Abstract
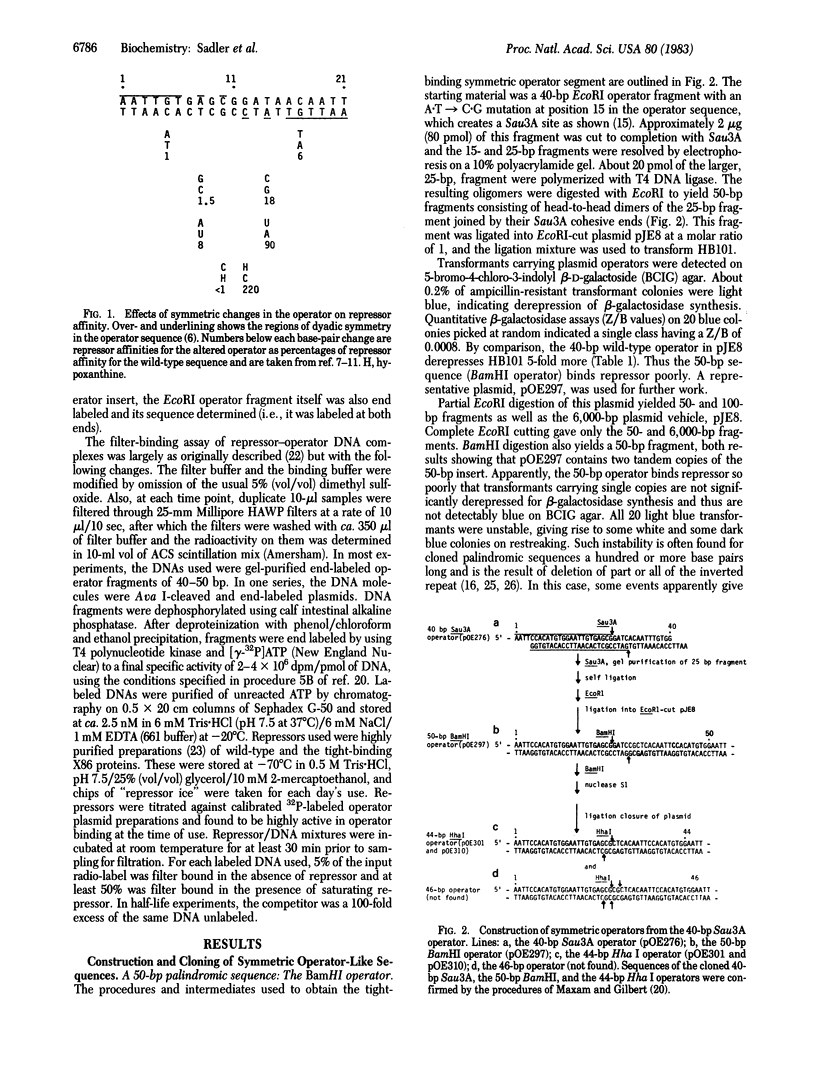
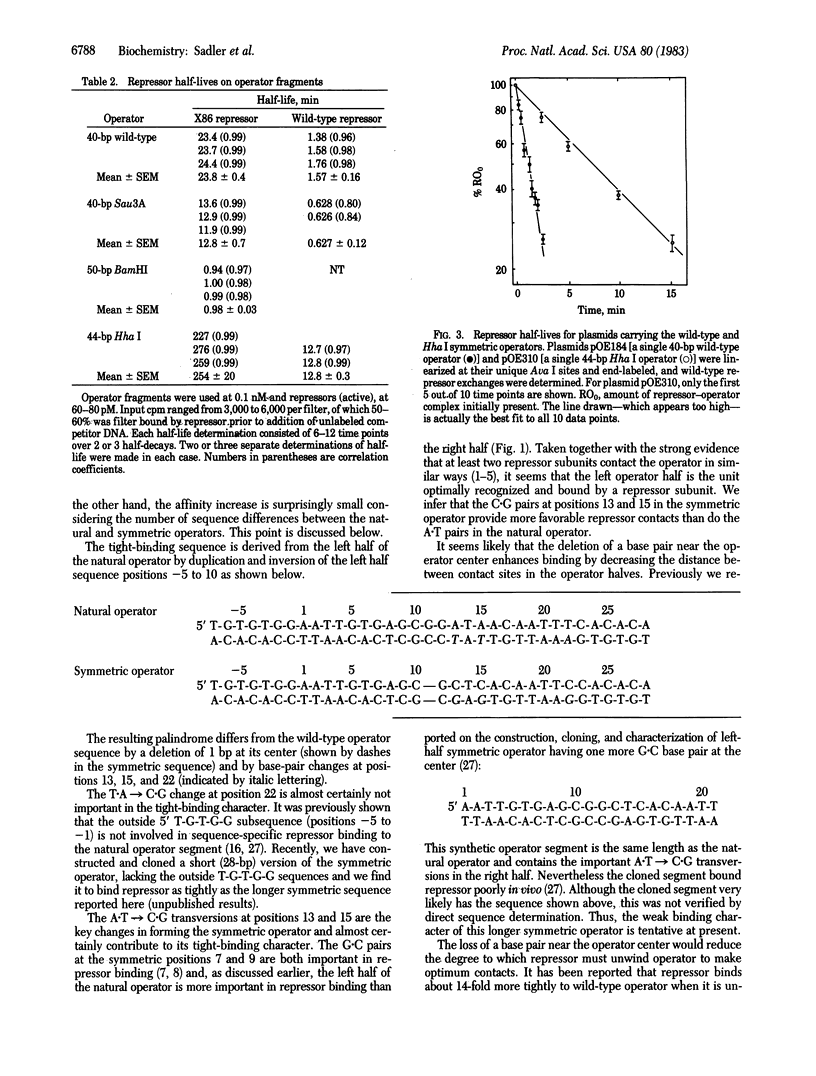
A completely symmetric DNA segment has been constructed that binds the lactose repressor of Escherichia coli 10-fold more tightly than does the natural lactose operator sequence. This tight-binding operator is an inverted repeat of a 15-base-pair segment from the left half of the natural operator sequence, the inversion being about the point indicated by the arrow shown below: (sequence in text) where the upper sequence is the natural operator and the lower sequence is the symmetric operator. The increased affinity of repressor for this symmetric sequence supports the idea that the tetrameric repressor is designed for a two-module binding to DNA, presumably via two (or two pairs) of its identical subunits. The natural operator is apparently "flawed" by "incorrect" base pairs in the right operator half and by an "incorrect" spacing between the operator halves with respect to maximal repressor binding.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betz J. L., Sadler J. R. Tight-binding repressors of the lactose operon. J Mol Biol. 1976 Aug 5;105(2):293–319. doi: 10.1016/0022-2836(76)90113-3. [DOI] [PubMed] [Google Scholar]
- Betz J. L., Sadler J. R. Variants of a cloned synthetic lactose operator. I. A palindromic dimer lactose operator derived from one stand of the cloned 40-base pair operator. Gene. 1981 Jan-Feb;13(1):1–12. doi: 10.1016/0378-1119(81)90038-x. [DOI] [PubMed] [Google Scholar]
- Betz J. L., Sadler J. R. Variants of a cloned synthetic lactose operator. II. Chloramphenicol-resistant revertants retaining a lactose operator in the CAT gene of plasmid pBR325. Gene. 1981 Nov;15(2-3):187–200. doi: 10.1016/0378-1119(81)90128-1. [DOI] [PubMed] [Google Scholar]
- Bolivar F. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique Eco RI sites for selection of Eco RI generated recombinant DNA molecules. Gene. 1978 Oct;4(2):121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Vournakis J. N., Donis-Keller H., Chaconas G., Dougall D. K., Kafatos F. C. End labeling of enzymatically decapped mRNA. Nucleic Acids Res. 1977 Dec;4(12):4165–4174. doi: 10.1093/nar/4.12.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler N., Weber K. Isolation of a set of hybrid lac repressors made in vitro between normal lac repressor and its homogeneous tryptic core. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3103–3106. doi: 10.1073/pnas.73.9.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W., Maxam A. The nucleotide sequence of the lac operator. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3581–3584. doi: 10.1073/pnas.70.12.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeddel D. V., Yansura D. G., Caruthers M. H. How lac repressor recognizes lac operator. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3578–3582. doi: 10.1073/pnas.75.8.3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobe A., Sadler J. R., Bourgeois S. lac Repressor-operator interaction. IX. The binding of lac repressor to operators containing Oc mutations. J Mol Biol. 1974 May 15;85(2):231–248. doi: 10.1016/0022-2836(74)90362-3. [DOI] [PubMed] [Google Scholar]
- Kania J., Brown D. T. The functional repressor parts of a tetrameric lac repressor-beta-galactosidase chimaera are organized as dimers. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3529–3533. doi: 10.1073/pnas.73.10.3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim R., Kim S. H. Direct measurement of DNA unwinding angle in specific interaction between lac operator and repressor. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):451–454. doi: 10.1101/sqb.1983.047.01.053. [DOI] [PubMed] [Google Scholar]
- Marinus M. G. Location of DNA methylation genes on the Escherichia coli K-12 genetic map. Mol Gen Genet. 1973 Dec 14;127(1):47–55. doi: 10.1007/BF00267782. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Nick H., Arndt K., Boschelli F., Jarema M. A., Lillis M., Sadler J., Caruthers M., Lu P. lac repressor-lac operator interaction: NMR observations. Proc Natl Acad Sci U S A. 1982 Jan;79(2):218–222. doi: 10.1073/pnas.79.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlendorf D. H., Anderson W. F., Fisher R. G., Takeda Y., Matthews B. W. The molecular basis of DNA-protein recognition inferred from the structure of cro repressor. Nature. 1982 Aug 19;298(5876):718–723. doi: 10.1038/298718a0. [DOI] [PubMed] [Google Scholar]
- Riggs A. D., Bourgeois S., Cohn M. The lac repressor-operator interaction. 3. Kinetic studies. J Mol Biol. 1970 Nov 14;53(3):401–417. doi: 10.1016/0022-2836(70)90074-4. [DOI] [PubMed] [Google Scholar]
- Rosenberg J. M., Khallai O. B., Kopka M. L., Dickerson R. E., Riggs A. D. Lac repressor purification without inactivation of DNA binding activity. Nucleic Acids Res. 1977 Mar;4(3):567–572. doi: 10.1093/nar/4.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler J. R., Betz J. L., Tecklenburg M., Goeddel D. V., Yansura D. G., Caruthers M. H. Cloning of chemically synthesized lactose operators. II. EcoRI-linkered operators. Gene. 1978 May;3(3):211–232. doi: 10.1016/0378-1119(78)90033-1. [DOI] [PubMed] [Google Scholar]
- Sadler J. R., Tecklenburg M., Betz J. L., Goeddel D. V., Yansura D. G., Caruthers M. H. Cloning of chemically synthesized lactose operators. Gene. 1977 Jul;1(5-6):305–321. doi: 10.1016/0378-1119(77)90036-1. [DOI] [PubMed] [Google Scholar]
- Sadler J. R., Tecklenburg M., Betz J. L. Plasmids containing many tandem copies of a synthetic lactose operator. Gene. 1980 Feb;8(3):279–300. doi: 10.1016/0378-1119(80)90005-0. [DOI] [PubMed] [Google Scholar]
- Sadler J. R., Tecklenburg M. Cloning and characterization of the natural lactose operator. Gene. 1981 Jan-Feb;13(1):13–23. doi: 10.1016/0378-1119(81)90039-1. [DOI] [PubMed] [Google Scholar]
- Sadler J. R., Tecklenburg M. Recovery of operator DNA binding activity from denatured lactose repressor. Biochemistry. 1976 Oct 5;15(20):4353–4356. doi: 10.1021/bi00665a001. [DOI] [PubMed] [Google Scholar]
- Schmitz A., Galas D. J. The interaction of RNA polymerase and lac repressor with the lac control region. Nucleic Acids Res. 1979 Jan;6(1):111–137. doi: 10.1093/nar/6.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinden R. R., Carlson J. O., Pettijohn D. E. Torsional tension in the DNA double helix measured with trimethylpsoralen in living E. coli cells: analogous measurements in insect and human cells. Cell. 1980 Oct;21(3):773–783. doi: 10.1016/0092-8674(80)90440-7. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Wang J. C., Barkley M. D., Bourgeois S. Measurements of unwinding of lac operator by repressor. Nature. 1974 Sep 20;251(5472):247–249. doi: 10.1038/251247a0. [DOI] [PubMed] [Google Scholar]
- Yansura D. G., Goeddel D. V., Kundu A., Caruthers M. H. Studied on gene control regions IX. The effect of hypoxanthine-substituted lac operators on the lac operator--lac repressor interaction. J Mol Biol. 1979 Sep 5;133(1):117–135. doi: 10.1016/0022-2836(79)90253-5. [DOI] [PubMed] [Google Scholar]


