Abstract
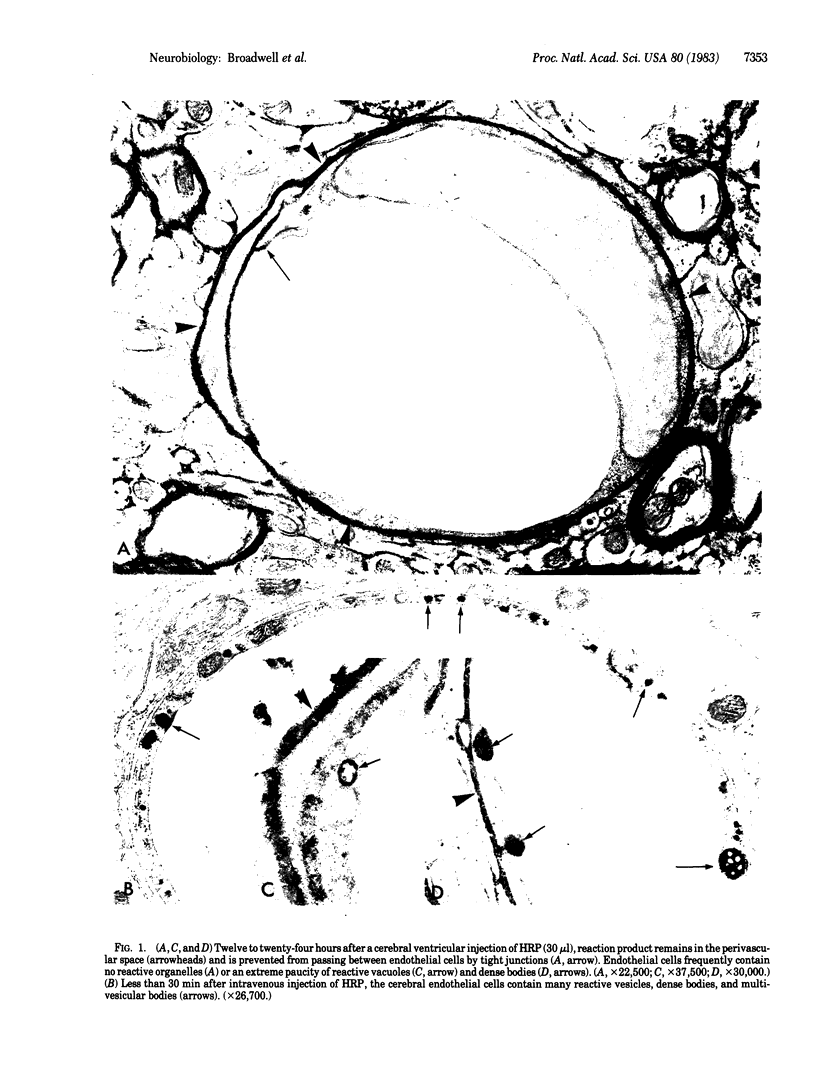
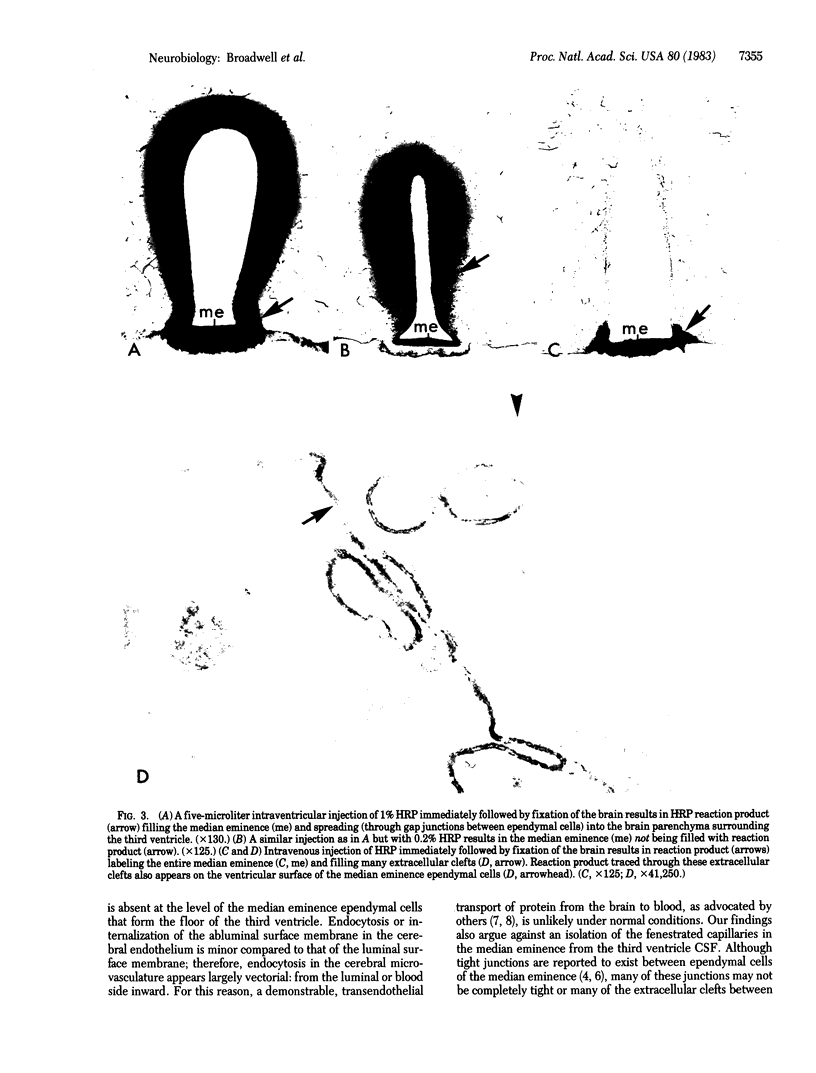
Ventriculo-cisternal perfusion of horseradish peroxidase (HRP) in the mouse brain has demonstrated that a brain-blood barrier exists at the microvascular endothelium in brain parenchyma but not in the median eminence of the hypothalamus. The brain-blood barrier is similar to the blood-brain barrier in that: tight junctions prevent the movement of protein between endothelial cells, HRP taken into the endothelial cells is directed to lysosomal dense bodies, and, contrary to the literature, a vesicular transendothelial transport of HRP from brain to blood does not occur under normal conditions. The endocytosis of ventricular injected HRP from the abluminal side of the endothelium is demonstrably less than the endocytosis of intravenous injected HRP from the luminal side; hence, the cerebral endothelium expresses a degree of polarity regarding the internalization of its cell surface membrane and extracellular protein. The passage of cerebrospinal fluid-borne or blood-borne HRP between some ependymal cells of the median eminence is not precluded by tight junctions. These patent extracellular channels offer a direct pathway for the exchange of substances between cerebrospinal fluid in the third ventricle and fenestrated capillaries in the median eminence.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brightman M. W. Morphology of blood-brain interfaces. Exp Eye Res. 1977;25 (Suppl):1–25. doi: 10.1016/s0014-4835(77)80008-0. [DOI] [PubMed] [Google Scholar]
- Broadwell R. D., Brightman M. W. Cytochemistry of undamaged neurons transporting exogenous protein in vivo. J Comp Neurol. 1979 May 1;185(1):31–73. doi: 10.1002/cne.901850104. [DOI] [PubMed] [Google Scholar]
- Broadwell R. D., Brightman M. W. Horseradish peroxidase: a tool for study of the neuroendocrine cell and other peptide-secreting cells. Methods Enzymol. 1983;103:187–218. doi: 10.1016/s0076-6879(83)03013-x. [DOI] [PubMed] [Google Scholar]
- Broadwell R. D., Salcman M. Expanding the definition of the blood-brain barrier to protein. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7820–7824. doi: 10.1073/pnas.78.12.7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundgaard M., Cserr H., Murray M. Impermeability of hagfish cerebral capillaries to horseradish peroxidase. An ultrastructural study. Cell Tissue Res. 1979 Apr 30;198(1):65–77. doi: 10.1007/BF00234835. [DOI] [PubMed] [Google Scholar]
- Deurs B. V. Vesicular transport of horseradish peroxidase from brain to blood in segments of the cerebral microvasculature in adult mice. Brain Res. 1977 Mar 18;124(1):1–8. doi: 10.1016/0006-8993(77)90859-9. [DOI] [PubMed] [Google Scholar]
- Krieger D. T., Martin J. B. Brain peptides (second of two parts). N Engl J Med. 1981 Apr 16;304(16):944–951. doi: 10.1056/NEJM198104163041605. [DOI] [PubMed] [Google Scholar]
- Wagner H. J., Pilgrim C., Brandl J. Penetration and removal of horseradish peroxidase injected into the cerebrospinal fluid: role of cerebral perivascular spaces, endothelium and microglia. Acta Neuropathol. 1974 Apr 30;27(4):299–315. doi: 10.1007/BF00690695. [DOI] [PubMed] [Google Scholar]
- Wagner H. J., Pilgrim C. Extracellular and transcellular transport of horseradish peroxidase (HRP) through the hypothalamic tanycyte ependyma. Cell Tissue Res. 1974;152(4):477–491. doi: 10.1007/BF00218933. [DOI] [PubMed] [Google Scholar]