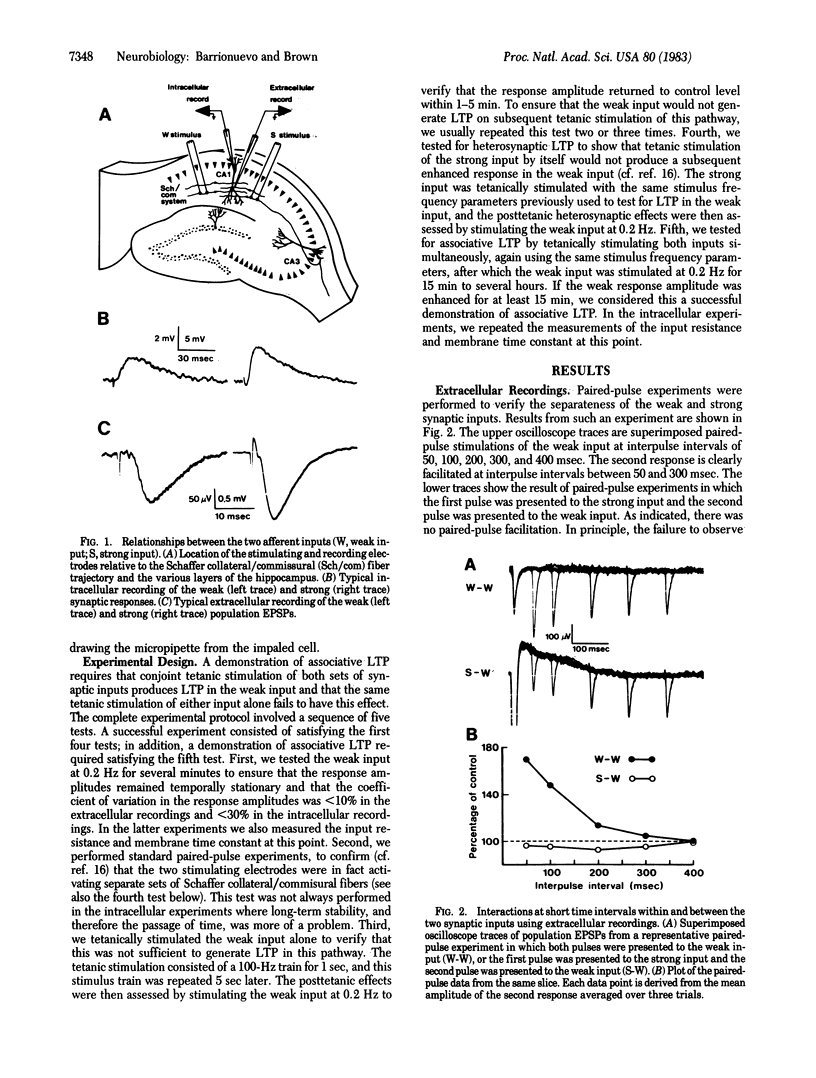
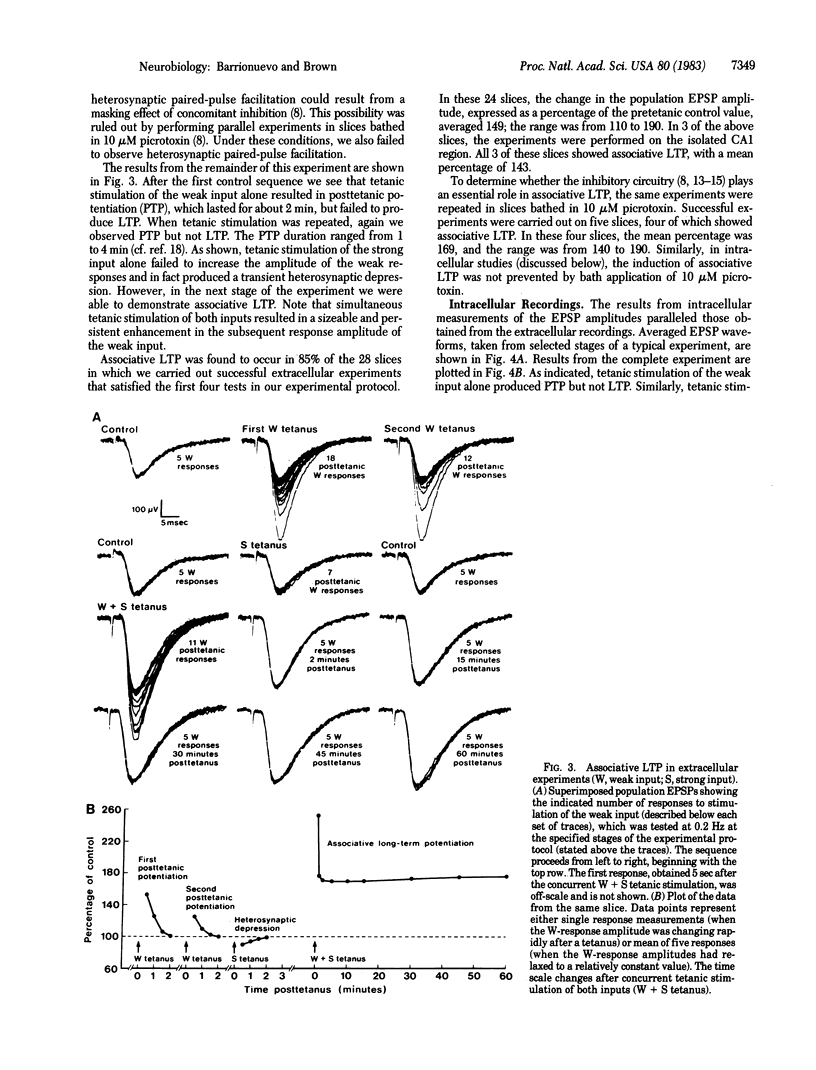
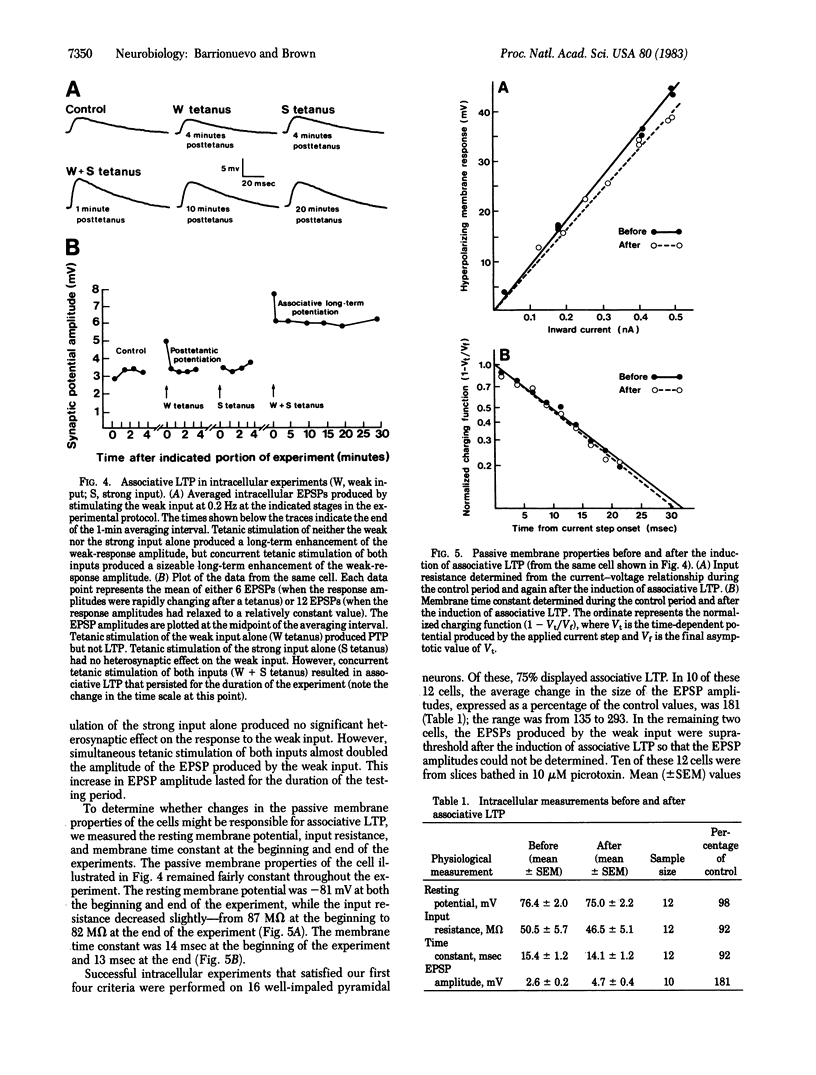
Abstract
Interactions between two excitatory monosynaptic inputs to hippocampal neurons of the CA1 region were examined in the in vitro slice. By adjusting the strengths of the electrical stimuli delivered to the two input pathways, one was made to generate a weak and the other a strong synaptic response. Simultaneous tetanic stimulation of both input pathways resulted in a subsequent long-term enhanced synaptic efficacy in the weak input under conditions in which the same tetanic stimulation of either input alone failed to have this effect. This form of long-term synaptic potentiation (LTP), known as associative LTP, was shown in some cases to last hours without decrement. The plastic changes were localized within the CA1 region and appear to reside in the pre- or postsynaptic elements of the monosynaptic excitatory input to the pyramidal neurons. The increased synaptic efficacy could not be accounted for by any of several measured postsynaptic passive membrane properties.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN P., ECCLES J. C., LOYNING Y. PATHWAY OF POSTSYNAPTIC INHIBITION IN THE HIPPOCAMPUS. J Neurophysiol. 1964 Jul;27:608–619. doi: 10.1152/jn.1964.27.4.608. [DOI] [PubMed] [Google Scholar]
- Alger B. E., Nicoll R. A. Feed-forward dendritic inhibition in rat hippocampal pyramidal cells studied in vitro. J Physiol. 1982 Jul;328:105–123. doi: 10.1113/jphysiol.1982.sp014255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T. H., Fricke R. A., Perkel D. H. Passive electrical constants in three classes of hippocampal neurons. J Neurophysiol. 1981 Oct;46(4):812–827. doi: 10.1152/jn.1981.46.4.812. [DOI] [PubMed] [Google Scholar]
- Brown T. H., Johnston D. Voltage-clamp analysis of mossy fiber synaptic input to hippocampal neurons. J Neurophysiol. 1983 Aug;50(2):487–507. doi: 10.1152/jn.1983.50.2.487. [DOI] [PubMed] [Google Scholar]
- Brown T. H., McAfee D. A. Long-term synaptic potentiation in the superior cervical ganglion. Science. 1982 Mar 12;215(4538):1411–1413. doi: 10.1126/science.6278593. [DOI] [PubMed] [Google Scholar]
- Brown T. H., Wong R. K., Prince D. A. Spontaneous miniature synaptic potentials in hippocampal neurons. Brain Res. 1979 Nov 9;177(1):194–199. doi: 10.1016/0006-8993(79)90931-4. [DOI] [PubMed] [Google Scholar]
- Dunwiddie T., Lynch G. Long-term potentiation and depression of synaptic responses in the rat hippocampus: localization and frequency dependency. J Physiol. 1978 Mar;276:353–367. doi: 10.1113/jphysiol.1978.sp012239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston D., Brown T. H. Giant synaptic potential hypothesis for epileptiform activity. Science. 1981 Jan 16;211(4479):294–297. doi: 10.1126/science.7444469. [DOI] [PubMed] [Google Scholar]
- Johnston D., Brown T. H. Interpretation of voltage-clamp measurements in hippocampal neurons. J Neurophysiol. 1983 Aug;50(2):464–486. doi: 10.1152/jn.1983.50.2.464. [DOI] [PubMed] [Google Scholar]
- KANDEL E. R., SPENCER W. A., BRINLEY F. J., Jr Electrophysiology of hippocampal neurons. I. Sequential invasion and synaptic organization. J Neurophysiol. 1961 May;24:225–242. doi: 10.1152/jn.1961.24.3.225. [DOI] [PubMed] [Google Scholar]
- Levy W. B., Brassel S. E., Moore S. D. Partial quantification of the associative synaptic learning rule of the dentate gyrus. Neuroscience. 1983 Apr;8(4):799–808. doi: 10.1016/0306-4522(83)90011-8. [DOI] [PubMed] [Google Scholar]
- Levy W. B., Steward O. Synapses as associative memory elements in the hippocampal formation. Brain Res. 1979 Oct 19;175(2):233–245. doi: 10.1016/0006-8993(79)91003-5. [DOI] [PubMed] [Google Scholar]
- Levy W. B., Steward O. Temporal contiguity requirements for long-term associative potentiation/depression in the hippocampus. Neuroscience. 1983 Apr;8(4):791–797. doi: 10.1016/0306-4522(83)90010-6. [DOI] [PubMed] [Google Scholar]
- Lynch G., Kessler M., Halpain S., Baudry M. Biochemical effects of high-frequency synaptic activity studied with in vitro slices. Fed Proc. 1983 Sep;42(12):2886–2890. [PubMed] [Google Scholar]
- Lynch G., Larson J., Kelso S., Barrionuevo G., Schottler F. Intracellular injections of EGTA block induction of hippocampal long-term potentiation. Nature. 1983 Oct 20;305(5936):719–721. doi: 10.1038/305719a0. [DOI] [PubMed] [Google Scholar]
- Lynch G., Schubert P. The use of in vitro brain slices for multidisciplinary studies of synaptic function. Annu Rev Neurosci. 1980;3:1–22. doi: 10.1146/annurev.ne.03.030180.000245. [DOI] [PubMed] [Google Scholar]
- McNaughton B. L., Douglas R. M., Goddard G. V. Synaptic enhancement in fascia dentata: cooperativity among coactive afferents. Brain Res. 1978 Nov 24;157(2):277–293. doi: 10.1016/0006-8993(78)90030-6. [DOI] [PubMed] [Google Scholar]


