn and MyD88 in B cells alone) reversed the disease process. These results demonstrate that loss of Lyn-mediated inhibitory signaling in B cells alone is sufficient to initiate systemic autoimmunity.
It has been assumed that the myeloproliferative phenotype observed in the lyn−/− mice was not B cell-mediated but rather due to the hyperresponsiveness of Lyn-deficient hematopoietic progenitors and myeloid cells to growth factors such as GM-CSF, G-CSF and IL-6 (2). In this line, lyn−/− μMT/μMT mice (lacking mature B cells) or lyn−/− Rag−/− mice (lacking mature B and T cells) still develop myeloproliferation indistinguishable from lyn−/− mice (2, 30). It is thus intriguing that myeloid expansion still occurs in B-lyn−/− mice in the presence of Lyn-sufficient myeloid cells. This observation suggests that myeloproliferation in lyn−/− mice is not only due intrinsic defects in myeloid progenitors and cells, but can also be a consequence of the inflammatory effects of immune complexes produced by self-reactive B-lymphocytes, as it has been also observed in other models of autoimmunity (31).
The observation that MyD88-deficiency in B cells alone reversed the autoimmune process in B-lyn−/− mice is consistent with a number of reports implicating TLR7 and TLR9 signaling in B cells as promoting the activation of B cells recognizing nuclear autoantigens, both in vitro and in vivo in a variety of mouse models of lupus (32-35). We found that loss of MyD88-dependent signals in Lyn-deficient B cells prevented class switching to pathogenic IgG2b and 2c autoreactive antibodies. In line with our findings, Ehlers et al. have shown that TLR9/MyD88 signaling is crucial for class switching to pathogenic autoreactive antibodies in the FcγRIIb−/− mouse model of lupus-like disease (33).
B1 cells produce most of the serum IgM -- expansion of B1 cell numbers and elevated IgM is often associated with autoimmunity (36). It is believed that BAFF protects B1 cells against FcγRIIb-mediated apoptosis and excessive BAFF levels in mice leads to the expansion of low affinity self-reactive B cells, including B1 cells, rather than to a dramatic breakdown in B cell tolerance (37-39). Interestingly, in the B-lyn−/− mice, deficiency of B cell MyD88 signaling abrogated the production of pathogenic IgG autoreactive antibodies but the B-lyn−/−myd88−/− mice still produced high levels of IgM self-reactive antibodies, high levels of serum BAFF and exhibit increased numbers of B1 cells in the peritoneal cavity, suggesting a similar mechanism in lyn−/− mice. This also suggests that the main role of TLR signaling in B cells is to promote class switching of self-reactive clones, which would normally take place in GC B cells. In agreement with this notion, previous studies of mice immunized with nucleic acid-containing virus particles also demonstrated that TLR signaling in B cells can promote the GC response (27). However lyn−/− animals, whether immunized or not, have altered lymphoid architecture lacking clear follicles and T cell zones, and exhibit poor splenic GC formation (9, 11, 25, 40, 41). These phenotypes were also observed in B-lyn−/− mice. The alteration in splenic architecture may be secondary to increased extramedullary myelopoiesis in these animals. The absolute numbers of GC B cells in lyn−/− or B-lyn−/− mice are substantially decreased, as is also true of mature naïve follicular B cells. It is possible that at least some fraction of the pathogenic IgGs in these animals are derived from extrafollicular B cells, under the influence of TLR signaling, as it has been suggested in other autoimmune strains (42). However, the observation that crossing the lyn−/− mice to signaling lymphocyte activation molecule (SLAM)-associated protein (SAP)-deficient animals, which lack T follicular helper cells and GC B cells, causes a large decrease in IgG ANA formation (Hua et al, submitted for publication), suggests a germinal center origin for the majority of pathogenic antibodies in this model. Therefore, the developmental history of autoreactive B cells in this model, and other lupus-like mouse models, remains an open and important question.
We have also examined the effects of deletion of Lyn selectively in DCs. Surprisingly, when the lynf/f mice were crossed to Cd11c-cre animals, to generate Lyn-deficiency in DCs specifically (DC-lyn−/− mice hereafter), the resulting mice developed a much more severe autoimmune/inflammatory disease than is present in either lyn−/− or B-lyn−/− animals (18). While the DC-lyn−/− mice developed similar titers of IgG anti-sm/RNP and anti-dsDNA as seen in the lyn−/− animals, they had much higher levels of T cell activation, myeloexpansion and much more severe glomerulonephritis. The DC-lyn−/− animals developed diffuse lymphoadenopathy as well as a number of tissue inflammatory infiltrates, including hepatitis, pneumonitis and dermatitis, which are rarely observed in lyn−/− animals (or in B-lyn−/− mice). As a result, the DC-lyn−/− mice showed decreased survival. By contrast, DC-lyn−/− mice showed none of the B cell homeostatic defects seen in lyn−/− or B-lyn−/− strains – in fact the DC-lyn−/− mice developed elevated levels of splenic and lymph node mature B cells, as well as increased plasma cell numbers. Importantly, deletion of myd88 in DCs alone in the DC-lyn−/− strain reversed the disease process completely, indicating that TLR signaling in DCs is necessary to sustain the inflammatory/autoimmune phenotype in this strain.
Together, these studies indicate that there are multiple pathways by which Lyn deficiency can initiate autoimmunity, leading to some similar and some distinct phenotypes. In the B-lyn−/− model, we propose that loss of inhibitory signaling in B cells leads to alterations in B cell homeostasis, which is likely caused by reduced survival of mature B cells with elevated tonic BCR signaling. In these mice, selection of self-reactive B cell clones by a mechanism requiring TLR signaling in the B cells leads to autoantibody production, probably in a T cell-dependent manner. This in turn leads to immune complex formation with kidney deposition, which we hypothesize drives the myeloexpansion and mild systemic inflammatory response (i.e. elevated cytokines) seen in the B-lyn−/− mice.
By contrast, in the DC-lyn−/− model the primary initiating response is dysregulated MyD88-mediated signaling in DCs probably in response to commensal organisms. This leads to exaggerated proinflammatory cytokine production, which drives T cell activation and production of IFN-γ (among other inflammatory cytokines) that in turn feeds back on the DCs to further activate them leading to a self-amplifying loop of inflammation (17). This proinflammatory state, which includes high BAFF levels even in the presence of elevated mature B cell numbers, leads to the selection of self-reactive B cell clones producing autoantibodies. The autoantibodies deposit in tissues, which we hypothesize further exacerbates the inflammatory disease. The very proinflammatory nature of the process in the DC-lyn−/− mice, with the lympho/myeloproliferation, the T cell activation and the high systemic cytokine production, likely accounts for why the disease is lethal in these mice compared to the B-lyn−/− animals. The MyD88 requirement for both the DC-initiated pathway of autoimmune and inflammatory disease and the B cell-initiated lupus-like autoimmunity is consistent with the earlier observation that the autoimmunity of the global lyn−/− mice is ablated by crossing to Myd88−/− animals (43).
Similar comparisons between B cell- versus DC-specific knockouts of SHP-1 and A20 have recently been reported and reinforce the conclusion that autoimmunity can be initiated by defective negative regulation of receptor signaling in either of these cell types. Deficiency of the tyrosine phosphatase SHP-1 selectively in B cells results in a mild autoimmunity with mild renal immune complex deposition (44). Lyn phosphorylation of the ITIM of CD22 leads to recruitment of SHP-1, and this is likely a key pathway affected by Lyn-deficiency of B cells since the phenotypes resulting from deletion of Lyn or SHP-1 in B cells or deletion of CD22 are all similar (45). Compared to deletion of SHP-1 in B cells, DC-specific deletion of SHP-1 produces a much more profound inflammatory and autoimmune process, which like the DC-lyn−/− mice, is dependent on expression of MyD88 in DCs (46). Similarly, mice lacking the tnfaip3 (A20) gene, specifically in B cells develop a mild autoimmunity similar to the B-lyn−/− animals (47). A20 is a negative regulator of NF-κB pathways downstream of the BCR, TLRs and a number of other cell types (48). DC-specific A20-deficient mice, as with DC-specific deletion of Lyn or SHP-1, have a stronger systemic inflammatory and autoimmune disease with rapid end-organ damage (49, 50). Thus, in all three cases, dysregulation of B cells or of DCs can lead to autoimmune and inflammatory disease. Understanding the mechanisms of the cellular interactions in genetic models of autoimmunity is critical for future studies. The approach of cell-type specific deletion of autoimmune susceptibility genes is clearly informative in this regard.
These studies illustrate that complex regulatory and counter regulatory networks are operating in the autoimmune Lyn-deficient model. It is very likely that similar processes are present in other autoimmune mice as well as in SLE patients. Indeed, since genome-wide associated studies have demonstrated that SLE is multigenic and likely genetically diverse amongst different groups of patients (51), it is likely that dysregulation of multiple cellular and molecular pathways can predispose to autoimmunity in different individuals, as is the case in mice defective in key negative regulators of receptor signaling such as Lyn, SHP-1 and A20. Therefore, defining which pathways are dysregulated in any given SLE patient will be required to develop appropriate therapy to that patient’s disease process – the variable results seen with many current SLE therapies may simply reflect the fact that one cannot treat a heterogeneous disease process with a single agent that affects a single pathway. Thus, disentangling the pathogenic mechanisms in mouse models such as the Lyn-deficient animal, may lead to practical improvements in our approach to treating complex autoimmune diseases in man.
Supplementary Material
Acknowledgments
1 This work was supported by the National Institutes of Health (AI65495 and AI68150 to C.A.L.; AI078869 to A.L.D).
3 Abbreviations used in this paper
- SFK
Src family kinase
- [Ca2+]
intracellular Ca2+ concentration
- T1
transitional stage 1
- T2
transitional stage 2
- T3
transitional stage 3
- Fol.
Follicular
- MZ
marginal zone
- GC
germinal center
- Ig
immunoglobulins
REFERENCES
- 1.Lowell CA. Src-family and Syk kinases in activating and inhibitory pathways in innate immune cells: signaling cross talk. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a002352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scapini P, Pereira S, Zhang H, Lowell CA. Multiple roles of Lyn kinase in myeloid cell signaling and function. Immunol Rev. 2009;228:23–40. doi: 10.1111/j.1600-065X.2008.00758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saijo K, Schmedt C, Su IH, Karasuyama H, Lowell CA, Reth M, Adachi T, Patke A, Santana A, Tarakhovsky A. Essential role of Src-family protein tyrosine kinases in NF-kappaB activation during B cell development. Nature immunology. 2003;4:274–279. doi: 10.1038/ni893. [DOI] [PubMed] [Google Scholar]
- 4.Ohashi PS, DeFranco AL. Making and breaking tolerance. Curr Opin Immunol. 2002;14:744–759. doi: 10.1016/s0952-7915(02)00406-5. [DOI] [PubMed] [Google Scholar]
- 5.DeFranco AL, Chan VW, Lowell CA. Positive and negative roles of the tyrosine kinase Lyn in B cell function. Semin Immunol. 1998;10:299–307. doi: 10.1006/smim.1998.0122. [DOI] [PubMed] [Google Scholar]
- 6.Gross AJ, Proekt I, DeFranco AL. Elevated BCR signaling and decreased survival of Lyn-deficient transitional and follicular B cells. Eur J Immunol. 2011;41:3645–3655. doi: 10.1002/eji.201141708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liossis SN, Solomou EE, Dimopoulos MA, Panayiotidis P, Mavrikakis MM, Sfikakis PP. B-cell kinase lyn deficiency in patients with systemic lupus erythematosus. Journal of investigative medicine : the official publication of the American Federation for Clinical Research. 2001;49:157–165. doi: 10.2310/6650.2001.34042. [DOI] [PubMed] [Google Scholar]
- 8.Harley JB, Alarcon-Riquelme ME, Criswell LA, Jacob CO, Kimberly RP, Moser KL, Tsao BP, Vyse TJ, Langefeld CD, Nath SK, Guthridge JM, Cobb BL, Mirel DB, Marion MC, Williams AH, Divers J, Wang W, Frank SG, Namjou B, Gabriel SB, Lee AT, Gregersen PK, Behrens TW, Taylor KE, Fernando M, Zidovetzki R, Gaffney PM, Edberg JC, Rioux JD, Ojwang JO, James JA, Merrill JT, Gilkeson GS, Seldin MF, Yin H, Baechler EC, Li QZ, Wakeland EK, Bruner GR, Kaufman KM, Kelly JA. Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nature genetics. 2008;40:204–210. doi: 10.1038/ng.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gutierrez T, Halcomb KE, Coughran AJ, Li QZ, Satterthwaite AB. Separate checkpoints regulate splenic plasma cell accumulation and IgG autoantibody production in Lyn-deficient mice. Eur J Immunol. 2010;40:1897–1905. doi: 10.1002/eji.200940043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan VW, Meng F, Soriano P, DeFranco AL, Lowell CA. Characterization of the B lymphocyte populations in Lyn-deficient mice and the role of Lyn in signal initiation and down-regulation. Immunity. 1997;7:69–81. doi: 10.1016/s1074-7613(00)80511-7. [DOI] [PubMed] [Google Scholar]
- 11.Hibbs ML, Tarlinton DM, Armes J, Grail D, Hodgson G, Maglitto R, Stacker SA, Dunn AR. Multiple defects in the immune system of Lyn-deficient mice, culminating in autoimmune disease. Cell. 1995;83:301–311. doi: 10.1016/0092-8674(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 12.Nishizumi H, Taniuchi I, Yamanashi Y, Kitamura D, Ilic D, Mori S, Watanabe T, Yamamoto T. Impaired proliferation of peripheral B cells and indication of autoimmune disease in lyn-deficient mice. Immunity. 1995;3:549–560. doi: 10.1016/1074-7613(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 13.Allman D, Lindsley RC, DeMuth W, Rudd K, Shinton SA, Hardy RR. Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J Immunol. 2001;167:6834–6840. doi: 10.4049/jimmunol.167.12.6834. [DOI] [PubMed] [Google Scholar]
- 14.Cornall RJ, Cyster JG, Hibbs ML, Dunn AR, Otipoby KL, Clark EA, Goodnow CC. Polygenic autoimmune traits: Lyn, CD22, and SHP-1 are limiting elements of a biochemical pathway regulating BCR signaling and selection. Immunity. 1998;8:497–508. doi: 10.1016/s1074-7613(00)80554-3. [DOI] [PubMed] [Google Scholar]
- 15.Meade J, Fernandez C, Turner M. The tyrosine kinase Lyn is required for B cell development beyond the T1 stage in the spleen: rescue by over-expression of Bcl-2. Eur J Immunol. 2002;32:1029–1034. doi: 10.1002/1521-4141(200204)32:4<1029::AID-IMMU1029>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 16.Gross AJ, Lyandres JR, Panigrahi AK, Prak ET, DeFranco AL. Developmental acquisition of the Lyn-CD22-SHP-1 inhibitory pathway promotes B cell tolerance. J Immunol. 2009;182:5382–5392. doi: 10.4049/jimmunol.0803941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scapini P, Hu Y, Chu CL, Migone TS, Defranco AL, Cassatella MA, Lowell CA. Myeloid cells, BAFF, and IFNγ establish an inflammatory loop that exacerbates autoimmunity in Lyn-deficient mice. J Exp Med. 2010;207:1757–1773. doi: 10.1084/jem.20100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lamagna C, Scapini P, van Ziffle JA, Defranco AL, Lowell CA. Hyperactivated MyD88 signaling in dendritic cells, through specific deletion of Lyn kinase, causes severe autoimmunity and inflammation. Proc Natl Acad Sci U S A. 2013;110:E3311–3320. doi: 10.1073/pnas.1300617110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hobeika E, Thiemann S, Storch B, Jumaa H, Nielsen PJ, Pelanda R, Reth M. Testing gene function early in the B cell lineage in mb1-cre mice. Proc Natl Acad Sci U S A. 2006;103:13789–13794. doi: 10.1073/pnas.0605944103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hou B, Reizis B, DeFranco AL. Toll-like receptors activate innate and adaptive immunity by using dendritic cell-intrinsic and -extrinsic mechanisms. Immunity. 2008;29:272–282. doi: 10.1016/j.immuni.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zikherman J, Parameswaran R, Weiss A. Endogenous antigen tunes the responsiveness of naive B cells but not T cells. Nature. 2012;489:160–164. doi: 10.1038/nature11311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shahaf G, Gross AJ, Sternberg-Simon M, Kaplan D, DeFranco AL, Mehr R. Lyn deficiency affects B-cell maturation as well as survival. Eur J Immunol. 2012;42:511–521. doi: 10.1002/eji.201141940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan VW, Lowell CA, DeFranco AL. Defective negative regulation of antigen receptor signaling in Lyn-deficient B lymphocytes. Current biology : CB. 1998;8:545–553. doi: 10.1016/s0960-9822(98)70223-4. [DOI] [PubMed] [Google Scholar]
- 24.Tsantikos E, Oracki SA, Quilici C, Anderson GP, Tarlinton DM, Hibbs ML. Autoimmune disease in Lyn-deficient mice is dependent on an inflammatory environment established by IL-6. J Immunol. 2010;184:1348–1360. doi: 10.4049/jimmunol.0901878. [DOI] [PubMed] [Google Scholar]
- 25.Tsantikos E, Quilici C, Harder KW, Wang B, Zhu HJ, Anderson GP, Tarlinton DM, Hibbs ML. Perturbation of the CD4 T cell compartment and expansion of regulatory T cells in autoimmune-prone Lyn-deficient mice. J Immunol. 2009;183:2484–2494. doi: 10.4049/jimmunol.0804346. [DOI] [PubMed] [Google Scholar]
- 26.Scapini P, Lamagna C, Hu Y, Lee K, Tang Q, DeFranco AL, Lowell CA. B cell-derived IL-10 suppresses inflammatory disease in Lyn-deficient mice. Proc Natl Acad Sci U S A. 2011;108:E823–832. doi: 10.1073/pnas.1107913108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hou B, Saudan P, Ott G, Wheeler ML, Ji M, Kuzmich L, Lee LM, Coffman RL, Bachmann MF, DeFranco AL. Selective utilization of Toll-like receptor and MyD88 signaling in B cells for enhancement of the antiviral germinal center response. Immunity. 2011;34:375–384. doi: 10.1016/j.immuni.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shlomchik MJ. Activating systemic autoimmunity: B’s, T’s, and tolls. Current opinion in immunology. 2009;21:626–633. doi: 10.1016/j.coi.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ganguly D, Haak S, Sisirak V, Reizis B. The role of dendritic cells in autoimmunity. Nat Rev Immunol. 2013 doi: 10.1038/nri3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harder KW, Parsons LM, Armes J, Evans N, Kountouri N, Clark R, Quilici C, Grail D, Hodgson GS, Dunn AR, Hibbs ML. Gain- and loss-of-function Lyn mutant mice define a critical inhibitory role for Lyn in the myeloid lineage. Immunity. 2001;15:603–615. doi: 10.1016/s1074-7613(01)00208-4. [DOI] [PubMed] [Google Scholar]
- 31.Liu K, Li QZ, Yu Y, Liang C, Subramanian S, Zeng Z, Wang HW, Xie C, Zhou XJ, Mohan C, Wakeland EK. Sle3 and Sle5 can independently couple with Sle1 to mediate severe lupus nephritis. Genes Immun. 2007;8:634–645. doi: 10.1038/sj.gene.6364426. [DOI] [PubMed] [Google Scholar]
- 32.Green NM, Marshak-Rothstein A. Toll-like receptor driven B cell activation in the induction of systemic autoimmunity. Seminars in immunology. 2011;23:106–112. doi: 10.1016/j.smim.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ehlers M, Fukuyama H, McGaha TL, Aderem A, Ravetch JV. TLR9/MyD88 signaling is required for class switching to pathogenic IgG2a and 2b autoantibodies in SLE. The Journal of experimental medicine. 2006;203:553–561. doi: 10.1084/jem.20052438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nickerson KM, Christensen SR, Shupe J, Kashgarian M, Kim D, Elkon K, Shlomchik MJ. TLR9 regulates TLR7- and MyD88-dependent autoantibody production and disease in a murine model of lupus. J Immunol. 2010;184:1840–1848. doi: 10.4049/jimmunol.0902592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teichmann LL, Schenten D, Medzhitov R, Kashgarian M, Shlomchik MJ. Signals via the adaptor MyD88 in B cells and DCs make distinct and synergistic contributions to immune activation and tissue damage in lupus. Immunity. 2013;38:528–540. doi: 10.1016/j.immuni.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol. 2011;11:34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 37.Amezcua Vesely MC, Schwartz M, Bermejo DA, Montes CL, Cautivo KM, Kalergis AM, Rawlings DJ, Acosta-Rodriguez EV, Gruppi A. FcgammaRIIb and BAFF differentially regulate peritoneal B1 cell survival. J Immunol. 2012;188:4792–4800. doi: 10.4049/jimmunol.1102070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thien M, Phan TG, Gardam S, Amesbury M, Basten A, Mackay F, Brink R. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. 2004;20:785–798. doi: 10.1016/j.immuni.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 39.Fletcher CA, Groom JR, Woehl B, Leung H, Mackay C, Mackay F. Development of autoimmune nephritis in genetically asplenic and splenectomized BAFF transgenic mice. J Autoimmun. 2011;36:125–134. doi: 10.1016/j.jaut.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 40.Kato J, Motoyama N, Taniuchi I, Takeshita H, Toyoda M, Masuda K, Watanabe T. Affinity maturation in Lyn kinase-deficient mice with defective germinal center formation. J Immunol. 1998;160:4788–4795. [PubMed] [Google Scholar]
- 41.Mirnics ZK, Caudell E, Gao Y, Kuwahara K, Sakaguchi N, Kurosaki T, Burnside J, Mirnics K, Corey SJ. Microarray analysis of Lyn-deficient B cells reveals germinal center-associated nuclear protein and other genes associated with the lymphoid germinal center. J Immunol. 2004;172:4133–4141. doi: 10.4049/jimmunol.172.7.4133. [DOI] [PubMed] [Google Scholar]
- 42.Sweet RA, Cullen JL, Shlomchik MJ. Rheumatoid factor B cell memory leads to rapid, switched antibody-forming cell responses. J Immunol. 2013;190:1974–1981. doi: 10.4049/jimmunol.1202816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silver KL, Crockford TL, Bouriez-Jones T, Milling S, Lambe T, Cornall RJ. MyD88-dependent autoimmune disease in Lyn-deficient mice. Eur J Immunol. 2007;37:2734–2743. doi: 10.1002/eji.200737293. [DOI] [PubMed] [Google Scholar]
- 44.Pao LI, Lam KP, Henderson JM, Kutok JL, Alimzhanov M, Nitschke L, Thomas ML, Neel BG, Rajewsky K. B cell-specific deletion of protein-tyrosine phosphatase Shp1 promotes B-1a cell development and causes systemic autoimmunity. Immunity. 2007;27:35–48. doi: 10.1016/j.immuni.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 45.O’Keefe TL, Williams GT, Batista FD, Neuberger MS. Deficiency in CD22, a B cell-specific inhibitory receptor, is sufficient to predispose to development of high affinity autoantibodies. The Journal of experimental medicine. 1999;189:1307–1313. doi: 10.1084/jem.189.8.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abram CL, Roberge GL, Pao LI, Neel BG, Lowell CA. Distinct roles for neutrophils and dendritic cells in inflammation and autoimmunity in motheaten mice. Immunity. 2013;38:489–501. doi: 10.1016/j.immuni.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tavares RM, Turer EE, Liu CL, Advincula R, Scapini P, Rhee L, Barrera J, Lowell CA, Utz PJ, Malynn BA, Ma A. The ubiquitin modifying enzyme A20 restricts B cell survival and prevents autoimmunity. Immunity. 2010;33:181–191. doi: 10.1016/j.immuni.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma A, Malynn BA. A20: linking a complex regulator of ubiquitylation to immunity and human disease. Nat Rev Immunol. 2012;12:774–785. doi: 10.1038/nri3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hammer GE, Turer EE, Taylor KE, Fang CJ, Advincula R, Oshima S, Barrera J, Huang EJ, Hou B, Malynn BA, Reizis B, DeFranco A, Criswell LA, Nakamura MC, Ma A. Expression of A20 by dendritic cells preserves immune homeostasis and prevents colitis and spondyloarthritis. Nature immunology. 2011;12:1184–1193. doi: 10.1038/ni.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kool M, van Loo G, Waelput W, De Prijck S, Muskens F, Sze M, van Praet J, Branco-Madeira F, Janssens S, Reizis B, Elewaut D, Beyaert R, Hammad H, Lambrecht BN. The ubiquitin-editing protein A20 prevents dendritic cell activation, recognition of apoptotic cells, and systemic autoimmunity. Immunity. 2011;35:82–96. doi: 10.1016/j.immuni.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 51.Rullo OJ, Tsao BP. Recent insights into the genetic basis of systemic lupus erythematosus. Ann Rheum Dis. 2013;72(Suppl 2):ii56–61. doi: 10.1136/annrheumdis-2012-202351. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.