Abstract
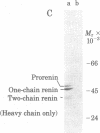
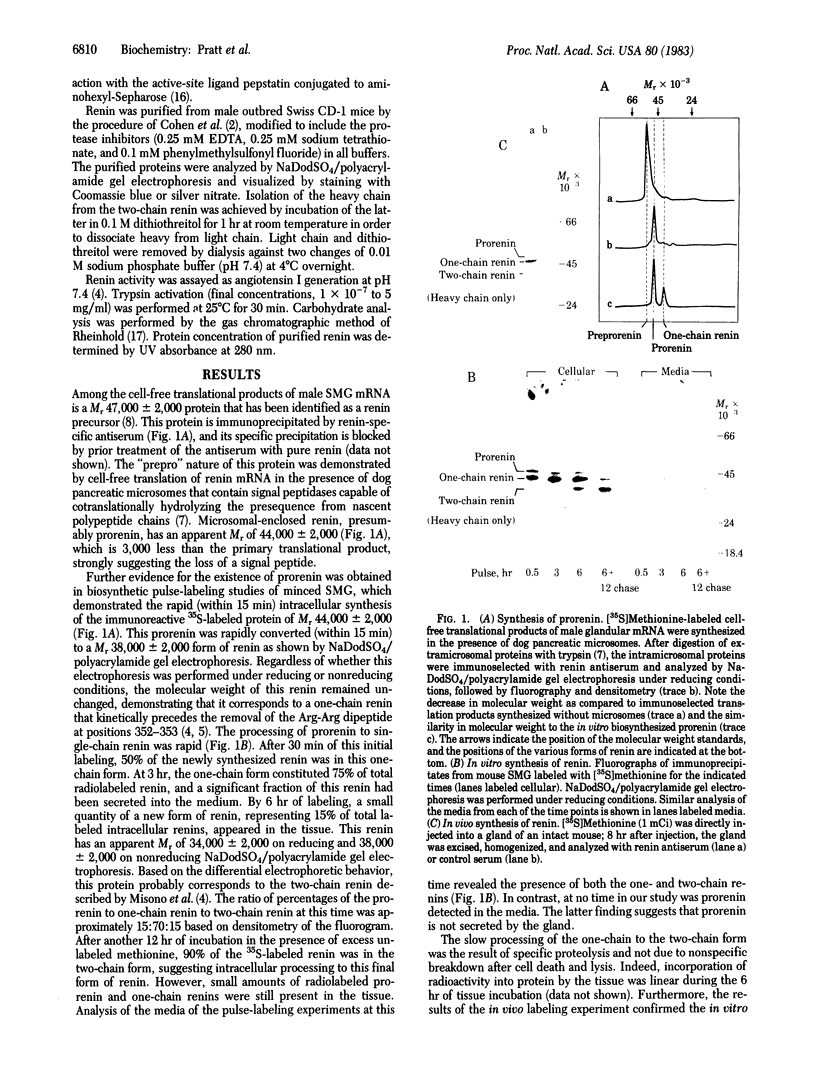
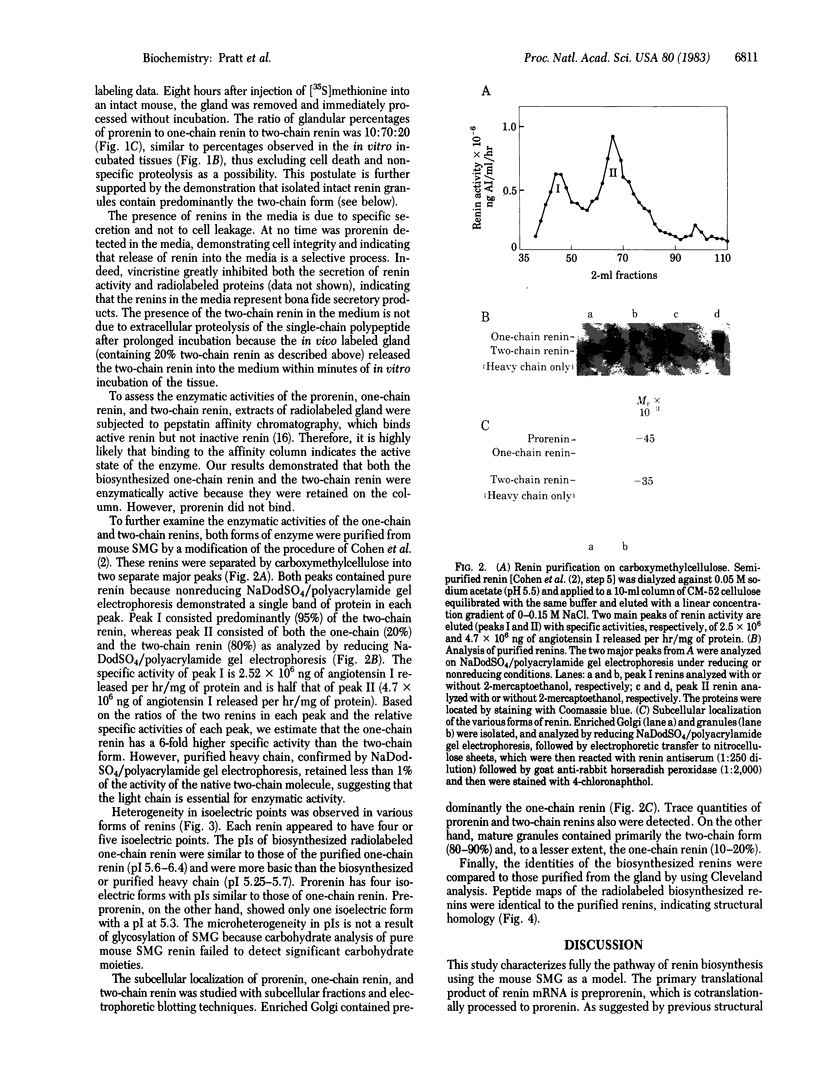
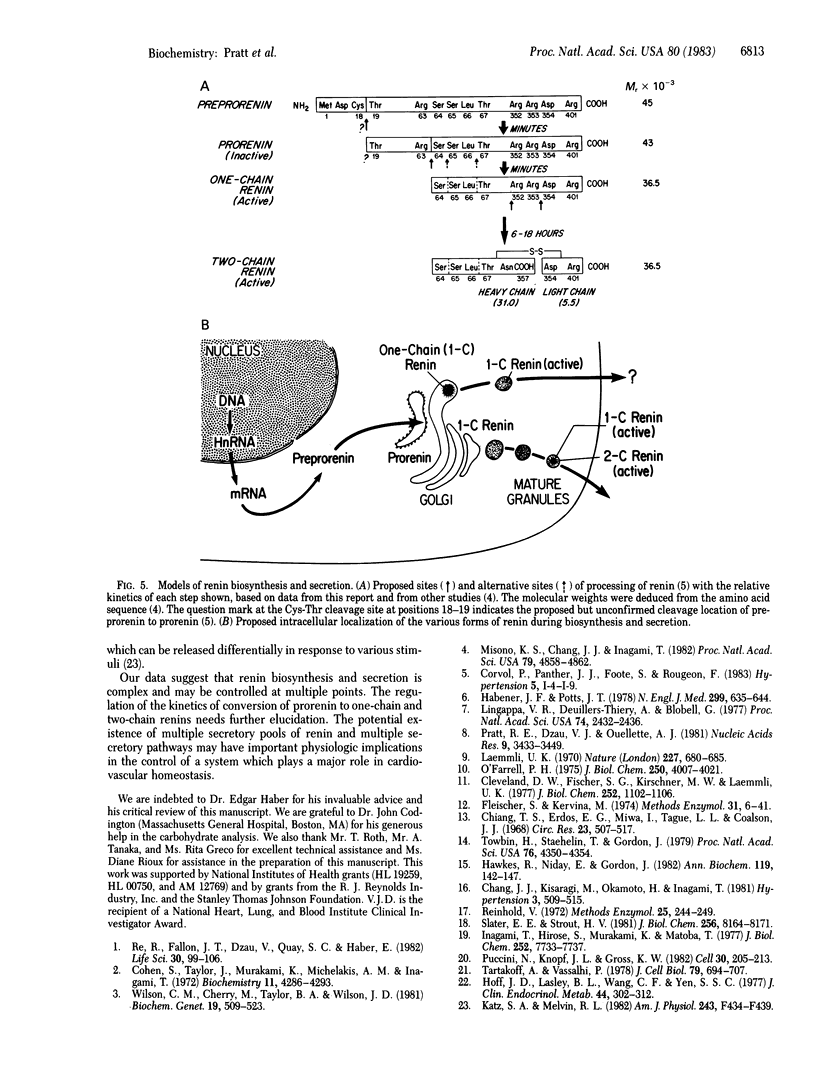
Processing of renin involves sequential proteolytic cleavages of a preproform to the active mature forms. Preprorenin is rapidly internalized cotranslationally into the rough endoplasmic reticulum and hydrolyzed by signal peptidase to produce prorenin. In the Golgi, prorenin is converted (within 15 min) to a form of renin that is enzymatically active. Over the next 12 hr, a slow intracellular process removes a dipeptide near the carboxyl terminus, converting the one-chain renin into two chains joined by a single disulfide bond. This conversion occurs during formation, condensation, and packaging of renin granules. The resultant two-chain renin is approximately one-sixth as active as the one-chain form. The intact renin molecule is obligatory for enzymatic activity because heavy chain alone has little or no activity. Both one- and two-chain renins are secreted, but prorenin is not. Multiple isoelectric forms of prorenin, one-chain renin, and two-chain renin are also observed. This microheterogeneity probably results from minor differences in amino acid composition as a consequence of variations in cleavage positions during processing. Thus, these data suggest that renin synthesis and secretion is complex and may be subject to regulation at multiple steps. Furthermore, based on the results of this study, we also propose that renin can be secreted by two different pathways.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang J. J., Kisaragi M., Okamoto H., Inagami T. Isolation and activation of inactive renin from human kidney and plasma. Plasma and renal inactive renins have different molecular weights. Hypertension. 1981 Sep-Oct;3(5):509–515. doi: 10.1161/01.hyp.3.5.509. [DOI] [PubMed] [Google Scholar]
- Chiang T. S., Erdös E. G., Miwa I., Tague L. L., Coalson J. J. Isolation from a salivary gland of granules containing renin and kallikrein. Circ Res. 1968 Oct;23(4):507–517. doi: 10.1161/01.res.23.4.507. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cohen S., Taylor J. M., Murakami K., Michelakis A. M., Inagami T. Isolation and characterization of renin-like enzymes from mouse submaxillary glands. Biochemistry. 1972 Nov 7;11(23):4286–4293. doi: 10.1021/bi00773a015. [DOI] [PubMed] [Google Scholar]
- Fleischer S., Kervina M. Subcellular fractionation of rat liver. Methods Enzymol. 1974;31:6–41. doi: 10.1016/0076-6879(74)31005-1. [DOI] [PubMed] [Google Scholar]
- Habener J. F., Potts J. T., Jr Biosynthesis of parathyroid hormone (second of two parts). N Engl J Med. 1978 Sep 21;299(12):635–644. doi: 10.1056/NEJM197809212991205. [DOI] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Hoff J. D., Lasley B. L., Wang C. F., Yen S. S. The two pools of pituitary gonadotropin: regulation during the menstrual cycle. J Clin Endocrinol Metab. 1977 Feb;44(2):302–312. doi: 10.1210/jcem-44-2-302. [DOI] [PubMed] [Google Scholar]
- Inagami T., Hirose S., Murakami K., Matoba T. Native form of renin in the kidney. J Biol Chem. 1977 Nov 10;252(21):7733–7737. [PubMed] [Google Scholar]
- Katz S. A., Malvin R. L. Independence of beta-adrenergic stimulation of renin release on renin synthesis. Am J Physiol. 1982 Nov;243(5):F434–F439. doi: 10.1152/ajprenal.1982.243.5.F434. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lingappa V. R., Devillers-Thiery A., Blobel G. Nascent prehormones are intermediates in the biosynthesis of authentic bovine pituitary growth hormone and prolactin. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2432–2436. doi: 10.1073/pnas.74.6.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misono K. S., Chang J. J., Inagami T. Amino acid sequence of mouse submaxillary gland renin. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4858–4862. doi: 10.1073/pnas.79.16.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Piccini N., Knopf J. L., Gross K. W. A DNA polymorphism, consistent with gene duplication, correlates with high renin levels in the mouse submaxillary gland. Cell. 1982 Aug;30(1):205–213. doi: 10.1016/0092-8674(82)90026-5. [DOI] [PubMed] [Google Scholar]
- Pratt R. E., Dzau V. J., Ouellette A. J. Abundant androgen regulated mRNAs in mouse submandibular gland: cell-free translation of renin precursor mRNA. Nucleic Acids Res. 1981 Jul 24;9(14):3433–3449. doi: 10.1093/nar/9.14.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re R., Fallon J. T., Dzau V., Quay S. C., Haber E. Renin synthesis by canine aortic smooth muscle cells in culture. Life Sci. 1982 Jan 4;30(1):99–106. doi: 10.1016/0024-3205(82)90641-5. [DOI] [PubMed] [Google Scholar]
- Slater E. E., Strout H. V., Jr Pure human renin. Identification and characterization and of two major molecular weight forms. J Biol Chem. 1981 Aug 10;256(15):8164–8171. [PubMed] [Google Scholar]
- Tartakoff A., Vassalli P., Détraz M. Comparative studies of intracellular transport of secretory proteins. J Cell Biol. 1978 Dec;79(3):694–707. doi: 10.1083/jcb.79.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. M., Cherry M., Taylor B. A., Wilson J. D. Genetic and endocrine control of renin activity in the submaxillary gland of the mouse. Biochem Genet. 1981 Jun;19(5-6):509–523. doi: 10.1007/BF00484623. [DOI] [PubMed] [Google Scholar]