Abstract
An increasing number of Shiga toxin 2f-producing Escherichia coli (STEC2f) infections in humans are being reported in Europe, and pigeons have been suggested as a reservoir for the pathogen. In Japan, there is very little information regarding carriage of STEC2f by pigeons, prompting the need for further investigation. We collected 549 samples of pigeon droppings from 14 locations in Kyushu, Japan, to isolate STEC2f and to investigate characteristics of the isolates. Shiga toxin stx 2f gene fragments were detected by PCR in 16 (2.9%) of the 549 dropping samples across four of the 14 locations. We obtained 23 STEC2f-isolates from seven of the original samples and from three pigeon dropping samples collected in an additional sampling experiment (from a total of seven locations across both sampling periods). Genotypic and phenotypic characteristics were then examined for selected isolates from each of 10 samples with pulsed-field gel electrophoresis profiles. Eight of the stx 2f gene fragments sequenced in this study were homologous to others that were identified in Europe. Some isolates also contained virulence-related genes, including lpfA O26, irp 2, and fyuA, and all of the 10 selected isolates maintained the eae, astA, and cdt genes. Moreover, five of the 10 selected isolates contained sfpA, a gene that is restricted to Shiga toxin-producing E. coli O165:H2 and sorbitol-fermenting Shiga toxin-producing E. coli O157:NM. We document serotypes O152:HNM, O128:HNM, and O145:H34 as STEC2f, which agrees with previous studies on pigeons and humans. Interestingly, O119:H21 was newly described as STEC2f. O145:H34, with sequence type 722, was described in a German study in humans and was also isolated in the current study. These results revealed that Japanese zoonotic STEC2f strains harboring several virulence-related factors may be of the same clonal complexes as some European strains. These findings provide useful information for public health-related disease management strategies in Japan.
Introduction
Recent reports have shown that cases of human infection by Shiga toxin 2f-producing Escherichia coli (STEC2f) are becoming more frequent, with pigeons being implicated as a possible source of infection [1], [2]. For example, van Duynhoven et al. [3] screened 211 human stool specimens suspected to contain Shiga toxin-producing E. coli (STEC), and reported that three of seven STEC strains were positive for STEC2f. Prager et al. [1] also reported that STEC2f was present in 2.5% of STEC strains identified in their study. In Japan, STEC2f strain O128:HNM was isolated from the feces of a 1-year-old boy with diarrhea and abdominal pain in 2002 [4]. In addition, Sonntag et al. [5] showed that a STEC2f O128:HNM strain from human diarrhea was identical to those of pigeon isolates belonging to the serotype O128:H2, based on the restriction fragment length polymorphism pattern identified in Germany. These studies have shown that the occurrence of STEC2f in humans is clearly higher than previously thought, and that zoonotic transfer from pigeons is a potentially serious threat.
In Europe, STEC2f strains among pigeons and humans were investigated using several methods, including isolation of the pathogen from city pigeons, analysis of the isolates' characteristics, and relationships between isolates. Isolation of stx 2f (or the toxin, Stx2f) from pigeon droppings was reported in Europe [6]. STEC2f serotypes are variable, and so far include the O15, O18ab:HNM, O25:H7, O45, O45:HNM, O66:HNM, O75:HNM, O128:H2, O132, O135:HNM, O152:HNM, and O-untypeable serotypes in Japanese [7] and European pigeons [5], [8], [9]. The stx 2f sequences were analyzed [2] and multi-locus sequence typing (MLST) of the pathogen in humans was conducted [1]. Given that the capacity of STEC strains to cause human disease is strongly associated with other virulence-related genes, in addition to stx [10], Sonntag et al. [5] evaluated the potential virulence of STEC2f strains containing such virulence-related genes, including lpfA O26 (major subunit of long polar fimbriae of STEC O26), sfpA (major fimbrial subunit of Sfp fimbriae), irp 2 (iron-repressible protein 2), and fyuA (ferric yersiniabactin uptake receptor). Other virulence-related genes, such as eae (intimin), EHEC-hlyA (enterohemorrhagic E. coli hemolysin), and cdt (cytolethal distending toxin), were also detected in STEC2f strains in Europe [1]; however, there is only one study of this emerging pathogen in Japan, which sampled only 67 pigeons [7]. The purpose of the present study was to investigate the prevalence of STEC2f-harboring pigeons in Kyushu, Japan, to determine the phenotypic and genotypic characteristics of any resulting isolates, and to compare the genotypes, including virulence-related genes, of Japanese isolates with those reported in Europe. Accordingly, we successfully isolated STEC2f strains from the pigeon droppings and identified some overlap with virulence gene clones identified in Europe, as well as documenting serotype O119:H21 as STEC2f for the first time. This study represents a critical appraisal of the occurrence of pigeons infected with the STEC2f zoonotic pathogen in Japan.
Materials and Methods
Sampling of pigeon droppings
Samples of droppings from city pigeons were collected for both experiment 1 (single dropping collection) and experiment 2 (pooled dropping collection) (see below) in Kyushu (Fig. 1), Japan, between December 2008 and March 2009. Kyushu is Japan's third largest island. It is located southwest of the main island of Honshu. For both experiments, droppings were collected from the ground while the pigeons were eating feed that we supplied, except at location K, where droppings were collected from plastic boards that had been sterilized with ethyl alcohol (80%). Therefore, not every sample came from a separate bird. Sampling locations were selected randomly from parks, station squares, and religious sites. In experiment 1, 549 individual pigeon droppings were collected from 14 locations in Kyushu (A–E, G–I, K–P in Fig. 1). Each dropping was collected in a 15-mL conical tube. In experiment 2, which was carried out for additional collection of STEC2f-isolates, 19 samples consisting of pooled pigeon droppings were collected from 13 locations (A–L, P in Fig. 1) in Kyushu.
Figure 1. Sampling locations in surveillance experiments in Kyushu, Japan.

Closed circles show locations of Shiga toxin 2f-producing Escherichia coli. Open circles indicate locations with no Shiga toxin 2f-producing E. coli.
Detection of stx 2f gene and isolation of STEC2f from pigeon droppings
Each dropping sample for experiment 1 was mixed with 10 mL of buffered peptone water (Oxoid, Hampshire, UK), while each pooled sample in experiment 2 was mixed with 225 mL of buffered peptone water. The collected samples in the buffered peptone water were incubated overnight at 42°C to reflect the body temperature of pigeons. Aliquots (0.1 mL in experiment 1 and 1 mL in experiment 2) of the cultures were centrifuged at 3,220× g for 20 min, and pellets were re-suspended in 0.5 mL of Tris-EDTA buffer. Cell suspensions were then boiled for 10 min and centrifuged at 3,220× g for 20 min for experiment 1 and at 20,814× g for 10 min for experiment 2. A 1-µL aliquot of each supernatant was used as a template for PCR-based detection of stx 2f. PCR was performed as described previously by Nakao et al. [11]. Primers G3-F (5′-TTT ACT GTG GAT TTC TCT TCG C-3′) and G3-R (5′-TCA GTA AGA TCC TGA GGC TTG-3′), at a final concentration of 0.5 µM, and GoTaq Green Master Mix (Promega, Madison, WI, USA) were used in the PCR reaction mixture.
The stx 2f PCR-positive mixed cultures were streaked onto deoxycholate-hydrogen sulfide-lactose agar (Eiken Chemical Co., Tokyo, Japan) and the plates were incubated at 37°C overnight. Presumptive E. coli colonies (25–96/sample) were isolated on nutrient agar plates (Eiken Chemical Co.) and incubated at 37°C overnight. The isolates were then re-tested for stx 2f using the PCR method described above. The biochemical characteristics of the stx 2f-positive isolates were examined using triple sugar iron agar (Eiken Chemical Co.), lysine decarboxylase broth (Eiken Chemical Co.), sulfide indole motility medium agar (Eiken Chemical Co.), methyl red-Voges-Proskauer medium (Nissui Pharmaceutical Co., Tokyo, Japan), Simmons citrate agar (Eiken Chemical Co.), and cytochrome-oxidase test strips (Nissui Pharmaceutical Co.) to determine whether the isolates were E. coli. Serological characteristics of the isolates were investigated using O- and H-antisera (Denka Seiken Co., Tokyo, Japan). The isolates were also tested using a previously described PCR-based method for discriminating between E. coli and Escherichia albertii [12], [13]. Genotyping of flagella antigen (H-typing), from isolates that were identified as having a type other than H- in the phenotyping assay, was also carried out by restriction fragment length polymorphism analysis, as previously described [14].
Pulsed-field gel electrophoresis
All of the STEC2f isolates (n = 23) were tested with pulsed-field gel electrophoresis (PFGE) to select representative isolates from each sample for further characterization. PFGE was carried out as previously described [15] for 11 isolates, while the other 12 isolates that showed Tris-dependent degradation were subsequently analyzed by PFGE with 50 µM thiourea described byRömling and Tümmler [16].
Full-length sequencing of stx 2f
Full-length sequence analysis of the stx 2f genes from the 10 selected isolates was carried out as previously described [17]. The sequences were assembled using the SeqManII program in the Lasergene software package (DNASTAR, Madison, WI, USA), and were compared with stx 2f reference sequences (AB232172, M29153, AJ010730, AJ270998, and AB472687 from the DNA Data Bank of Japan, or DDBJ). Cluster analysis was performed using the unweighted pair-group method with arithmetic averages using MEGA 4 software [18].
Genotypic and phenotypic characterization of isolates
The following genes were amplified using primers described by Sonntag et al. [5]: lpfA O26, iha (iron-regulated gene A homologue adhesin), efa1 (enterohemorrhagic E. coli factor for adherence), sfpA, EHEC-hlyA, espP (extracellular serine protease), pagC (outer membrane invasion protein), irp 2, and fyuA. cdt was amplified using the method described by Pickett et al. [19], and eae, bfpA (major pilin structural unit bundling), and astA (heat-stable toxin 1) were amplified as described by Kobayashi et al. [20]. Heteroduplex mobility assay typing of the 5′ terminal region of the eae gene was performed as described by Ito et al. [21]. Typing of the 3′ terminal region of eae was conducted using the PCR method described by Blanco et al. [22]. Full-length sequence analysis of the sfpA genes from isolates G2-1c, G5-1, H1-1b, H3-1a, and H4-1b was carried out as previously described [17] using novel primers designed for this study, AJ131667-1036F: 5′-CATAAGCACAGAGAGCAGACTAAC-3′ and EU980315-1479R: 5′-GCAGCACTCCAGAGAAATGTTAC-3′ to confirm whether the genes detected by PCR were sfpA.
Toxin assay
Filtrates of cultures of each of the isolates, with mitomycin C (MMC) (0.2 mg/L), were tested for cytotoxic activity in Vero cells [23], and in a reversed passive latex agglutination assay (RPLA) using a VTEC-RPLA kit (Denka Seiken) to detect Shiga toxin, as previously described [17]. E. coli O157:H7 (ATCC 43894), harboring stx 1 and stx 2, was also examined as a reference strain in both tests.
Multi-locus sequence typing (MLST)
Housekeeping genes adk (adenylate kinase), fumC (fumarate hydratase), gyrB (DNA gyrase), icd (isocitrate/isopropylmalate dehydrogenase), mdh (malate dehydrogenase), purA (adenylosuccinate dehydrogenase), and recA (ATP/GTP binding motif) were used for MLST analysis. Cell treatment and sequencing were performed as previously described [24]. Primers for the sequencing reactions are listed in the MLST Database at the Environmental Research Institute, University College Cork, Ireland (http://mlst.ucc.ie/, available April 1, 2012). Sequences were submitted to the database website and were assigned existing or novel allele type numbers and sequence type (ST) numbers defined by the database. Composite STs were assigned based on the set of allele types derived from each of the seven loci. Allele sequences for each strain were then concatenated in the order adk–fumC–gyrB–icd–mdh–purA–recA for a final composite length of 3,423 bp. The concatenated sequences were aligned using ClustalW software [25], and a phylogenetic tree was constructed using the neighbor-joining method to compare with other published STs (Center for Information Biology and DDBJ). Phylogenetic analyses were performed using NJplot (http://pbil.univ-lyon1.fr/software/njplot.html). A minimum spanning tree generated from the data for ST 20, ST 2684, ST 2685, ST 28, ST 722, ST 40, and ST 11 was also described using Prim's algorithm from the PubMLST site (http://pubmlst.org/, accessed May 2, 2013).
Ethics statement
No specific permits were required for the described field studies, locations, and activities. The sampled locations are not privately-owned or protected in any way, and the field studies did not involve endangered or protected species. At no point did the researchers come into physical contact with the pigeons. This study was carried out in strict accordance with the guidelines of the Regulations for the Ethical and Humane Use of Experimental Animals at Fukuoka Institute of Health and Environmental Sciences, which is based on domestic standards, and approved by the Animal Ethics Committee of Fukuoka Institute of Health and Environmental Sciences under permit number 250802.
Results
Detection of Shiga toxin 2f-producing E. coli
In experiment 1, stx 2f was detected in 16 (2.9%) of 549 dropping cultures from four of the 14 locations by PCR. The incidence varied from 0–17.0% (Table 1). In experiment 2, stx 2f was detected in four of 19 pooled dropping samples at four (A, H, I and K) of the 13 locations. stx 2f was detected in one out of the two samplings and one out of five samplings at locations I and K, respectively. The combined results of both the experiments showed stx 2f was detected at seven of the 16 locations (Fig. 1). No common characteristics or features were found in the seven locations where stx 2f was detected.
Table 1. stx2f PCR-positive droppings from pigeons from 12 locations in Kyushu, Japan (experiment 1).
| Sampling location | No. of pigeon droppings tested | No. of PCR-positive droppings (%) | |
| G | 47 | 8 | (17.0%) |
| H | 47 | 4 | (8.5%) |
| N | 50 | 3 | (6.0%) |
| O | 25 | 1 | (4.0%) |
| A | 49 | 0 | (0.0%) |
| B | 6 | 0 | (0.0%) |
| C | 50 | 0 | (0.0%) |
| D | 29 | 0 | (0.0%) |
| E | 50 | 0 | (0.0%) |
| I | 50 | 0 | (0.0%) |
| K | 8 | 0 | (0.0%) |
| L | 50 | 0 | (0.0%) |
| M | 38 | 0 | (0.0%) |
| P | 50 | 0 | (0.0%) |
| Total | 549 | 16 | (2.9%) |
Phenotyping and genotyping of selected isolates chosen by pulsed-field gel electrophoresis
We obtained 23 STEC2f isolates from 10 samples at seven locations in total: 14 isolates from seven samples from four locations in experiment 1, and nine isolates from three samples from three locations in experiment 2. These isolates were identified as STEC2f-positive by PCR and as E. coli by biochemical tests. None of the isolates were identified as E. albertii by PCR [12], [13].
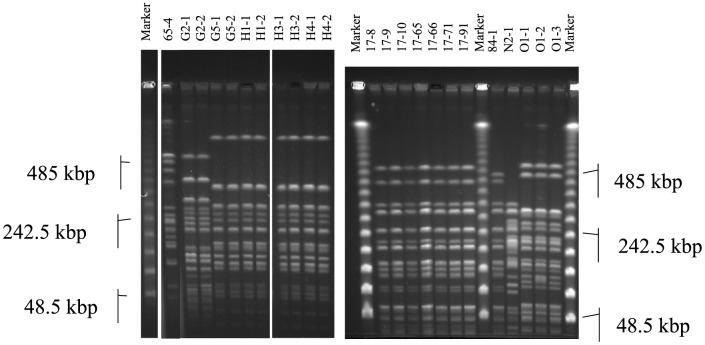
In the PFGE analyses, no remarkable differences in fragment patterns (defined as more than three fragments [26]) were observed between isolates from the same samples, suggesting that these isolates were the same or closely related strains (Fig. 2). Isolates from sample location G (G5 in Fig. 2) and H (H1, H2, and H3 in Fig. 2) showed the same PFGE profiles. Therefore, we selected one isolate from each of the 10 unique samples covering all STEC2f-positive locations (n = 7) across the two experiments. The 10 selected STEC2f-isolates showed several serotypes and characteristics (Table 2). The results of genotyping and phenotyping H-type assays were identical in each of six isolates. The 10 selected isolates showed a variety of O serogroups that were specific to location, and no O serogroup was shared between the different locations, except for untypeable. Selected isolates from each sample showed the following characteristics: supernatants from the isolate cultures containing MMC had titers of 16–256 in the RPLA test for Stx2 only, while ATCC 43894, the control strain, had titers of 16 and 8,192 for Stx1 and Stx2, respectively, in the MMC treatment assay. For the Vero cell assays using culture supernatants, the titers were between 4,096 and 262,144 with MMC, while ATCC 43894 with MMC treatment showed a titer of 65,536 (Table 2). Normal Vero cells were observed in a control plate containing only medium and MMC.
Figure 2. Comparison of pulsed-field gel electrophoresis analysis of fingerprints from Shiga toxin 2f-producing Escherichia coli isolates digested with XbaI.
Isolates 65-4 to H4-2 were tested with Tris-borate-EDTA buffer. Isolates 17-8 to O1-3 were tested using buffer with 50 µM thiourea because they showed degradation in the presence of Tris.
Table 2. Characteristics of Shiga toxin 2f-producing Escherichia coli isolates that were isolated in this study.
| Isolate No. | Location | Serotype | stx 2f sequence type | Sequence types from multi locus sequence typing | Gene detection* | eae typing | Toxin assay (titer) | ||||||||
| astA | lpfA O26 | sfpA | irp 2 | fyuA | cdt | EHEC-hlyA, bfpA, iha, espP, efa1, or pagC | Heteroduplex mobility assay typing in 5′ region | 3′ region typing | Vero cell assay +MMC 0.2 mg/L† | RPLA +MMC 0.2 mg/L | |||||
| N2-1 | N | O152:HNM | Type A‡ | 2685 | +§ | + | −§ | − | − | + | − | b1 | β 1 | 262,144 | 256 |
| O1-3 | O | O128:HNM | Type B‡ | 2684 | + | + | − | − | − | + | − | b1 | β 1 | Not done | Not done |
| 84-1 | K | OUT:HNM‖ | Type A | 20 | + | + | − | − | − | + | − | b1 | β 1 | Not done | Not done |
| 17-8 | A | OUT:HNM | Type A | 20 | + | + | − | − | − | + | − | b1 | β 1 | 16,384 | 64 |
| G2-1c | G | O119:H21 | Type A | 40 | + | − | + | − | − | + | − | d1 | θ/γ 2 | 262,144 | 64 |
| 65-4 | I | O145:H34 | Type B | 722 | + | − | − | − | − | + | − | a1 | ι1 | 4,096 | 16 |
| H1-1b | H | OUT:H6 | Type A | 28 | + | − | + | + | + | + | − | a1 | ξ/β 2 | 262,144 | 256 |
| H3-1a | H | OUT:H6 | Type A | 28 | + | − | + | + | + | + | − | a1 | ξ/β 2 | 262,144 | 256 |
| H4-1b | H | OUT:H6 | Type A | 28 | + | − | + | + | + | + | − | a1 | ξ/β 2 | 262,144 | 256 |
| G5-1 | G | OUT:H6 | Type A | 28 | + | − | + | + | + | + | − | a1 | ξ/β 2 | 262,144 | 64 |
*astA, heat-stable toxin 1; lpfAO26, major subunit of long polar fimbriae of STEC O26; sfpA, major fimbrial subunit of Sfp fimbriae; irp 2, iron-repressible protein 2; fyuA, ferric yersiniabactin uptake receptor; cdt, cytolethal distending toxin; EHEC-hlyA, enterohemorrhagic E. coli hemolysin: bfpA, major pilin structural unit bundling; iha, iron-regulated gene A homolog adhesin; espP, extracellular serine protease; efa1, enterohemorrhagic E. coli factor for adherence (Efa-1); pagC, outer membrane invasion protein.
MMC, mitomycin C.
Type A is as same as the sequence of O128:H2 isolates (AJ270998 and AJ 010730) in Europe, compared across 1,230 bp; Type B is as same as the sequence of an O63 isolate (AB232172) from Nagasaki, Japan, compared across 1,230 bp.
+, positive; −, negative.
OUT, untypeable in O serogroup.
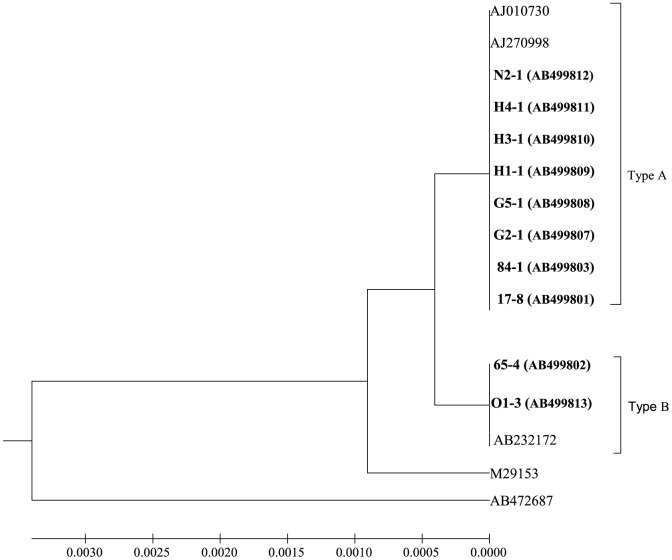
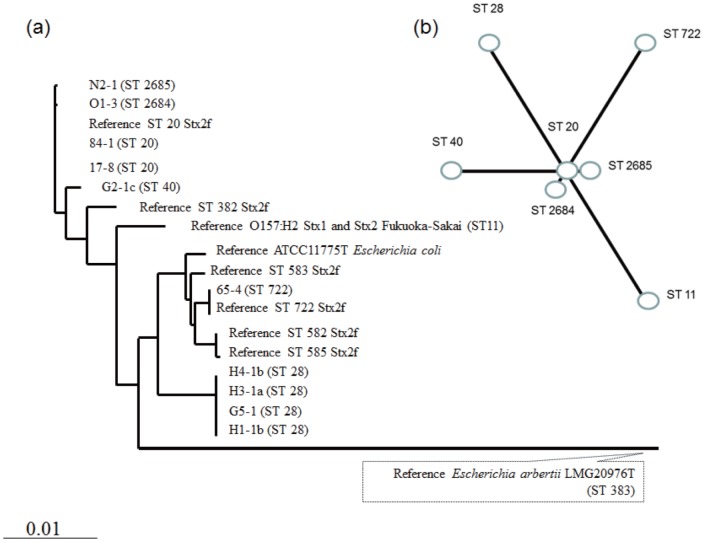
The isolates contained several virulence factors, as well as two different stx 2f STs (Table 2). Eight out of 10 isolates had stx 2f STs that were previously identified in European isolates [2], whereas two other isolates had sequences that showed identity to a human isolate from Nagasaki, Kyushu [27] (Fig. 3). The major characteristics of the virulence factors are summarized in Table 2. The gene that was detected by PCR with the primers for sfpA was confirmed as sfpA by DNA sequencing. The sequences from five isolates were completely identical to each other (525/525 bp, 100%), and only differed at 10 bases (10/525) from the sfpA sequence reported for E. coil O157:H- (AF401292). All of the isolates harbored cdt (we did not perform sub-typing) and astA, as well as eae. The dominant intimin type was type β in the 3′ terminal region, and b1 type in the 5′ terminal region. The MLST results revealed six STs in the selected isolates (Table 2, Fig. 4). No correlation was observed among stx 2f-sequence patterns, eae-types, and STs, although presence-absence patterns of virulence related genes might be correlated with intra-isolate STs (Table 2).
Figure 3. Phylogenetic tree showing stx 2f nucleotide sequence clusters in this study and stx 2f sequences in other Escherichia coli.
The stx 2f sequence in this study belonged to the branch of stx 2f genogroups, based on approximately 1.23 kb of sequence from the start codon of subunit A to the stop codon of subunit B. Accession numbers for reference sequences are in parentheses. Nucleotide sequence clusters identified in the current study are indicated in bold. The scale bar indicates the number of nucleotide substitutions per site. Cluster analysis was performed using an unweighted pair-group method with arithmetic average using MEGA 4 software [18]. “Type A” and “Type B” are described in footnote “‡” of Table 2.
Figure 4. Results of multi-locus sequence typing of Shiga toxin 2f-producing Escherichia coli isolates.
(a) Phylogenetic tree showing nucleotide sequence clusters of selected isolates with multi-locus sequence typing. Allele sequences for each strain were concatenated in the order adk–fumC–gyrB–icd–mdh–purA–recA for a final composite length of 3,423 bp. Reference sequence types (ST) 20, ST 382, ST 583, ST 722, ST 582, and ST 585 are available from the study by Prager et al. [1]. Other reference sequences were tested in the present study. The scale bar indicates the number of nucleotide substitutions per site. Escherichia coli type strain ATCC11775T and Escherichia albertii type strain LMG20976T are included as reference. (b) A minimum spanning tree was also constructed using Prim's algorithm from the PubMLST site (http://pubmlst.org/, accessed May 2, 2013).
Database accession numbers
The DDBJ accession numbers for the stx2f sequences of the isolates are AB499801–AB499803 and AB499807–AB499813. The sfpA sequences are submitted under numbers AB826561–AB826565. The sequences generated by multi-locus sequence typing (MLST) are available from the MLST database (http://mlst.ucc.ie/).
Discussion
The current study produced three important findings in regard to the isolation and characteristics of STEC2f in pigeons in Japan. First, STEC2f was isolated from pigeon excrement in Kyushu, Japan. The percentage of stx 2f-positive droppings varied from 0–17% across the 14 locations examined. Second, some of the stx 2f-gene fragment sequences identified in the current study were previously identified in Europe [2], and STEC2f O145:H34, ST 722, which has been isolated in Europe [1], was also obtained from pigeon droppings at location I in the present study. Last, the isolates varied in their serotypes, phenotypes, and genotypes. In particular, some isolates contained sfpA, which is reportedly restricted to some STEC strains [28], suggesting horizontal transfer of this virulence gene.
The isolation of STEC2f from pigeon droppings in Kyushu adds to the global surveillance data on STEC2f from pigeons. STEC2f from pigeons has previously been reported in other countries, including Finland [8], Germany [6], Italy [9], and India [29]. Kobayashi et al [7] reported that the frequency of STEC2f carriage in pigeons in Tokyo Bay, Japan, was 3.0%, while 3.5% of 29 studied pigeons in Finland carried STEC2f [8] Groβmann et al. [6] reported that 30.0% and 37.0% of 366 German pigeon dropping samples contained STEC2f, as analyzed by PCR and DNA-DNA hybridization, respectively. However, as not all of our samples came from separate pigeons, comparing our results with previous studies is difficult. Further investigation with direct sampling from pigeon cloaca is required to measure carriage rates of STEC2f in pigeons in Kyushu, Japan. The present STEC2f-positive isolates showed various lineages and harbored several virulence factors. All of the selected isolates contained eae, astA, and cdt, while some of these isolates also had “the high virulence pathogenicity island” characterized by the genes irp 2 and fyuA, in addition to genes for the major fimbrial subunit, LPFO26 (lpfA O26) [5]. Moreover, one O119:H21 and four O-untypeable:H16 isolates harbored sfpA (Table 2), which encodes the Sfp protein, the major pilin subunit of STEC O165:H2 and sorbitol-fermenting STEC O157:NM strains [28]. This indicates the horizontal transfer of this virulence gene to other serotypes, which is a novel and an important finding. The presence of eae, the locus coding for enterocyte effacement, in the STEC2f-positive strains is similar to previous findings [6], [9]. In particular, the confirmation of the β eae subtype in some of the present isolates is consistent with reports by Morabito et al. [9]. In other bacteria, some E. albertii strains examined in a report by Ooka et al. [30] encoded both eae (subtype N1.1 or N2) and stx 2f. In the present study, tested strains were confirmed to be E. coli, not E. albertii, using specific PCR and MLST analyses (Fig. 4) used previously by Ooka et al. to identify E. albertii [30]. In the current study, none of the isolates contained efa1, which is expressed following expression of eae and plays an important role in intestinal colonization by STEC strains [31]. Sonntag et al. reported the presence of lpfA O26 but not iha, efa1, sfpA, espP, pagC, irp 2, fyuA, or cdt in their STEC2f-isolates, while our results showed the presence of sfpA, irp 2, fyuA, and cdt, as well as lpfA O26, in some isolates. Further studies are needed to evaluate potential pathogenicities of the STEC2f strains. Six STs were identified in this study. ST 28, ST 2684, and ST 2685 are newly documented STs for STEC2f. These results suggest that several lineages of STEC2f are present in Kyushu, Japan, some of which may be hazardous to humans. The discrepancies among stx 2f-sequences, eae-types, and presence-absence patterns of virulence-related genes in each of the STEC2f lineages, as defined by MLST, suggest that further studies are needed to determine how and when each E. coli lineage acquired Shiga toxin phage, eae, or virulence-related genes.
Serotyping and MLST revealed that there may be a relationship between Japanese and European STEC2f strains. This is the first study to document serotype O119:H21 as being STEC2f, while O128:HNM, O145:H34, and O152:HNM were previously reported as human or pigeon STEC2f isolates [1], [3]–[5], [27], [29], [32], [33]. In particular, the O128 group, including O128:HNM, have been isolated by many laboratories in Japan [4] and Europe [1], [5], [32]. In addition, the 1,230-bp stx 2f sequence of some of the present isolates was the same as that identified for European isolates. STEC2f O145:H34, which is ST 722, was detected in both German human samples [1] and in Japanese pigeon excrement. These results suggest that STEC2f strains in Kyushu, Japan, and those in Europe may be in the same clonal complexes or lineage. It is important to identify the source of transmission of this pathogen, harboring several virulence-related genes, between Japan and Europe. Our findings suggest that STEC2f strains in pigeons represent a potential hazard in Japan. The findings contribute to public health in Kyushu and other parts of Japan, where STEC2f, and its relation to pigeons has not been reported. Because commercial stx PCR primers seldom detect the stx 2f gene [17], we recommend testing E. coli isolates with stx 2f-specific PCR primers. This knowledge is particularly useful for public health in Japan.
Acknowledgments
We are grateful to Dr. Ooka, Faculty of Medicine, University of Miyazaki, and Ms. Honda and Ms. Asoshima, Fukuoka City Institute of Hygiene and the Environment, for their invaluable advice and technical help. We also thank Dr. Hirata, Dr. Chijiwa, and Dr. Sera, Fukuoka Institute of Health and Environmental Sciences, for invaluable advice, and Dr. Saeki, Ms. Maruta, Mr. Kimoto and Ms. Uemura, Fukuoka Institute of Health and Environmental Sciences, for their technical assistance. The MLST data are publicly available at http://mlst.ucc.ie, which is currently supported by a grant from the Science Foundation of Ireland (05/FE1/B882).
Funding Statement
This work was supported by the JSPS KAKENHI, Grant No. 24590846, to Murakami, and was also supported in part by a grant to Etoh from the Daido Life Welfare Foundation, Osaka, Japan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. URL: JSPS, http://www.jsps.go.jp/english/index.html; Daido Life Welfare Foundation, http://www.daido-life-welfare.or.jp/.
References
- 1. Prager R, Fruth A, Siewert U, Strutz U, Tschäpe H (2009) Escherichia coli encoding Shiga toxin 2f as an emerging human pathogen. Int J Med Microbiol 299: 343–353. [DOI] [PubMed] [Google Scholar]
- 2. Schmidt H, Scheef J, Morabito S, Caprioli A, Wieler LH, et al. (2000) A new Shiga toxin 2 variant (Stx2f) from Escherichia coli isolated from pigeons. Appl Environ Microbiol 66: 1205–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Duynhoven YT, Friesema IH, Schuurman T, Roovers A, van Zwet AA, et al. (2008) Prevalence, characterisation and clinical profiles of Shiga toxin-producing Escherichia coli in The Netherlands. Clin Microbiol Infect 14: 437–445. [DOI] [PubMed] [Google Scholar]
- 4. Isobe J, Kimata K, Shimojima M, Hosorogi S, Tanaka D, et al. (2004) Isolation of Escherichia coli O128:HNM harboring stx2f gene from diarrhea patients. Kansenshogaku Zasshi 78: 1000–1005. [DOI] [PubMed] [Google Scholar]
- 5. Sonntag AK, Zenner E, Karch H, Bielaszewska M (2005) Pigeons as a possible reservoir of Shiga toxin 2f-producing Escherichia coli pathogenic to humans. Berl Münch Tierärztl Wochenschr 118: 464–470. [PubMed] [Google Scholar]
- 6. Groβmann K, Weniger B, Baljer G, Brenig B, Wieler LH (2005) Racing, ornamental and city pigeons carry shiga toxin producing Escherichia coli (STEC) with different Shiga toxin subtypes, urging further analysis of their epidemiological role in the spread of STEC. Berl Münch Tierärztl Wochenschr 118: 456–463. [PubMed] [Google Scholar]
- 7. Kobayashi H, Kanazaki M, Hata E, Kubo M (2009) Prevalence and characteristics of eae- and stx-positive strains of Escherichia coli from wild birds in the immediate environment of Tokyo Bay. Appl Environ Microbiol 75: 292–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kobayashi H, Pohjanvirta T, Pelkonen S (2002) Prevalence and characteristics of intimin- and Shiga toxin-producing Escherichia coli from gulls, pigeons and broilers in Finland. J Vet Med Sci 64: 1071–1073. [DOI] [PubMed] [Google Scholar]
- 9. Morabito S, Dell'Omo G, Agrimi U, Schmidt H, Karch H, et al. (2001) Detection and characterization of Shiga toxin-producing Escherichia coli in feral pigeons. Vet Microbiol 82: 275–283. [DOI] [PubMed] [Google Scholar]
- 10. Prager R, Annemüller S, Tschäpe H (2005) Diversity of virulence patterns among shiga toxin-producing Escherichia coli from human clinical cases-need for more detailed diagnostics. Int J Med Microbiol 295: 29–38. [DOI] [PubMed] [Google Scholar]
- 11. Nakao H, Kimura K, Murakami H, Maruyama T, Takeda T (2002) Subtyping of Shiga toxin 2 variants in human-derived Shiga toxin-producing Escherichia coli strains isolated in Japan. FEMS Immunol Med Microbiol 34: 289–297. [DOI] [PubMed] [Google Scholar]
- 12. Hyma KE, Lacher DW, Nelson AM, Bumbaugh AC, Janda JM, et al. (2005) Evolutionary genetics of a new pathogenic Escherichia species: Escherichia albertii and related Shigella boydii strains. Journal of Bacteriology 187: 619–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oaks LJ, Besser TE, Walk ST, Gordon DM, Beckmen KB, et al. (2010) Escherichia albertii in wild and domestic birds. Emerg Infect Dis 16: 638–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Machado J, Grimont F, Grimont PA (2000) Identification of Escherichia coli flagellar types by restriction of the amplified fliC gene. Res Microbiol 151: 535–546. [DOI] [PubMed] [Google Scholar]
- 15. Murakami K, Ishihara T, Horikawa K, Oda T (2007) Features of Salmonella serovars among food handlers in Kyushu, Japan. New Microbiol 30: 155–159. [PubMed] [Google Scholar]
- 16. Römling U, Tümmler B (2000) Achieving 100% typeability of Pseudomonas aeruginosa by pulsed-field gel electrophoresis. J Clin Microbiol 38: 464–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Etoh Y, Murakami K, Ichihara S, Sera N, Hamasaki M, et al. (2009) Isolation of Shiga Toxin 2f-producing Escherichia coli (O115:HNM) from an adult symptomatic patient in Fukuoka Prefecture, Japan. Jpn J Infect Dis 62: 315–317. [PubMed] [Google Scholar]
- 18. Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution 24: 1596–1599. [DOI] [PubMed] [Google Scholar]
- 19. Pickett CL, Pesci EC, Cottle DL, Russell G, Erdem AN, et al. (1996) Prevalence of cytolethal distending toxin production in Campylobacter jejuni and relatedness of Campylobacter sp. cdtB gene. Infection and Immunity 64: 2070–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kobayashi K, Seto K, Yatsuyanagi J, Saito S, Terao M, et al. (2002) Presence of the genes regarding adherence factors of Escherichia coli isolates and a consideration of the procedure for detection of diarrheagenic strain. Kansenshogaku Zasshi 76: 911–920. [DOI] [PubMed] [Google Scholar]
- 21. Ito K, Iida M, Yamazaki M, Moriya K, Moroishi S, et al. (2007) Intimin types determined by heteroduplex mobility assay of intimin gene (eae)-positive Escherichia coli strains. Journal of Clinical Microbiology 45: 1038–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Blanco M, Blanco JE, Dahbi G, Mora A, Alonso MP, et al. (2006) Typing of intimin (eae) genes from enteropathogenic Escherichia coli (EPEC) isolated from children with diarrhoea in Montevideo, Uruguay: identification of two novel intimin variants (μB and ξR/β2B). J Med Microbiol 55: 1165–1174. [DOI] [PubMed] [Google Scholar]
- 23. Konowalchuk J, Speirs JI, Stavric S (1977) Vero response to a cytotoxin of Escherichia coli . Infection and Immunity 18: 775–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Noda T, Murakami K, Asai T, Etoh Y, Ishihara T, et al. (2011) Multi-locus sequence typing of Salmonella enterica subsp. enterica serovar Enteritidis strains in Japan between 1973 and 2004. Acta Veterinaria Scandinavica 53: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22: 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, et al. (1995) Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol 33: 2233–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yamaguchi Y, Yamasaki S, Noguchi H (2001) Molecular analysis about the pathogenicity of Shiga toxin-producing Escherichia coli (STEC) O63:HNM isolated from infantile diarrhea. Annual report of Nagasaki Prefectural Institute of Publich health and Environmental Sciences 47: 21–28. [Google Scholar]
- 28. Bardiau M, Szalo M, Mainil JG (2010) Initial adherence of EPEC, EHEC and VTEC to host cells. Vet Res 41: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farooq S, Hussain I, Mir MA, Bhat MA, Wani SA (2009) Isolation of atypical enteropathogenic Escherichia coli and Shiga toxin 1 and 2f-producing Escherichia coli from avian species in India. Letters in Applied Microbiology. [DOI] [PubMed]
- 30. Ooka T, Seto K, Kawano K, Kobayashi H, Etoh Y, et al. (2012) Clinical significance of Escherichia albertii . Emerg Infect Dis 18: 488–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Deacon V, Dziva F, van Diemen PM, Frankel G, Stevens MP (2010) Efa-1/LifA mediates intestinal colonization of calves by enterohaemorrhagic Escherichia coli O26:H- in a manner independent of glycosyltransferase and cysteine protease motifs or effects on type III secretion. Microbiology 156: 2527–2536. [DOI] [PubMed] [Google Scholar]
- 32. Jenkins C, Willshaw GA, Evans J, Cheasty T, Chart H, et al. (2003) Subtyping of virulence genes in verocytotoxin-producing Escherichia coli (VTEC) other than serogroup O157 associated with disease in the United Kingdom. J Med Microbiol 52: 941–947. [DOI] [PubMed] [Google Scholar]
- 33. Seto K, Taguchi M, Kobayashi K, Kozaki S (2007) Biochemical and molecular characterization of minor serogroups of Shiga toxin-producing Escherichia coli isolated from humans in Osaka prefecture. J Vet Med Sci 69: 1215–1222. [DOI] [PubMed] [Google Scholar]