Abstract
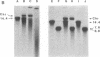
The mechanism by which Haemophilus protects donor DNA from cellular restriction and degradative enzymes during transformation is unclear. In this report, we demonstrate that donor DNA enters Haemophilus influenzae through specialized membranous extensions, which we have termed "transformasomes." DNA within transformasomes is in a protected state--resistant to external DNase and cellular restriction enzymes, although remaining unmodified and double-stranded. The ability of donor DNA to exit from transformasomes is dependent on its topological conformation. Circular DNA remains intact within transformasomes, while linear DNA rapidly exits and undergoes homologous recombination. Protected donor DNA can be preferentially removed from the surface of competent cells by extraction with organic solvents. Structurally intact transformasomes containing donor DNA could be partitioned into the organic layer and can be further purified by density centrifugation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concino M. F., Goodgal S. H. DNA-binding vesicles released from the surface of a competence-deficient mutant of Haemophilus influenzae. J Bacteriol. 1982 Oct;152(1):441–450. doi: 10.1128/jb.152.1.441-450.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concino M. F., Goodgal S. H. Haemophilus influenzae polypeptides involved in deoxyribonucleic acid uptake detected by cellular surface protein iodination. J Bacteriol. 1981 Oct;148(1):220–231. doi: 10.1128/jb.148.1.220-231.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danner D. B., Smith H. O., Narang S. A. Construction of DNA recognition sites active in Haemophilus transformation. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2393–2397. doi: 10.1073/pnas.79.7.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodgal S. H. DNA uptake in Haemophilus transformation. Annu Rev Genet. 1982;16:169–192. doi: 10.1146/annurev.ge.16.120182.001125. [DOI] [PubMed] [Google Scholar]
- Gromkova R., Goodgal S. Uptake of plasmid deoxyribonucleic acid by Haemophilus. J Bacteriol. 1981 Apr;146(1):79–84. doi: 10.1128/jb.146.1.79-84.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herriott R. M., Meyer E. M., Vogt M. Defined nongrowth media for stage II development of competence in Haemophilus influenzae. J Bacteriol. 1970 Feb;101(2):517–524. doi: 10.1128/jb.101.2.517-524.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
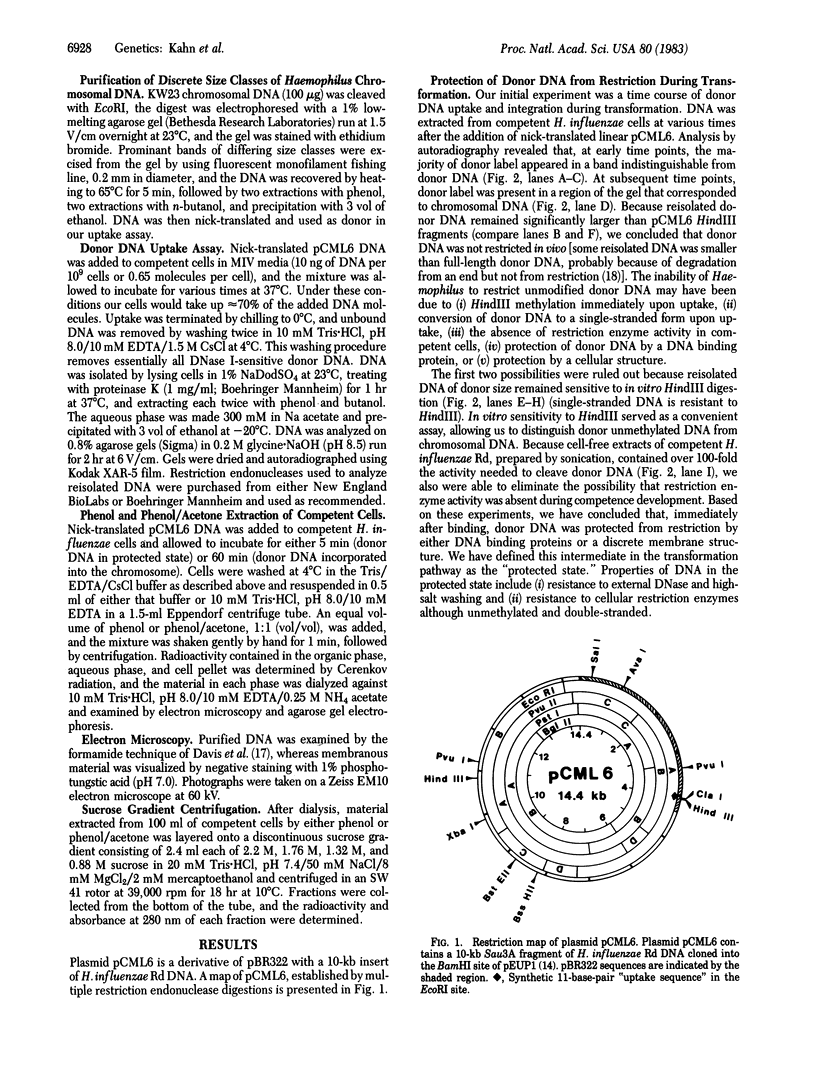
- Kahn M. E., Maul G., Goodgal S. H. Possible mechanism for donor DNA binding and transport in Haemophilus. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6370–6374. doi: 10.1073/pnas.79.20.6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn M., Concino M., Gromkova R., Goodgal S. DNA binding activity of vesicles produced by competence deficient mutants of Haemophilus. Biochem Biophys Res Commun. 1979 Apr 13;87(3):764–772. doi: 10.1016/0006-291x(79)92024-2. [DOI] [PubMed] [Google Scholar]
- Lundquist R. C., Olivera B. M. Transient generation of displaced single-stranded DNA during nick translation. Cell. 1982 Nov;31(1):53–60. doi: 10.1016/0092-8674(82)90404-4. [DOI] [PubMed] [Google Scholar]
- Notani N. K., Setlow J. K. Mechanism of bacterial transformation and transfection. Prog Nucleic Acid Res Mol Biol. 1974;14(0):39–100. doi: 10.1016/s0079-6603(08)60205-6. [DOI] [PubMed] [Google Scholar]
- Notani N., Goodgal S. H. On the nature of recombinants formed during transformation in Hemophilus influenzae. J Gen Physiol. 1966 Jul;49(6):197–209. doi: 10.1085/jgp.49.6.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O., Danner D. B., Deich R. A. Genetic transformation. Annu Rev Biochem. 1981;50:41–68. doi: 10.1146/annurev.bi.50.070181.000353. [DOI] [PubMed] [Google Scholar]
- Wilcox K. W., Smith H. O. Isolation and characterization of mutants of Haemophilus influenzae deficient in an adenosine 5'-triphosphate-dependent deoxyribonuclease activity. J Bacteriol. 1975 May;122(2):443–453. doi: 10.1128/jb.122.2.443-453.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoon K. C., Habersat M., Scocca J. J. Synthesis of envelope polypeptides by Haemophilus influenzae during development of competence for genetic transformation. J Bacteriol. 1976 Jul;127(1):545–554. doi: 10.1128/jb.127.1.545-554.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoon K. C., Scocca J. J. Constitution of the cell envelope of Haemophilus influenzae in relation to competence for genetic transformation. J Bacteriol. 1975 Aug;123(2):666–677. doi: 10.1128/jb.123.2.666-677.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]