Abstract
Objectives
We tested whether the myocardial extracellular volume (ECV) is increased in hypertension (HTN) and atrial fibrillation (AF) undergoing pulmonary vein isolation, and to determine if there was an association between the ECV and post-procedural recurrence of AF.
Background
Hypertension (HTN) is associated with myocardial fibrosis, an increase in the ECV, and AF. Data linking these are limited. T1 measurements pre and post-contrast in a cardiac magnetic resonance (CMR) study provide a method for quantification of the ECV.
Methods
Consecutive patients with HTN and recurrent AF referred for pulmonary vein isolation underwent a contrast CMR study with measurement of the ECV, and were followed prospectively for a median of 18 months. The end-point of interest was late recurrence of AF.
Results
Patients had elevated left ventricular (LV) volumes, LV mass, left atrial volumes, and an increased ECV (AF, 0.34±0.03 vs. 0.29±0.03, healthy controls, p < 0.001). There were positive associations between the ECV and left atrial volume (r=0.46, p < 0.01) and the LV mass, and a negative association between the ECV and diastolic function (early mitral annular relaxation, E′, r=−0.55, p < 0.001). In the best overall multi-variable model, the ECV was the strongest predictor of the primary outcome of recurrent AF (HR 1.29, 95% CI 1.15–1.44, p < 0.0001) and the secondary composite outcome of recurrent AF, heart failure admission, and death (HR 1.35, 95% CI 1.21–1.51, p < 0.0001). Each 10% increase in the ECV was associated with a 29% increased risk of recurrent AF.
Conclusions
In patients with AF and HTN, expansion of the ECV is associated with diastolic function and LA remodeling, and is a strong independent predictor of recurrent AF post pulmonary vein isolation.
Keywords: Myocardial Fibrosis, Cardiac Magnetic Resonance, T1 measurements
Systemic arterial hypertension (HTN) is one of the commonest risk factors for the development of atrial fibrillation (AF) (1). An early myocardial response in the adjustment to pressure overload in HTN is an increase in the myocardial extracellular volume (ECV) due to the development of pathological myocardial fibrosis (2). Myocardial fibrosis is associated with myocardial stiffening, diastolic dysfunction, and elevated left atrial pressure, all key mediators for the development of AF. However, there are limited data directly linking myocardial fibrosis with AF (3,4), and data suggest that myocardial fibrosis in HTN is potentially reversible, especially at an early stage (5). The gold standard for detection of myocardial fibrosis, endomyocardial biopsy, is invasive. The current optimal non-invasive invasive test for detection of replacement myocardial fibrosis, such as that which occurs with a myocardial infarction, is cardiac magnetic resonance (CMR) with late gadolinium enhancement (LGE) (6). However, LGE-CMR depends on focal contrast enhancement relative to a normal area of myocardium. Disease processes such as HTN are likely diffuse and lack a normal reference myocardium (7). Consistent with this are published data on the presence of LGE in patients with HTN report an incidence ranging from 0% to approximately 50% in high-risk populations (8,9), underestimating both the presence and extent of fibrosis suggested by pathological data (10–12).
These limitations have prompted research into novel CMR-based quantitative techniques for quantification of the myocardial ECV, which is derived from pre- and post-contrast T1 measures (13–17). The ECV has been validated as a non-invasive estimate of myocardial fibrosis (15,17) and an elevated ECV is associated with increased mortality (18). However, there are limited data on whether the ECV derived from T1 measurements is abnormal in patients with HTN (19), furthermore, there are limited data linking expansion of the ECV with adverse clinical outcomes (18). However, testing a broad group of patients with HTN for both expansion of the ECV and linking expansion of the ECV in patients with isolated HTN would require preliminary data. Prior to pulmonary vein isolation (PVI), we routinely perform imaging of pulmonary vein anatomy with CMR and HTN is one of the primary etiologies for AF in patients requiring PVI. Therefore we aimed to test whether T1 measurements could detect expansion of the ECV in patients with HTN undergoing PVI for recurrent AF, to test whether the ECV in this population was associated with other measures of cardiovascular structure and function, and to test whether an elevated ECV in this population was associated with the risk of recurrent AF after PVI.
Methods
Study population
We performed a prospective, observational study of consecutive patients with HTN undergoing a PVI for recurrent AF. The cohort underwent a CMR that included gadolinium between July 2009 and January 2012. Patients were referred for a CMR study specifically for imaging of pulmonary veins prior to PVI for treatment of recurrent AF. We choose this population for two reasons: first, the incidence of isolated HTN is high among patients with AF; second, patients in whom PVI is being planned for treatment of AF are routinely referred, at this institution, for CMR-based imaging of the pulmonary veins. To limit the contribution of other pathologies that expand the myocardial extracellular volume, we excluded patients with diabetes mellitus, myocardial infarction by either history, according to the presence of pathological Q waves on an EKG, or typical LGE infarct pattern, severe renal failure (glomerular filtration rate < 30 ml/m2), a history of heart failure or any prior documented reduced left ventricular ejection fraction (<50%), significant valvular heart disease (moderate AS, AI, MR, and MS), an infiltrative cardiomyopathy by history or imaging, and patients who were not in sinus rhythm at the time of the CMR. All patients meeting the inclusion and exclusion criteria were included, including those who had an ablation for AF prior to the study period or at another institution. Hypertension and the presence of LVH were defined using published criteria (20,21).For the active cohort, screening consisted of a comprehensive questionnaire detailing medical history (including current medications) and standard anthropometric data, as well as a clinical evaluation which included blood pressure, heart rate, serum creatinine, hematocrit, EKG, and echocardiogram. Healthy volunteer controls were recruited by open enrollment. For healthy controls, we excluded patients with a history of HTN, known cardiac disease, renal failure (glomerular filtration rate < 60 ml/m2) or diabetes. Screening for volunteers similarly consisted of a comprehensive questionnaire detailing medical and medication history, standard anthropometric data, and measurement of blood pressure, heart rate, serum creatinine, and hematocrit. Healthy controls did not undergo an EKG or an echocardiogram. Data was entered into a registry at the time of the CMR study. The protocol was approved by the Partners Healthcare System Institutional Review Board.
Standard CMR protocol
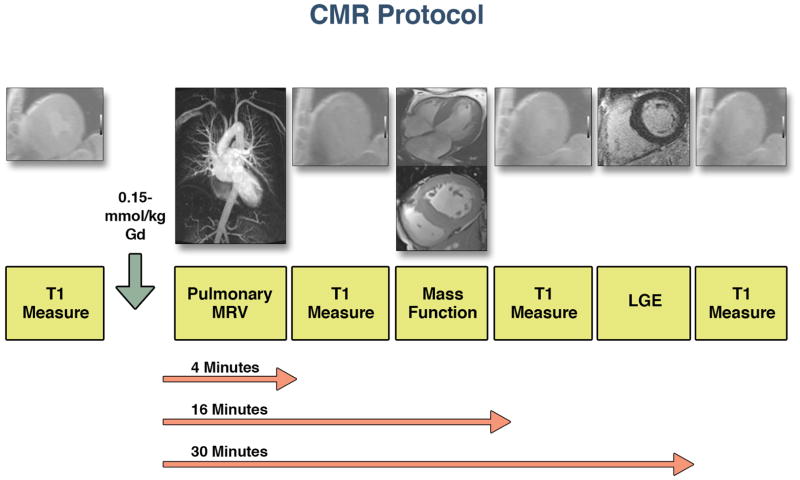
All images were acquired with EKG gating, breath-holding, and with the patient in a supine position. Subjects were imaged on 3.0-T CMR system (Tim Trio, Siemens Medical Systems, Erlangen, Germany). The basic CMR protocol consisted of cine steady-state free precession (SSFP) imaging as previously described (22). All patients underwent an LGE imaging protocol (repetition time, 4.8 ms; echo time, 1.3 ms; inversion time, 200 to 300 ms) for focal myocardial fibrosis. A segmented inversion-recovery pulse sequence for LGE was used starting 10–15 minutes after a 0.15mmol/kg single bolus dose of gadolinium DTPA (Magnevist, Bayer HealthCare Phamaceuticals Inc, Wayne, New Jersey).
Myocardial extracellular volume fraction
T1 measurements were performed with an ECG-gated Look-Locker sequence with a non-slice-selective adiabatic inversion pulse, followed by a gradient-echo acquisition as previously described (19,22). We have previously tested and validated this sequence as being independent of heart rate in both clinical studies and with phantom models (23,24). The T1 sequence was performed in a single slice in the mid-ventricle and was repeated in the same mid LV short-axis slice once before and 3 additional times after the injection of gadolinium spanning an approximately 30 minute period (Figure 1). For each Look-Locker sequence the endo- and epicardial borders of the LV were traced and divided into 6 standard segments; segments were numbered 1 through 6 starting from the anterior RV insertion point and proceeding in a clockwise direction (Medis®, Leiden, The Netherlands). The reciprocal of T1 (R1=1/T1) was used to plot the myocardial R1 against the R1 in the blood pool, and calculate the slope by linear regression, using all measurement points with an R1 of < 3.0 s-1(19). The slope of the linear relationship (the partition coefficient for gadolinium, λGd) was calculated and an ECV for all 6 myocardial segments was quantified as reported previously (13,14). A global ECV for each patient or healthy volunteer was then calculated by averaging the 6 myocardial segmental values. Regions with visible LGE were not excluded from ECV measurements.
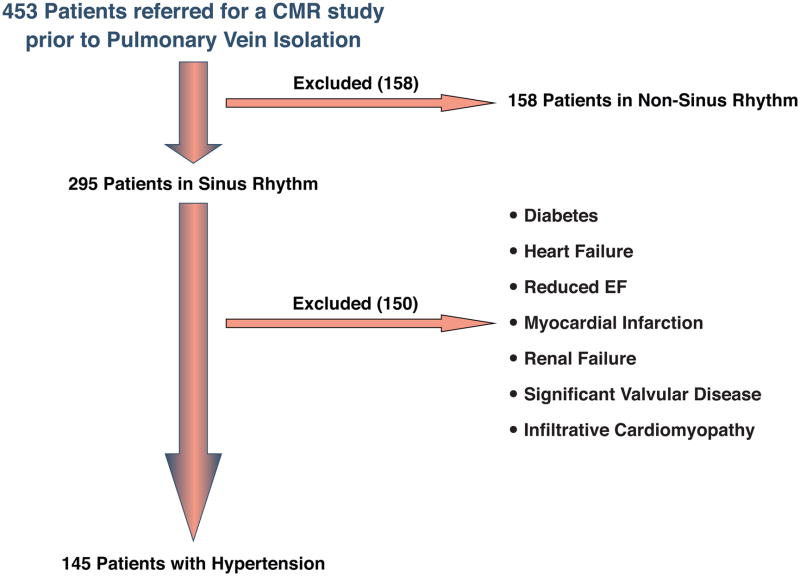
Figure 1. Flow diagram for study.
In general, 4–5 T1 measurements were recorded for each study patients. We recorded 1 T1 measurement pre-contrast and 3–4 post-contrast. We also completed a magnetic resonance angiogram for pulmonary vein identification, an LGE sequence, and SSFP imaging of the LV and RV.
Echocardiography
A Vivid-7 ultrasound system (GE Medical Systems, Milwaukee, WI) was used for standard and tissue Doppler (TDI) echocardiography. Pulmonary artery systolic pressure was estimated from the tricuspid regurgitant velocity plus an estimate of right atrial pressure derived from the inferior vena cava. Tissue Doppler myocardial velocities were acquired in early diastole (E′) at the lateral and septal aspects of the mitral annulus in the apical 4-chamber view. Measurements for the E′ were averaged from three successive heart beats. The ratio of the transmitral E wave velocity to the tissue Doppler E′, was calculated as a measure of left atrial pressure.
Clinical follow-up
The primary outcome of interest was recurrence of AF. The secondary outcome of interest was a composite of death, admission for decompensated heart failure, and a recurrence of AF. Recurrence of AF was defined as AF occurring > 3 months after pulmonary vein isolation and confirmed by either EKG or cardiac monitoring. Patients underwent electrocardiography at all clinical visits, but routine prolonged cardiac monitoring was not performed in the absence of symptoms. We ascertained mortality using the Social Security Death Index and confirmed using electronic chart review and if necessary contact with the primary providers. Hospitalization with worsening heart failure was defined by the occurrence of symptoms consistent with heart failure, together with the absence of recognized AF as a cause, and the need for treatment with diuretics (25). Patients were followed post procedure at 3- to 6-month intervals via clinic visits. We contacted patients who were no longer being followed in clinic by telephone and confirmed data via correspondence with the patient’s physicians. Patents without events were censored at the date of the last available follow-up. Complete follow-up was obtained for all patients.
Statistical analysis
Continuous data are presented as mean ± SD. Continuous data were compared using an unpaired Student t-test or Mann–Whitney non-parametric test as appropriate. Nominal data are presented as number and percentages and were compared with a Chi-squared test. We determined the association between the ECV and other cohort characteristics using a Pearson correlation. Comparison between multiple groups was made using an ANOVA, and if the findings were significant, post-hoc comparison between groups was performed with a Tukey test. The hazard ratio was calculated for the primary and secondary outcomes using a Cox regression model. Considering all the significant variables in the univariable analysis, we sought the best-overall multivariable models for the primary and secondary end-points by stepwise-forward selection with a probability to enter set at p < 0.05 and to remove the effect from the regression at p < 0.05. Event-free survival was determined according to the Kaplan–Meier method using the median ECV value and comparisons of cumulative event rates were performed by the Log-Rank test. We assessed the inter- and intra-observer variability in a randomly selected group of 15 subjects. Comparison of the inter-observer and intra-observer characteristics of the myocardial ECV were made using Bland-Altman plots and determination of the 95% limits of agreement between methods. To test whether there were differences in the ECV at the base, mid, and apex of the left ventricle among patients, we performed a pilot study of the initial 20 patients enrolled and performed the T1 measurements at corresponding three slices. Values were compared with an ANOVA for repeated measures. A two-tailed p value of < 0.05 was deemed significant. SAS 9.2 was used for statistical analysis (SAS Institute Inc, Cary, NC).
Results
Between July 2009 and January 2012, 453 patients with AF were referred for a CMR study for identification of pulmonary vein anatomy prior to PVI. Of these patients, 295 (65%) were in sinus rhythm at the time of the study; 150 were excluded from our analytic sample based on pre-specified criteria, leaving a final study cohort of 145 patients who met the inclusion criteria (Figure 2). There were 96 males (66%) with an average age of 58 ± 12 years (range 36 to 85 years). The average body mass index (BMI) was 30 ± 4 kg/m2, systolic blood pressure was 133 ± 10 mmHg, diastolic blood pressure was 81 ± 6 mmHg (Table 1). The control group consisted of 65% males, an average age of 57 ± 11 years, and had an average BMI of 28 ± 6 kg/m2 (Table 1). Overall, 28 patients (19%) had a prior ablation.
Figure 2. CMR study protocol.
In brief, 453 patients underwent a CMR prior to AF ablation. We excluded patients who were not in sinus rhythm at the time of the study and patients who had known alternate contributors to myocardial fibrosis.
Table 1.
Baseline Characteristics in Cohort with Hypertension vs. Healthy Controls
| Hypertension (145) | Control (20) | p Value | |
|---|---|---|---|
| Mean Age (yrs) | 58 (12) | 57 (11) | 0.71 |
| Male (number, %) | 96 (66) | 13 (65) | 0.99 |
| Body Mass Index (kg/m2) | 30 (4) | 28 (6) | 0.07 |
| Heart Rate (beats/min) | 75 (16) | 69 (11) | 0.12 |
| Systolic Blood Pressure (mmHg) | 133 (10) | 123 (10) | 0.001 |
| Diastolic Blood Pressure (mmHg) | 81 (6) | 74 (8) | 0.001 |
| Hematocrit (%) | 40 (3) | 41 (4) | 0.30 |
| Glomerular Filtration Rate (ml/m2) | 78 (21) | 95 (11) | 0.01 |
All data are mean (SD) unless otherwise indicated; AH = arterial hypertension; Glomerular filtration rate was estimated using the Modification of Diet in Renal Disease formula.
Clinical, EKG, and echocardiographic parameters in the study group with hypertension and recurrent AF
Hypertension was diagnosed a median of 52 months prior to the CMR study (interquartile range (IQR), 28–68 months). Atrial fibrillation was diagnosed a median of 37 months prior to the CMR study (IQR, 26–62 months) (Table 2). Eighteen patients (12%) had LVH. By echocardiography, patients had normal LV (left ventricular) size and function, normal estimated pulmonary artery pressure, and normal estimated left atrial filling pressure (Table 2).
Table 2.
Clinical, EKG, and Echocardiographic Characteristics in the Cohort with Hypertension
| Hypertension (145) | |
|---|---|
| Clinical Variables: n (%) | |
| Family History of Hypertension | 26 (18) |
| Hyperlipidemia | 52 (36) |
| Cigarette Smoking (hx or active) | 14 (10) |
| Obstructive Sleep Apnea | 25 (17) |
| Medications: n (%) | |
| ● Angiotensin converting enzyme inhibitor | 78 (54) |
| ● Angiotensin receptor blocker | 37 (26) |
| ● Beta-blocker | 103 (71) |
| ● Thiazide Diuretics | 59 (41) |
| ● Calcium Channel Blockers | 55 (38) |
| ● Spironolactone | 15 (10) |
| ● Alpha-Blockers | 13 (9) |
| ● Other | 10 (7) |
| ● Coumadin | 145 (100) |
| ● Aspirin | 46 (32) |
| ● Statin | 51 (35) |
| EKG: | |
| ● PR Interval (ms) | 171 (28) |
| ● QRS Duration (ms) | 101 (15) |
| ● QTc Duration (ms) | 437 (33) |
| ● LVH, n (%) | 18 (12) |
| Echocardiography: | |
| ● LVEF (%) | 59 (7) |
| ● LVIDd (mm) | 48 (6) |
| ● Estimated PASP (mmHg) | 29 (7) |
| ● LA dimension (mm) | 41 (5) |
| ● E/A ratio | 1.1 (0.5) |
| ● Deceleration Time (msec) | 216 (49) |
| ● e’ Medial (cm/sec) | 6.1 (1.2) |
| ● a’ Medial (cm/sec) | 7.6 (1.6) |
| ● e’ Lateral (cm/sec) | 6.3 (1.6) |
| ● a’ Lateral (cm/sec) | 8.0 (2.0) |
| ● E/e’ | 12.2 (5.1) |
All data are mean (SD) unless otherwise indicated; ACE = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker; LVEF = left ventricular ejection fraction; LVIDd = left ventricular internal dimension in diastole; PASP = pulmonary artery systolic pressure; LA dimension = left atrial anterior-posterior dimension; E/A ratio = the ratio of early transmitral filling to late transmitral filling; e’ medial = early medial mitral annular relaxation velocity; a’ medial = late medial mitral annular relaxation velocity; e’ lateral = early lateral mitral annular relaxation velocity; a’ lateral = late lateral mitral annular relaxation velocity; E/e’ = ratio of early transmitral filling to early mitral annular velocity.
CMR parameters
CMR parameters for patients and healthy volunteers are presented in Table 3. In brief, patients had increased LV, right ventricular (RV), left atrial (LA) volumes and LV mass in comparison to age and gender-matched healthy volunteers. Late gadolinium enhancement was an infrequent finding and observed in 3 patients. Of these, 1 patient had mid-myocardial LGE involving the basal antero-septal segment, 1 patient had diffuse LGE involving the mid infero-septal segment and the mid antero-septal segment, and 1 patient had LGE involving the posterior right ventricular insertion point in the basal slice.
Table 3.
CMR Variables
| Hypertension (145) | Control (20) | p Value | |
|---|---|---|---|
| LVEDV (ml) | 155 (35) | 131 (27) | 0.01 |
| LVESV (ml) | 64 (20) | 46 (14) | 0.001 |
| LVEDV indexed (ml/m2) | 79 (18) | 68 (12) | 0.009 |
| LVESV indexed (ml/m2) | 32 (10) | 24 (8) | 0.003 |
| LV Mass Index (mg/m2) | 68 (20) | 45 (8) | < 0.001 |
| RVEDV (ml) | 153 (37) | 154 (27) | 0.009 |
| RVESV (ml) | 73 (23) | 76 (8) | 0.015 |
| RVEDV indexed (ml/m2) | 76 (16) | 67 (12) | 0.031 |
| RVESV indexed (ml/m2) | 36 (10) | 31 (7) | 0.04 |
| Maximal LA Volume (ml) | 118 (28) | 66 (15) | <0.001 |
| Minimal LA Volume (ml) | 64 (18) | 23 (9) | <0.001 |
| Max LA Volume indexed (ml/m2) | 45 (15) | 36 (9) | <0.001 |
| Min LA Volume indexed (ml/m2) | 25 (9) | 13 (5) | <0.001 |
| LVEF (Median, IQR, %) | 60 (57,67) | 60 (57,66) | 0.71 |
| RVEF (%) | 53 (6) | 54 (5) | 0.53 |
| λGd, Partition Coefficient | 0.56 (0.06) | 0.48 (0.05) | <0.001 |
| Extracellular volume fraction | 0.34 (0.03) | 0.28 (0.03) | <0.001 |
All data are mean (SD) unless otherwise indicated; LVEDV = left ventricular end diastolic volume; LVESV = left ventricular end systolic volume; RVEDV = right ventricular end diastolic volume; RVESV = right ventricular end systolic volume; Indexed Max LA Volume = maximum left atrial volume indexed to body surface area; Indexed Min LA Volume = minimum left atrial volume indexed to body surface area; LVEF = left ventricular ejection fraction; RVEF = right ventricular ejection fraction.
Myocardial extracellular volume fraction
The myocardial ECV was increased in patients (Table 3). The ECV was similar in males and females (Males vs. Females, 0.33±0.04 vs. 0.34±0.03 vs, p = 0.18). The ECV was not different between the 6 segments within the mid-ventricular slice (anterior segment through antero-septal segment, 0.34 ± 0.04, 0.33 ± 0.04, 0.33 ± 0.04, 0.33 ± 0.04, 0.34 ± 0.04, 0.34 ± 0.04, p = 0.38 for trend). In a sub-study of the initial 20 patients, we tested the ECV at the base, mid, and apex of the LV. The ECV measured 0.34±0.03 at the base, 0.34±0.03 at the mid-LV slice, and 0.35±0.04 at the apex of the LV (p = 0.56). We measured the inter- and intra-observer variability in 15 randomly selected studies. The limits of agreement were – 0.017 to + 0.015 for the inter-observer agreement and – 0.015 to + 0.015 for the intra-observer agreement (Figure 3). In patients with HTN, there was a positive association between the ECV and indexed left atrial volume (r=0.46, p <0.01), and the LV mass index (r=0.45, p <0.01), and a negative association between the ECV and early mitral annular relaxation (E′, r = −0.55, p <0.001).
Figure 3. Bland-Altman plots showing the 95% limits of agreement between two observers (A) and within a single observer (B) for 15 randomly selected patients with hypertension.
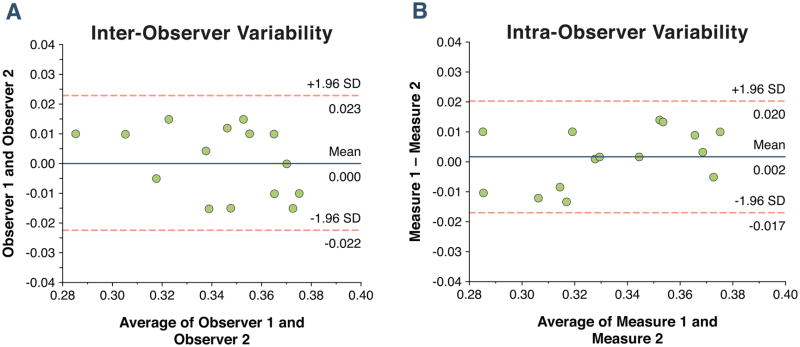
The mean percentage difference between observers was 5.9% with a standard deviation of the absolute difference of 0.02. The mean percentage intra-observer variability was 4.8% with an absolute standard deviation of 0.015.
Clinical follow-up
Patients were followed for a median duration of 18 months. During the follow-up period there were 45 episodes of recurrent AF. For the composite outcome of death, heart failure admission, and AF, there were 52 events (2 deaths, 5 heart failure admissions, and 45 late recurrences of AF). On univariate analysis to test the association between variables and the recurrence of AF, the presence of sleep apnea (HR 1.89, 95% CI 1.01–3.74, chi-squared 3.97, p = 0.04), LV mass index (HR 1.02, 95% CI 1.00–1.03, chi-squared 4.36, p = 0.04), indexed LA volume (HR 1.03, 95% CI 1.00–1.06, chi-squared 4.74, p = 0.03), and the ECV (HR 1.30, 95% CI 1.16–1.46, chi-squared 20.0, p < 0.0001) were associated with the recurrence of AF (Table 4). In the best overall multivariable model, the ECV was the only variable selected for association with recurrent AF (HR 1.29, 95% CI 1.15–1.44, chi-squared 18.8, p <0.0001). Kaplan-Meier curves were generated for the comparison of event-free survival from recurrent AF according to the median ECV value of 0.34 (Figure 4).Data from the secondary outcome analysis are presented in supplemental Table 1 and 2, as well as supplemental Figure 1 and Figure 2. In an analysis extending the outcome of interest to include death and heart failure admissions, the presence of sleep apnea, LV mass index, LA volume and the ECV provided the strongest association with the combined outcome. In a multivariable model, the presence of sleep apnea and the ECV remained significantly associated with the composite outcome (Supplemental Table 2 and Figure 1). For the secondary outcome of death, heart failure admission, and recurrence of AF, each 10% increase in the ECV was associated with a 24% increase in the risk of an adverse event. Kaplan-Meier curves were generated for the comparison of event-free survival from the composite outcome according to the median ECV value of 0.34 (Supplemental Figure 2).
Table 4.
Univariate Analyses for Association with Recurrence of Atrial Fibrillation
| HR | CI | LRχ2 | p Value | |
|---|---|---|---|---|
| Age | 1.02 | 0.99–1.03 | 3.01 | 0.10 |
| Male | 0.81 | 0.44–1.49 | 0.45 | 0.65 |
| Hypertension Duration | 1.00 | 0.99–1.01 | 1.02 | 0.31 |
| Atrial Fibrillation Duration | 1.00 | 0.99–1.01 | 0.06 | 0.80 |
| Cigarette Smoking | 0.99 | 0.35–2.73 | 0.01 | 0.97 |
| Sleep Apnea | 1.89 | 1.01–3.74 | 3.97 | 0.04 |
| Prior Pulmonary Vein Isolation | 1.21 | 0.72–2.17 | 1.56 | 0.23 |
| Hyperthyroidism | 0.82 | 0.19–3.38 | 0.08 | 0.78 |
| Excess Alcohol Intake | 0.79 | 0.25–2.58 | 0.14 | 0.70 |
| Family History of Hypertension | 1.02 | 0.48–2.19 | 0.01 | 0.95 |
| Hyperlipidemia | 0.79 | 0.42–1.46 | 0.56 | 0.45 |
| Medications: | ||||
| ● Thiazide Diuretic | 1.04 | 0.57–1.88 | 0.01 | 0.91 |
| ● Loop Diuretic | 1.12 | 0.35–3.62 | 0.04 | 0.85 |
| ● ACE | 0.94 | 0.52–1.69 | 0.04 | 0.84 |
| ● ARB | 0.82 | 0.41–1.65 | 0.31 | 0.58 |
| ● Beta-Blocker | 1.11 | 0.57–2.14 | 0.09 | 0.76 |
| ● Calcium Channel Blocker | 0.65 | 0.34–1.25 | 1.66 | 0.20 |
| ● ASA | 1.01 | 0.54–1.90 | 0.01 | 0.97 |
| ● Class 1/Class 3 Anti-Arrhythmic | 1.32 | 0.66–2.68 | 0.61 | 0.43 |
| ● Statin Therapy | 0.87 | 0.42–1.60 | 0.21 | 0.65 |
| ● Inhibition of aldosterone receptor | 1.03 | 0.43–2.44 | 0.01 | 0.94 |
| ● Alpha Blocker | 0.53 | 0.13–2.17 | 0.79 | 0.34 |
| ● Digoxin | 0.95 | 0.13–6.92 | 0.01 | 0.96 |
| BMI | 1.01 | 0.95–1.08 | 0.18 | 0.67 |
| Systolic Blood Pressure | 0.99 | 0.98–1.01 | 0.76 | 0.38 |
| Diastolic Blood Pressure | 1.01 | 0.98–1.04 | 0.72 | 0.40 |
| Heart Rate | 1.00 | 0.98–1.02 | 0.08 | 0.77 |
| Glomerular Filtration Rate | 0.97 | 0.93–1.03 | 2.10 | 0.15 |
| Hematocrit | 1.01 | 0.99–1.01 | 0.05 | 0.86 |
| EKG Parameters: | ||||
| ● PR Interval | 1.00 | 0.99–1.01 | 0.53 | 0.46 |
| ● QRS Interval | 1.01 | 0.99–1.03 | 0.71 | 0.40 |
| ● QTc Interval | 1.00 | 0.99–1.01 | 0.11 | 0.74 |
| ● LVH (EKG) | 1.33 | 0.66–2.02 | 1.23 | 0.26 |
| Echocardiographic Parameters: | ||||
| ● LVEF | 0.98 | 0.94–1.02 | 0.28 | 1.17 |
| ● LVIDd | 1.01 | 0.96–1.06 | 0.16 | 0.68 |
| ● Estimated PASP | 1.05 | 1.00–1.10 | 3.76 | 0.05 |
| ● Transmitral E velocity | 1.00 | 0.98–1.02 | 0.01 | 0.97 |
| ● Transmitral A velocity | 1.00 | 0.99–1.02 | 0.26 | 0.61 |
| ● E/A ratio | 1.03 | 0.49–2.18 | 0.01 | 0.93 |
| ● Deceleration Time | 1.00 | 0.99–1.01 | 0.28 | 0.60 |
| ● e’ Lateral | 0.85 | 0.69–1.05 | 2.29 | 0.13 |
| ● e’ Medial | 0.82 | 0.71–1.03 | 2.51 | 0.09 |
| ● E/e’ | 1.04 | 0.99–1.08 | 2.45 | 0.10 |
| Cardiac Magnetic Resonance: | ||||
| ● LVEDV | 1.00 | 0.99–1.01 | 0.38 | 0.53 |
| ● LVESV | 1.00 | 0.99–1.02 | 0.83 | 0.36 |
| ● LVEF | 1.00 | 0.96–1.05 | 0.02 | 0.87 |
| ● LV Mass index | 1.02 | 1.00–1.03 | 4.36 | 0.04 |
| ● RVEDV | 1.00 | 0.99–1.01 | 0.27 | 0.60 |
| ● RVESV | 1.00 | 0.99–1.02 | 0.08 | 0.78 |
| ● RVEF | 1.01 | 0.96–1.07 | 0.29 | 0.59 |
| ● Maximal LA volume indexed | 1.05 | 0.99–1.08 | 3.71 | 0.05 |
| ● Minimal LA Volume indexed | 1.03 | 1.00–1.06 | 4.74 | 0.03 |
| ● Partition coefficient for gadolinium, λGd | 1.25 | 1.13–1.37 | 17.0 | <0.0001 |
| ● Extracellular Volume Fraction | 1.30 | 1.16–1.46 | 20.0 | <0.0001 |
Figure 4. Kaplan-Meier curve showing the survival free of recurrent AF in patients separated according to the median ECV value of 0.34.
Results were compared using a Log-Rank test. Time is recorded in months.
Discussion
In this study, we assessed the prognostic impact of the ECV on the recurrence of AF in a cohort of patients with HTN undergoing PVI for management of their arrhythmia. The major finding of this study is that the ECV was the strongest predictor in the best overall model for recurrent AF post PVI in patients. This prognostic finding was supported by several potential mechanistic observations: Expansion of the ECV was associated with increased LV mass, left atrial volume, and reduced diastolic function. These data provide imaging evidence of an association between expansion of the myocardial extracellular volume, altered myocardial function, elevated left atrial volumes and adverse cardiovascular outcomes.
There are extensive data suggesting a strong interplay between diastolic function, left atrial size, and the occurrence of AF (26,27). Expansion of the myocardial ECV due to myocardial fibrosis is one of the key determinants in the development of diastolic dysfunction (10,28). Using T1 measurements, we measured the HTN-associated expansion of the ECV, and found an association between the ECV, diastolic function, and left atrial size, suggesting a mechanistic link between HTN, myocardial fibrosis, diastolic function, atrial remodeling, and recurrence of AF. The increase in the ECV is likely due to a combination of HTN and AF as there are data supporting both as a cause of expansion of the ECV (10,29,30). However, there are limited pathological and imaging data providing a link between expansion of the ECV and AF (3,4,31), between the ECV and HTN (19), and testing the association between the ECV and adverse outcomes. Swartz and colleagues demonstrated a link between serum measures of collagen turnover, atrial fibrosis, and the occurrence of AF (31). Also, in a broad cohort of patients referred for CMR study prior to pulmonary vein isolation, Ling and colleagues recently demonstrated an association between T1 measures post-contrast with both age and persistent AF (4). Our data provide support and extend these findings. Important differences exist which merit some discussion. We specifically looked at patients with isolated HTN and not all patients with AF and found in patients with HTN, that the ECV was associated with LV mass, diastolic function, and LA size. We also found clinically important consequences to the ECV measure. We found that the ECV was the strongest independent predictor of recurrent AF in a relatively homogenous population. These data suggests an association between HTN, myocardial fibrosis, diastolic function, adverse LA remodeling, and recurrent AF.
We studied patients with recurrent AF with HTN being referred for PVI. We did not study patients with isolated asymptomatic HTN. However, in broad groups of patients with HTN, left ventricular hypertrophy (LVH) or an increase in LV mass is a maladaptive response phenotype to chronic pressure overload observed in selected patients. Patients display a range of markedly different cardiac phenotype responses to HTN, and it is clear that the presence of this abnormal cardiac phenotype marks a group with HTN at significantly increased risk of adverse outcomes(32–34). Therapy based on either the presence of LVH or increased LVH mass has prognostic significance (32). An increase in LV mass or LVH in patients with HTN is the macroscopic representation of a combination of increased cardiac size, an increase in cell size, and myocardial fibrosis (35). Some of the current standard methods for assessment of the cardiac adaptation to HTN include measurement of cardiac size. Methods for measurement of cardiac size are limited by a combination of poor sensitivity (21), acoustic windows (36), geometric assumptions (37), criteria for normalization (38), and the definitions of normal (39). Indeed, in this study, only 12% of patients had LVH by EKG criteria and only 24 patients (17%) from our cohort had LVH as determined using published CMR criteria (40). These data suggest that a more accurate method examining tissue phenotypes in response to such stressors as HTN may be of benefit for stratifying risk. In this study, the ECV predicted a far stronger risk of either recurrence of AF, or a combination of death, heart failure admission, or recurrence of AF, than either the presence of LVH on EKG, CMR-derived LV mass, or indeed measurement of diastolic function or left atrial volume. These data suggests that more accurate tissue characterization of response of patients with HTN may be beneficial and warrants further investigation. We believe that this measurement may represent an extension of current methods in assessment of patients with pressure-overload, and a extension of this hypothesis may be to measure the ECV in patients with HTN with normal LV mass, normal LA volume, and normal measures of cardiac function.
Data on the presence of LGE in patients with HTN report a wide incidence (0% to 50%) (8,9), underestimating both the presence and extent of fibrosis suggested by pathological data (10–12). The ECV derived by CMR is based on the serial measurement of myocardial T1 pre-and post-contrast (13–16,18,41,42), has been validated against histology (15,17), and is a marker of adverse outcomes among a broad group of patients (18). In the healthy state, between 25–33% of the normal myocardium consists of the three-dimensional extracellular space (2,43) and closely aligning with the normal value reported in this study. Here, we provide additive data in a homogenous cohort of patients with HTN. In patients with HTN, there was an association between the ECV, and diastolic function, and LA size. These data are additive to other populations, where an association between T1 values and markers or measures of diastolic function has been shown in patients with heart failure (17), diabetes (44), and aortic stenosis (45). Ultimately, data provided by quantification of the myocardial extracellular volume could have therapeutic implications. Data on the pharmacological intervention to reducing atrial fibrillation via targeting myocardial fibrosis have yielded conflicting results (46,47) suggesting that identification of a high-risk group with more extensive extracellular volume expansion may be of benefit.
This study has to be interpreted within the context of the design format. We did not study a broad group of patients with isolated asymptomatic HTN. We looked specifically at a population of patients with recurrent AF and HTN being referred for pulmonary vein isolation. While pathological validation for the ECV as a surrogate for myocardial fibrosis has been published (15,17), we did not perform endomyocardial biopsies in our population to verify the presence of myocardial fibrosis and correlate the extent of extent of pathological fibrosis with the ECV. We also did not perform serial prolonged monitoring for recurrence of AF; Confirmation of recurrence was performed based on patient symptoms and likely underestimates the true incidence of AF recurrence. We did attempt to remove known modifiers of the ECV such as diabetes, coronary disease, reduced ejection fraction and valvular disease, however, the inclusion of unknown modifiers of the ECV cannot be excluded.
In conclusion, the CMR-derived ECV is elevated in patients with HTN and recurrent AF requiring PVI, is associated with other adverse structural and functional consequences, and is a strong independent predictor of adverse cardiovascular outcomes. These data may support further investigation into whether intervention based on the ECV can improve outcomes.
Supplementary Material
Acknowledgments
We would like to acknowledge the advice and editorial comments of Dr. William G Stevenson in production of this manuscript.
Funding: Dr. Neilan is supported by an American Heart Association Fellow to Faculty Grant (12FTF12060588). Dr. Jerosch-Herold and Dr. Kwong are supported by research grants from the National Institutes of Health (RO1HL090634, RO1HL091157, respectively).
Footnotes
Conflict of Interest: The Authors have no relevant conflicts of interest related to this submission to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. Jama. 1996;275:1557–62. [PubMed] [Google Scholar]
- 2.Weber KT. Cardiac interstitium in health and disease: the fibrillar collagen network. J Am Coll Cardiol. 1989;13:1637–52. doi: 10.1016/0735-1097(89)90360-4. [DOI] [PubMed] [Google Scholar]
- 3.Frustaci A, Caldarulo M, Buffon A, Bellocci F, Fenici R, Melina D. Cardiac biopsy in patients with “primary” atrial fibrillation. Histologic evidence of occult myocardial diseases. Chest. 1991;100:303–6. doi: 10.1378/chest.100.2.303. [DOI] [PubMed] [Google Scholar]
- 4.Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation. 2002;106:2085–90. doi: 10.1161/01.cir.0000033824.02722.f7. [DOI] [PubMed] [Google Scholar]
- 5.Diez J, Querejeta R, Lopez B, Gonzalez A, Larman M, Martinez Ubago JL. Losartan-dependent regression of myocardial fibrosis is associated with reduction of left ventricular chamber stiffness in hypertensive patients. Circulation. 2002;105:2512–7. doi: 10.1161/01.cir.0000017264.66561.3d. [DOI] [PubMed] [Google Scholar]
- 6.Kim RJ, Fieno DS, Parrish TB, et al. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100:1992–2002. doi: 10.1161/01.cir.100.19.1992. [DOI] [PubMed] [Google Scholar]
- 7.Mewton N, Liu CY, Croisille P, Bluemke D, Lima JA. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol. 2011;57:891–903. doi: 10.1016/j.jacc.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rudolph A, Abdel-Aty H, Bohl S, et al. Noninvasive detection of fibrosis applying contrast-enhanced cardiac magnetic resonance in different forms of left ventricular hypertrophy relation to remodeling. J Am Coll Cardiol. 2009;53:284–91. doi: 10.1016/j.jacc.2008.08.064. [DOI] [PubMed] [Google Scholar]
- 9.Puntmann VO, Jahnke C, Gebker R, et al. Usefulness of magnetic resonance imaging to distinguish hypertensive and hypertrophic cardiomyopathy. Am J Cardiol. 2010;106:1016–22. doi: 10.1016/j.amjcard.2010.05.036. [DOI] [PubMed] [Google Scholar]
- 10.Brilla CG, Funck RC, Rupp H. Lisinopril-mediated regression of myocardial fibrosis in patients with hypertensive heart disease. Circulation. 2000;102:1388–93. doi: 10.1161/01.cir.102.12.1388. [DOI] [PubMed] [Google Scholar]
- 11.Querejeta R, Lopez B, Gonzalez A, et al. Increased collagen type I synthesis in patients with heart failure of hypertensive origin: relation to myocardial fibrosis. Circulation. 2004;110:1263–8. doi: 10.1161/01.CIR.0000140973.60992.9A. [DOI] [PubMed] [Google Scholar]
- 12.Rossi MA. Pathologic fibrosis and connective tissue matrix in left ventricular hypertrophy due to chronic arterial hypertension in humans. J Hypertens. 1998;16:1031–41. doi: 10.1097/00004872-199816070-00018. [DOI] [PubMed] [Google Scholar]
- 13.Jerosch-Herold M, Sheridan DC, Kushner JD, et al. Cardiac magnetic resonance imaging of myocardial contrast uptake and blood flow in patients affected with idiopathic or familial dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2008;295:H1234–H1242. doi: 10.1152/ajpheart.00429.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broberg CS, Chugh SS, Conklin C, Sahn DJ, Jerosch-Herold M. Quantification of diffuse myocardial fibrosis and its association with myocardial dysfunction in congenital heart disease. Circ Cardiovasc Imaging. 2010;3:727–34. doi: 10.1161/CIRCIMAGING.108.842096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flett AS, Hayward MP, Ashworth MT, et al. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans. Circulation. 2010;122:138–44. doi: 10.1161/CIRCULATIONAHA.109.930636. [DOI] [PubMed] [Google Scholar]
- 16.Ugander M, Oki AJ, Hsu LY, et al. Extracellular volume imaging by magnetic resonance imaging provides insights into overt and sub-clinical myocardial pathology. Eur Heart J. 2011 doi: 10.1093/eurheartj/ehr481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iles L, Pfluger H, Phrommintikul A, et al. Evaluation of diffuse myocardial fibrosis in heart failure with cardiac magnetic resonance contrast-enhanced T1 mapping. J Am Coll Cardiol. 2008;52:1574–80. doi: 10.1016/j.jacc.2008.06.049. [DOI] [PubMed] [Google Scholar]
- 18.Wong TC, Piehler K, Meier CG, et al. Association between extracellular matrix expansion quantified by cardiovascular magnetic resonance and short-term mortality. Circulation. 2012;126:1206–16. doi: 10.1161/CIRCULATIONAHA.111.089409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coelho-Filho OR, Mongeon FP, Mitchell R, et al. The Role of Transcytolemmal Water Exchange in Magnetic Resonance Measurements of Diffuse Myocardial Fibrosis in Hypertensive Heart Disease. Circ Cardiovasc Imaging. 2012 doi: 10.1161/CIRCIMAGING.112.979815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. Jama. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 21.Levy D, Labib SB, Anderson KM, Christiansen JC, Kannel WB, Castelli WP. Determinants of sensitivity and specificity of electrocardiographic criteria for left ventricular hypertrophy. Circulation. 1990;81:815–20. doi: 10.1161/01.cir.81.3.815. [DOI] [PubMed] [Google Scholar]
- 22.Mongeon FP, Jerosch-Herold M, Coelho-Filho OR, Blankstein R, Falk RH, Kwong RY. Quantification of Extracellular Matrix Expansion by CMR in Infiltrative Heart Disease. JACC Cardiovasc Imaging. 2012;5:897–907. doi: 10.1016/j.jcmg.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neilan TG, Coelho-Filho OR, Shah RV, et al. The Myocardial Extracellular Volume Fraction From T1 Measurements in Healthy Volunteers and Mice. JACC Cardiovasc Imaging. 2013 doi: 10.1016/j.jcmg.2012.09.020. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ho CY, Abbasi SA, Neilan TG, et al. T1 Measurements Identify Extracellular Volume Expansion in Hypertrophic Cardiomyopathy Sarcomere Mutation Carriers With and Without Left Ventricular Hypertrophy. Circ Cardiovasc Imaging. 2013 doi: 10.1161/CIRCIMAGING.112.000333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–49. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 26.Tsang TS, Gersh BJ, Appleton CP, et al. Left ventricular diastolic dysfunction as a predictor of the first diagnosed nonvalvular atrial fibrillation in 840 elderly men and women. J Am Coll Cardiol. 2002;40:1636–44. doi: 10.1016/s0735-1097(02)02373-2. [DOI] [PubMed] [Google Scholar]
- 27.Vaziri SM, Larson MG, Benjamin EJ, Levy D. Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study. Circulation. 1994;89:724–30. doi: 10.1161/01.cir.89.2.724. [DOI] [PubMed] [Google Scholar]
- 28.Kuwahara F, Kai H, Tokuda K, et al. Transforming growth factor-beta function blocking prevents myocardial fibrosis and diastolic dysfunction in pressure-overloaded rats. Circulation. 2002;106:130–5. doi: 10.1161/01.cir.0000020689.12472.e0. [DOI] [PubMed] [Google Scholar]
- 29.Shinbane JS, Wood MA, Jensen DN, Ellenbogen KA, Fitzpatrick AP, Scheinman MM. Tachycardia-induced cardiomyopathy: a review of animal models and clinical studies. J Am Coll Cardiol. 1997;29:709–15. doi: 10.1016/s0735-1097(96)00592-x. [DOI] [PubMed] [Google Scholar]
- 30.He X, Gao X, Peng L, et al. Atrial fibrillation induces myocardial fibrosis through angiotensin II type 1 receptor-specific Arkadia-mediated downregulation of Smad7. Circ Res. 2011;108:164–75. doi: 10.1161/CIRCRESAHA.110.234369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swartz MF, Fink GW, Sarwar MF, et al. Elevated Pre-Operative Serum Peptides for Collagen I and III Synthesis Result in Post-Surgical Atrial Fibrillation. J Am Coll Cardiol. 2012;60:1799–806. doi: 10.1016/j.jacc.2012.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dahlof B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 33.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–6. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 34.Bluemke DA, Kronmal RA, Lima JA, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148–55. doi: 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anversa P, Ricci R, Olivetti G. Quantitative structural analysis of the myocardium during physiologic growth and induced cardiac hypertrophy: a review. J Am Coll Cardiol. 1986;7:1140–9. doi: 10.1016/s0735-1097(86)80236-4. [DOI] [PubMed] [Google Scholar]
- 36.Gardin JM, Arnold A, Gottdiener JS, et al. Left ventricular mass in the elderly The Cardiovascular Health StudyHypertension 1997291095–103. [DOI] [PubMed] [Google Scholar]
- 37.Armstrong AC, Gidding S, Gjesdal O, Wu C, Bluemke DA, Lima JA. LV mass assessed by echocardiography and CMR, cardiovascular outcomes, and medical practice. JACC Cardiovasc Imaging. 2012;5:837–48. doi: 10.1016/j.jcmg.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 39.Missouris CG, Forbat SM, Singer DR, Markandu ND, Underwood R, MacGregor GA. Echocardiography overestimates left ventricular mass: a comparative study with magnetic resonance imaging in patients with hypertension. J Hypertens. 1996;14:1005–10. [PubMed] [Google Scholar]
- 40.Maceira AM, Prasad SK, Khan M, Pennell DJ. Normalized left ventricular systolic and diastolic function by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2006;8:417–26. doi: 10.1080/10976640600572889. [DOI] [PubMed] [Google Scholar]
- 41.Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med. 2004;52:141–6. doi: 10.1002/mrm.20110. [DOI] [PubMed] [Google Scholar]
- 42.Lee JJ, Liu S, Nacif MS, et al. Myocardial T1 and extracellular volume fraction mapping at 3 tesla. J Cardiovasc Magn Reson. 2011;13:75. doi: 10.1186/1532-429X-13-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Polimeni PI. Extracellular space and ionic distribution in rat ventricle. Am J Physiol. 1974;227:676–83. doi: 10.1152/ajplegacy.1974.227.3.676. [DOI] [PubMed] [Google Scholar]
- 44.Jellis C, Wright J, Kennedy D, et al. Association of imaging markers of myocardial fibrosis with metabolic and functional disturbances in early diabetic cardiomyopathy. Circ Cardiovasc Imaging. 2011;4:693–702. doi: 10.1161/CIRCIMAGING.111.963587. [DOI] [PubMed] [Google Scholar]
- 45.Flett AS, Sado DM, Quarta G, et al. Diffuse myocardial fibrosis in severe aortic stenosis: an equilibrium contrast cardiovascular magnetic resonance study. Eur Heart J Cardiovasc Imaging. 2012 doi: 10.1093/ehjci/jes102. [DOI] [PubMed] [Google Scholar]
- 46.Schneider MP, Hua TA, Bohm M, Wachtell K, Kjeldsen SE, Schmieder RE. Prevention of atrial fibrillation by Renin-Angiotensin system inhibition a meta-analysis. J Am Coll Cardiol. 2012;55:2299–307. doi: 10.1016/j.jacc.2010.01.043. [DOI] [PubMed] [Google Scholar]
- 47.Yusuf S, Teo K, Anderson C, et al. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet. 2008;372:1174–83. doi: 10.1016/S0140-6736(08)61242-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.