Abstract
The water-soluble zinc salts gluconate, sulfate, and acetate are commonly used as supplements in tablet or syrup form to prevent zinc deficiency and to treat diarrhea in children in combination with oral rehydration. Zinc citrate is an alternative compound with high zinc content, slightly soluble in water, which has better sensory properties in syrups but no absorption data in humans. We used the double-isotope tracer method with 67Zn and 70Zn to measure zinc absorption from zinc citrate given as supplements containing 10 mg of zinc to 15 healthy adults without food and compared absorption with that from zinc gluconate and zinc oxide (insoluble in water) using a randomized, double-masked, 3-way crossover design. Median (IQR) fractional absorption of zinc from zinc citrate was 61.3% (56.6–71.0) and was not different from that from zinc gluconate with 60.9% (50.6–71.7). Absorption from zinc oxide at 49.9% (40.9–57.7) was significantly lower than from both other supplements (P < 0.01). Three participants had little or no absorption from zinc oxide. We conclude that zinc citrate, given as a supplement without food, is as well absorbed by healthy adults as zinc gluconate and may thus be a useful alternative for preventing zinc deficiency and treating diarrhea. The more insoluble zinc oxide is less well absorbed when given as a supplement without food and may be minimally absorbed by some individuals. This trial was registered at clinicaltrials.gov as NCT01576627.
Introduction
Zinc is an essential trace element that has a critical role in maintaining structural and catalytic functions of >200 enzymes involved in major metabolic pathways, including nucleic acid metabolism, protein synthesis, and cell division (1). Although it remains difficult to define zinc status, zinc deficiency appears to be common among children in many developing countries, negatively affecting physical growth, immune competence, neural development, and reproductive outcomes, and increasing morbidity and mortality (2). The WHO considers zinc deficiency to be a major contributor to the burden of disease in developing countries, especially in those with a high mortality rate (3). Several factors contribute to the development of zinc deficiency, including increased requirements at certain stages of the life cycle, malabsorption, impaired utilization, and increased losses attributable to repeated diarrhea. However, most often the primary cause of zinc deficiency is inadequate dietary zinc intake and low bioavailability of zinc attributable to the consumption of plant-based diets that are high in phytic acid, thus inhibiting zinc absorption (2).
Zinc is lost in greater quantities during diarrhea, and zinc supplements have been successfully used to treat diarrhea (4). WHO guidelines for the treatment of diarrhea recommend zinc supplementation in combination with oral rehydration salts solution (5). The WHO recommends the use of the water-soluble compounds zinc sulfate (23% zinc), zinc acetate (30% zinc), or zinc gluconate (14% zinc) in the form of syrups or dispersible tablets for diarrhea management in infants (6). However, zinc sulfate and zinc acetate have a strong metallic, bitter, and astringent taste that needs to be masked. Moreover, the low zinc content of zinc gluconate makes this compound more expensive. Of the zinc compounds permitted in the European Union for use as supplements or for food fortification, zinc sulfate (water soluble, zinc content of 23%) and zinc oxide (water insoluble, zinc content of 80%) are the least expensive and most commonly used (2). An alternative zinc compound with promising sensory properties is zinc citrate (Markus Gerhart, Jungbunzlauer Ladenburg, Ladenburg, Germany, personal communication). This compound has a high zinc content of 31%, is slightly soluble in water, is odorless, and has a relatively low cost (2). However, there are no human absorption data to support the use of zinc citrate.
From the limited published data on the absorption of zinc from supplements fed to humans, it would appear that zinc gluconate, zinc citrate, and zinc sulfate are absorbed to a similar extent and that zinc oxide is slightly less well absorbed (7, 8). In a recent rat study, however, zinc citrate was found to be equally as bioavailable as zinc gluconate, zinc sulfate, zinc acetate, and zinc oxide (9); although in an in vitro dialyzability study, zinc citrate in infant formula was found to have a lower dialyzability than zinc gluconate and zinc oxide (10).
The goal of the present trial was to use the double-isotope tracer ratio (DITR)7 method (11–13) to compare zinc absorption from zinc citrate with zinc gluconate and zinc oxide when these zinc compounds were given as supplements without food. This is recommended to maximize zinc absorption and is usually advised for diarrhea treatment. The used isotope tracer technique included the administration of 2 stable zinc isotopes (1 orally and 1 i.v.), followed by the quantification of the 2 isotopes in 1 spot urine sample.
Materials and Methods
Participants.
Fifteen adults were included in this randomized, double-masked, 3-way crossover study. Healthy male and female participants aged 18–45 y, with a BMI of 19–25 kg/m2, who were not vegan, smoking, pregnant, or lactating, were recruited for the study among the students and staff of the Swiss Federal Institute of Technology (ETH) Zurich. Participants were asked to stop the intake of any vitamin/mineral supplements 2 wk before the study start and for the whole study duration. Individuals with any known metabolic, gastrointestinal, or chronic disease and those taking long-term medication (except for contraceptives) were excluded from the study. Eligibility to participate and written informed consent from all participants was obtained at ETH Zurich before the start of the study. The study was conducted at the Clinical Trials Center of the University Hospital Zurich. The study was approved by the Ethics Committee of the Canton of Zurich (KEK Zürich). This trial was registered at clinicaltrials.gov as NCT01576627.
Test compounds and stable isotope labels.
Zinc gluconate, zinc oxide, and zinc citrate were administered as supplements at a dose of 10 mg of zinc each. Each dose consisted of 9 mg of nonlabeled and 1 mg of 67Zn-labeled zinc. The supplements were preweighed at a precision of 0.1 mg on a 4-digit analytical scale (Mettler-Toledo AT200) into gelatin capsules Nr 00 provided by the Cantonal Pharmacy Zurich at ETH Zurich. All test supplements were individually packed into small zip-lock bags and labeled with a number and a color code identifying the compound.
Nonlabeled zinc oxide was provided by Brüggemann Chemical. Isotopically enriched 67ZnO (67Zn-metal: 90.59% enriched) was purchased from Chemgas. The isotopically enriched ZnO powder was premixed at ETH Zurich with the nonlabeled ZnO at a ratio of 1:9 to avoid the imprecise dosage of the small quantity of the isotope. Homogeneity was tested by analyzing isotopic composition of samples taken at different locations of the mixture. From this mixture of nonlabeled and labeled ZnO, the isotopically labeled zinc citrate and zinc gluconate were prepared by Jungbunzlauer Ladenburg in collaboration with Wolfgang Schubert from the Mannheim University of Applied Sciences, Mannhein, Germany. For zinc citrate, anhydrous citric acid (Jungbunzlauer), was dissolved in deionized water and neutralized with stoichiometric amounts of the mixture of nonlabeled and labeled ZnO. Precipitated zinc citrate was filtered and dried at 105°C for 15 h. The procedure was similar for zinc gluconate using glucono-delta-lactone (Jungbunzlauer) and drying the clear zinc gluconate solution by a rotary evaporator at 95°C and 15 mbar vacuum.
Isotopic abundances and ratios were measured in triplicate by inductively coupled mass spectrometry (ICP-MS) (Neptune; Thermo Fisher Scientific), with the bracketing standard method to correct for mass bias, after dissolution of the compounds in 0.05 mol/L nitric acid. Zinc contents were determined by isotopic dilution, in triplicate, using a zinc standard solution of natural isotopic composition (Titrisol; Merck).
Doses for i.v. administration containing 0.2 mg of 70Zn in 9 mL of saline were prepared from 70ZnO (70Zn-metal: 95.4% enriched) in a sterile environment at the Cantonal Pharmacy of the University Hospital Zurich according to Good Manufacturing Practice. The isotopically enriched zinc oxide was converted to ZnCl2 by dissolution in HCl, adjusted to pH 6 by adding NaHCO3 and diluted with physiologic saline. Individual doses of 9.5 g of solution were transferred to glass vials, septum sealed, labeled, sterilized, and checked for sterility and pyrogens.
Study design.
A randomized crossover design was used with each participant acting as his/her own control (Supplemental Fig. 1). At the screening visit, medical history and dietary intake data using a short questionnaire were assessed, the individual’s height and weight were measured, inclusion/exclusion criteria were checked, and the individual’s eligibility was judged accordingly. On study day 1, a baseline (baseline 1) spot urine sample was collected, and blood was drawn by direct venipuncture for the analyses of plasma zinc (PZn) and C-reactive protein (CRP) concentration after an overnight fast. A pregnancy test was applied to urine samples from female participants. The participants then received the first gelatin capsule containing the supplement labeled with 67Zn, which they consumed together with 300 mL of high-purity water (18.2 MΩ⋅cm purified water, Nanopure; Skan). Immediately thereafter, 9 of the 9.5 mL in the vial containing the 70Zn-labeled saline was aspirated into a sterile syringe. The dose was injected i.v. over a period of 5 min through a sterile injection system consisting of a 2-way catheter and a septum injection port. The syringe was weighed before and after the injection to calculate the exact administered dose. The injection system was flushed with 10 mL of physiologic saline to ensure quantitative isotope administration. A spot urine sample was collected on study day 5, 4 d after supplement administration. A washout period of 4 wk followed to ensure adequate decay of the isotopes administered on day 1. On study day 28, after an overnight fast, a spot urine sample serving as the new baseline sample (baseline 2) was collected to account for residual enrichment from the first administration, followed by the administration of the second supplement and the second i.v. 70Zn dose. A spot urine sample was collected on study day 33, 4 d after the consumption of the second supplement. On study day 57, again a spot urine sample serving as new baseline sample (baseline 3) was collected after an overnight fast, followed by the third supplement and i.v. dose. A spot urine sample was collected on study day 61.
No consumption of foods or drinks was allowed within the first 3 h after oral and i.v. isotope administration. Thereafter, the participants were allowed to live according to their normal food habits until the evening before the next test supplement administration.
Analytical methods.
The venous blood samples drawn from each study participant immediately before the first test supplement administration into trace element free heparinized tubes (Ref-01.1604.400; Sarstedt) were refrigerated at 4°C immediately after blood collection until separation. The plasma was separated within 1 h after blood collection by centrifugation at 3000 × g for 10 min, aliquoted into acid-washed plastic vials, and frozen at −25°C for the later analysis of PZn and CRP. PZn concentration was measured by flame atomic absorption spectrophotometry (AA240FS; Varian) using commercial aqueous standards (Titrisol; Merck) for external calibration. Accuracy was checked by analysis of commercially available serum controls (Seronorm Trace Elements Serum L-1 and L-2; Sero). CRP was measured on an IMMULITE 2000 automatic system (Siemens Healthcare Diagnostics).
A total of 6 spot urine samples (1 before and 1 96 h after each of the 3 test supplement administrations) were collected from each study participant and were stored at −25°C until analysis. Each urine sample was analyzed in duplicate for its zinc isotopic composition under chemical blank monitoring at the Human Nutrition Laboratory. After concentration by freeze drying, urine samples were mineralized using an HNO3/H2O2 mixture and microwave heating, followed by separation of zinc from the sample matrix by anion-exchange chromatography according to a slightly modified method as described by Pinna et al. (14). The resin (AG 1-X8 Resin, 100–200 mesh, chloride form; Bio-Rad Laboratories) was equilibrated in ultrapure water and packed into polypropylene minicolumns (Spectra/Chrom, 45 μm filter size; Spectrum Chromatography) to a volume of 1 mL. The resin was washed with 15 mL of 2 mol/L NHO3, rinsed with 2 mL of ultrapure water, and conditioned adding first 5 mL of 0.5 mol/L HCl and then 10 mL of 6 mol/L HCl. The digest (in 6 mol/L HCl) was added to the columns and washed with 10 mL of 6 mol/L HCl, 5 mL of 2.5 mol/L HCl, and 15 mL of 0.5 mol/L HCl, and zinc was eluted from columns using 10 mL of 0.005 mol/L HCl. This fraction was collected in Teflon beakers. The eluent was evaporated to dryness, dissolved twice in 2 mL of NHO3, and finally redissolved in 2 mL of 0.05 mol/L HNO3. All acids used for the preparation of samples were ultrapure. All isotopic analysis were performed by ICP-MS using a high-resolution double focusing magnetic sector field multicollector mass spectrometer (Neptune; Thermo Fisher Scientific). 70Zn-to-66Zn and 67Zn-to-66Zn ratios were measured by the bracketing standard method to determine 70Zn and 67Zn enrichment.
Calculation of zinc absorption.
Fractional absorption (FA) of zinc was determined and calculated by measuring isotopic enrichment in a spot urine sample (15) after oral and i.v. administration of the stable isotopes from the 3 different zinc supplements according to the method of Friel et al. (11). The effect of the observed zinc contamination throughout the analytical procedure on the correction for residual enrichment in baselines 2 and 3 was found negligible.
Statistical analyses.
All statistical analyses were performed with the per-protocol individuals. Analyses were conducted with SPSS statistical software (version 19; IBM). For the comparison of mean FA of zinc between the different test supplements, the Friedman test followed by post hoc analysis with Wilcoxon’s signed-rank test conducted with a Bonferroni’s correction was used. Results are presented as medians (IQRs). Study participants’ characteristics (anthropometry, PZn, CRP) are reported as means ± SDs or geometric means (ranges). Significance was set at P < 0.05.
Fifteen eligible individuals were required for the study to allow the detection of a 40% difference in FA of zinc between the compounds, with 80% power at a level of significance of 0.05, taking into account a 20% dropout rate. Sample size calculation was based on the pooled results of 5 previous zinc absorption studies done at the Human Nutrition Laboratory (SD of the log-transformed differences between pairs = 0.20).
Results
Participant characteristics.
Of the 15 participants, 1 male and 3 females had a PZn concentration below the suggested lower cutoffs indicating mild zinc deficiency (74 μg/dL for males and 70 μg/dL for nonpregnant females for morning fasting blood samples in adults) (2). Anthropometry, PZn, and CRP concentrations before the first test supplement was administered are shown in Table 1. In 1 of the participants, a slightly elevated CRP concentration (3.2 mg/L) was measured (16).
TABLE 1.
Anthropometry and PZn and CRP concentrations of male and female study participants before administration of the first test supplement1
| Males (n = 7) | Females (n = 8) | |
| Age, y | 23.1 ± 2.3 | 25.1 ± 1.8 |
| Height, cm | 180.5 ± 4.7 | 164.7 ± 5.6 |
| Weight, kg | 71.9 ± 4.5 | 57.9 ± 5.7 |
| BMI, kg/m2 | 22.1 ± 1.2 | 21.4 ± 1.8 |
| PZn, μg/dL | 84.6 ± 13.1 | 72.6 ± 9.3 |
| Plasma CRP, mg/L | 0.4 (0.2–2.2) | 0.9 (0.2–3.2) |
Values are means ± SDs or geometric means (ranges). CRP, C-reactive protein; PZn, plasma zinc.
Zinc absorption.
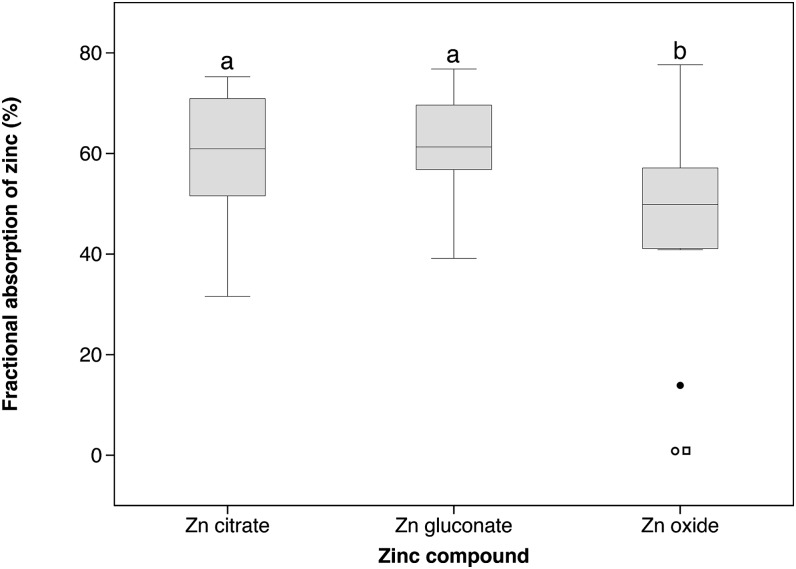
We found a statistically significant difference in zinc absorption depending on the type of zinc compound used as supplement when all individuals who had completed the study per protocol (n = 15) were included for statistical analysis (P = 0.041). Median (IQR) zinc absorption in percentage from zinc citrate, zinc gluconate, and zinc oxide were 61.3 (56.6–71.0), 60.9 (50.6–71.7), and 49.9 (40.9–57.7), respectively (Table 2). There was a significantly higher absorption of zinc from zinc citrate (P = 0.006) and zinc gluconate (P = 0.009) when compared with that from zinc oxide (Fig. 1). The absorption of zinc citrate did not differ from that of zinc gluconate.
TABLE 2.
Fractional absorption of zinc from zinc citrate, zinc gluconate, and zinc oxide supplements, including and excluding outlier participants of zinc oxide1
| Population | n | FAZ from zinc citrate | FAZ from zinc gluconate | FAZ from zinc oxide |
| % | % | % | ||
| Per-protocol population | 15 | 61.3 (56.6, 71.0)a | 60.9 (50.6, 71.7)a | 49.9 (40.9, 57.7)b |
| Non-absorbers excluded | 13 | 61.3 (56.8, 70.5) | 60.9 (51.6, 70.9) | 50.9 (42.6, 59.2) |
| All outliers excluded | 12 | 61.9 (56.7, 71.6) | 64.0 (52.3, 71.3) | 53.5 (44.2, 60.0) |
Values are medians (IQRs). Labeled medians in a row without a common letter differ, P < 0.01 (Friedman test, followed by Wilcoxon’s signed-rank test and Bonferroni’s correction). FAZ, fractional absorption of zinc.
FIGURE 1.
Fractional absorption of zinc from zinc citrate, zinc gluconate, and zinc oxide supplements consumed with water, including all per-protocol individuals (n = 15). Labeled plots without a common letter differ, P < 0.01. The box plots show the median and 25th and 75th percentiles. Whiskers in the plots represent the highest and lowest values, and circles and squares represent outliers (values >1.5 interquartile ranges away from the 25th percentile). The open square and open circle are values below the limit of detection of 1.5% from 2 individuals; the filled circle is a value from an individual who absorbed zinc oxide at a low level (14%).
Two (1 male, 1 female) of the 15 participants who had completed the study per protocol did not absorb zinc from zinc oxide. The measured zinc FA was below the limit of detection of the method, which was 1.5%, calculated from the lowest measurable 67Zn enrichment in urine by ICP-MS. Although there is no reason for excluding these 2 participants because they were apparently healthy on the day of administration of the zinc oxide supplement and up to 4 d later when the urine sample was collected, these are outliers, and statistical analysis was also performed without these 2 participants. When excluding these 2 participants from the statistical analysis (n = 13), the Friedman test no longer showed an overall statistical significance (Table 2). There was an additional outlier participant (female) who absorbed zinc from zinc oxide at a low level (14%) (Fig. 1). Excluding this participant as well (n = 12) also resulted in no statistical difference between the different zinc compounds (Table 2).
For all 3 outliers, the differences in isotope enrichment between duplicate measures were similar to those determined for other samples/participants. Additionally, because the enrichment in 70Zn (i.v. tracer) was similar to other participants at the same time point, a technical problem (sample preparation or isotopic measurement) or a sample mislabeling/confusion can be ruled out.
Discussion
The principal finding of this study is that zinc absorption from zinc citrate is relatively high and is equivalent to that from zinc gluconate. The measured value of 61% zinc absorption from zinc citrate is similar to the previously reported 71% zinc absorption from zinc sulfate for adults consuming a 10-mg zinc dose that was measured using the same DITR method (17). Additionally, our results indicate that zinc oxide, the cheapest and most insoluble zinc supplementation compound, is significantly less well absorbed than citrate or gluconate, although admittedly only when the 3 outliers are included in the statistical analysis. However, we could find no sound reason to exclude them. They all appeared healthy, and their absorption from zinc gluconate and zinc citrate was comparable with that of other study participants. Two previous studies also reported lower zinc absorption from zinc oxide supplements than from zinc acetate or sulfate supplements. Henderson et al. (18) measured zinc absorption using mean PZn AUC and reported that absorption of zinc from zinc oxide was lower than from zinc acetate at both high and low intragastric pH and that increasing intragastric pH significantly reduced absorption from zinc oxide. Similarly, PZn concentration in pregnant women after 4 wk of consumption of a supplement containing zinc sulfate was higher than when consuming a supplement of zinc oxide (8).
Our results indicate that zinc citrate could be a useful compound for zinc supplementation. At the present time, WHO recommends the use of the water-soluble compounds zinc sulfate, zinc acetate, or zinc gluconate in the form of syrups or dispersible tablets in the management of diarrhea (6). Zinc citrate might be a useful addition to this list and be especially suitable for chewable/crushable tablets because it has better sensory properties than zinc acetate, zinc sulfate (2), and zinc gluconate (Markus Gerhart and Jungbunzlauer Ladenburg, personal communication), which have an astringent, bitter, or metallic taste. In relation to price and zinc content, zinc citrate would have an advantage over zinc gluconate, because the costs per kilogram of zinc are $30 vs. $78, respectively (Markus Gerhart, Jungbunzlauer Ladenburg, personal communication). The advantage of zinc citrate over zinc sulfate is related to its better sensory qualities and the higher zinc content of citrate (31% vs. 23%) at a similar price and presumably for a similarly high absorption (17).
We used stable isotopes to quantify zinc absorption with the DITR technique, which was developed by Friel et al. (11) and has been validated by several research groups. It is recommended as the method of choice for determination of fractional zinc absorption (12, 13, 15, 19). The technique applied to a spot urine specimen is easy to implement and not sensitive to participant compliance. The DITR technique has been more frequently used to estimate absorption of zinc sulfate and oxide from zinc-fortified foods than from zinc supplements. The presence of food results in lower zinc absorption values ranging from 10 to 25% (20–22) and also shows little or no difference in absorption between zinc oxide and zinc sulfate, possibly because of gastric acid stimulation from the food. Additionally, 1 study investigated zinc absorption from a beverage fortified with zinc gluconate and reported absorption in the same range at slightly <25% but did not measure the absorption of other zinc compounds (23). Supplemental Table 1 summarizes the results of absorption studies with different zinc compounds added to foods and measured using the DITR technique.
There are no human absorption data to compare absorption of zinc citrate or zinc gluconate with zinc sulfate or zinc acetate, the other zinc supplements recommended by the WHO for diarrhea treatment (6). The WHO recommendations are based on randomized placebo-controlled trials investigating the effect of zinc supplementation on diarrhea treatment (24). All the trials used zinc sulfate, zinc acetate, or zinc gluconate and reported similar efficacy. Zinc citrate supplements would also be expected to improve diarrheal treatment, but additional studies are needed to confirm this.
A surprising finding from the present study is that 3 participants of the 15 absorbed little or no zinc from zinc oxide, suggesting that there is a portion of the population that is not able to dissolve zinc oxide in the gastric juice, probably because of a high intragastric pH making it poorly absorbable. Because the low absorption was measured in 3 individuals who absorbed zinc gluconate and zinc citrate normally and because all were apparently healthy at the time of supplement consumption, it is unlikely that this was a coincidence. Additional repeated measurements of their urine samples excluded an analytical error.
Several studies have suggested that Helicobacter pylori-associated hypochlorhydria will decrease gastric acid and decrease iron absorption (25); however, results in relation to iron are contradictory (26), and the prevalence of H. pylori infection in the Swiss population is low, affecting 7.3% of adolescents (27). Similarly, data on hypochlorhydria in young Japanese adults show that only ∼5% are affected (28).
Additionally, Serfaty-Lacrosniere et al. (29) showed that, although induced hypochlorhydria in healthy volunteers significantly increased gastric pH, net absorption of native food zinc from a meal was not negatively affected. Similarly, we found no indication of non-absorbers in a zinc absorption study with Swiss adults consuming a maize porridge fortified with zinc oxide (Marica Brnić, ETH Zurich, Zurich, Switzerland, personal communication), and other studies have shown no difference between fortification with zinc oxide and zinc sulfate (20, 22). Thus, the effect seen in the present study may only be present in case of administration of supplements and would not affect its use as a food fortificant, in which it is often the preferred compound because of its low price ($5.6 per kilogram of zinc compared with $24 for zinc sulfate; Markus Gerhart, Jungbunzlauer Ladenburg, personal communication). Additional investigations in relation to the influence of gastric pH on zinc absorption from zinc oxide supplements are recommended.
In conclusion, our results indicate that zinc is as well absorbed from zinc citrate as from zinc gluconate and that zinc citrate should be as effective as zinc gluconate in the prevention of zinc deficiency and presumably also in the treatment of diarrhea. Its higher zinc content, good sensory properties, and lower price make it an attractive alternative to gluconate and other water-soluble zinc compounds. Zinc oxide appears to be less well absorbed than other zinc compounds when given without food and may be minimally absorbed by some individuals.
Supplementary Material
Acknowledgments
R.W., C.Z., and R.F.H. designed research; R.W., F.T., and M.B. conducted research; R.W. analyzed data; R.W. and R.F.H. wrote the paper, and R.W. and R.F.H. had primary responsibility for final content. We thank A. Krzystek for technical assistance and the Clinical Trials Center team (N. Leu-Möckli, M. Spitaleri, and R. Grossmann) for their assistance in performing the study. The authors’ thanks also go to Markus Gerhart, Jungbunzlauer Ladenburg, for providing information regarding the sensory properties and prices of the different zinc compounds. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: CRP, C-reactive protein; DITR, double-isotope tracer ratio; ETH, Swiss Federal Institute of Technology; FA, fractional absorption; ICP-MS, inductively coupled plasma mass spectrometry; PZn, plasma zinc.
Literature Cited
- 1.Gibson RS, Hess SY, Hotz C, Brown KH. Indicators of zinc status at the population level: a review of the evidence. Br J Nutr. 2008;99(Suppl 3):S14–23 [DOI] [PubMed] [Google Scholar]
- 2.IZiNCG; Brown KH, Rivera JA, Bhutta Z, Gibson RS, King JC, Lönnerdal B, Ruel MT, Sandtröm B, Wasantwisut E, Hotz C. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr Bull. 2004;25:S99–S203 [PubMed] [Google Scholar]
- 3.WHO. The World Health Report 2002: reducing risks, promoting healthy lives. Geneva: World Health Organization; 2002.
- 4.Haider BA, Bhutta ZA. The effect of therapeutic zinc supplementation among young children with selected infections: a review of the evidence. Food Nutr Bull. 2009;30:S41–59 [DOI] [PubMed] [Google Scholar]
- 5. WHO. Diarrhoea treatment guidelines for clinical-based healthcare workers; Including new recommendations for the use of ORS and zinc supplementation. Geneva: World Health Organization; 2005.
- 6. WHO, UNICEF, Johns Hopkins Bloomberg School of Public Health, USAID. Implementing the new recommendations on the clinical management of diarrhoea: guidelines for policy makers and programme managers. Geneva (Switzerland): World Health Organization; 2006.
- 7.Siepmann M, Spank S, Kluge A, Schappach A, Kirch W. The pharmacokinetics of zinc from zinc gluconate: a comparison with zinc oxide in healthy men. Int J Clin Pharmacol Ther. 2005;43:562–5 [DOI] [PubMed] [Google Scholar]
- 8.Wolfe SA, Gibson RS, Gadowsky SL, O'Connor DL. Zinc status of a group of pregnant adolescents at 36 weeks gestation living in southern Ontario. J Am Coll Nutr. 1994;13:154–64 [DOI] [PubMed] [Google Scholar]
- 9.Bertinato J, Sherrard L, Plouffe LJ. EDTA disodium zinc has superior bioavailability compared to common inorganic or chelated zinc compounds in rats fed a high phytic acid diet. J Trace Elem Med Biol. 2012;26:227–33 [DOI] [PubMed] [Google Scholar]
- 10.Guillem A, Alegria A, Barbera R, Farre R, Lagarda MJ, Clemente G. In vitro dialyzability of zinc from different salts used in the supplementation of infant formulas. Biol Trace Elem Res. 2000;75:11–9 [DOI] [PubMed] [Google Scholar]
- 11.Friel JK, Naake VL, Jr, Miller LV, Fennessey PV, Hambidge KM. The analysis of stable isotopes in urine to determine the fractional absorption of zinc. Am J Clin Nutr. 1992;55:473–7 [DOI] [PubMed] [Google Scholar]
- 12.Lowe NM, Woodhouse LR, Matel JS, King JC. Comparison of estimates of zinc absorption in humans by using 4 stable isotopic tracer methods and compartmental analysis. Am J Clin Nutr. 2000;71:523–9 [DOI] [PubMed] [Google Scholar]
- 13.Yang L, Yang X, Piao J, Tian Y, Li P, Wang Y, Wang J. Studies on zinc bioavailability from a representative diet in Chinese urban women of childbearing age using a double label stable isotope technique. J Trace Elem Med Biol. 2005;19:159–64 [DOI] [PubMed] [Google Scholar]
- 14.Pinna K, Woodhouse LR, Sutherland B, Shames DM, King JC. Exchangeable zinc pool masses and turnover are maintained in healthy men with low zinc intakes. J Nutr. 2001;131:2288–94 [DOI] [PubMed] [Google Scholar]
- 15.Shames DM, Woodhouse LR, Lowe NM, King JC. Accuracy of simple techniques for estimating fractional zinc absorption in humans. J Nutr. 2001;131:1854–61 [DOI] [PubMed] [Google Scholar]
- 16.Biasucci LM. CDC/AHA Workshop on Markers of Inflammation and Cardiovascular Disease: application to clinical and public health practice: clinical use of inflammatory markers in patients with cardiovascular diseases: a background paper. Circulation. 2004;110:e560–7 [DOI] [PubMed] [Google Scholar]
- 17.Tran CD, Miller LV, Krebs NF, Lei S, Hambidge KM. Zinc absorption as a function of the dose of zinc sulfate in aqueous solution. Am J Clin Nutr. 2004;80:1570–3 [DOI] [PubMed] [Google Scholar]
- 18.Henderson LM, Brewer GJ, Dressman JB, Swidan SZ, DuRoss DJ, Adair CH, Barnett JL, Berardi RR. Effect of intragastric pH on the absorption of oral zinc acetate and zinc oxide in young healthy volunteers. JPEN J Parenter Enteral Nutr. 1995;19:393–7 [DOI] [PubMed] [Google Scholar]
- 19.Sparacino G, Shames DM, Vicini P, King JC, Cobelli C. Double isotope tracer method for measuring fractional zinc absorption: theoretical analysis. Am J Physiol Endocrinol Metab. 2002;282:E679–87 [DOI] [PubMed] [Google Scholar]
- 20.Herman S, Griffin IJ, Suwarti S, Ernawati F, Permaesih D, Pambudi D, Abrams SA. Cofortification of iron-fortified flour with zinc sulfate, but not zinc oxide, decreases iron absorption in Indonesian children. Am J Clin Nutr. 2002;76:813–7 [DOI] [PubMed] [Google Scholar]
- 21.López de Romaña DL, Salazar M, Hambidge KM, Penny ME, Peerson JM, Krebs NF, Brown KH. Longitudinal measurements of zinc absorption in Peruvian children consuming wheat products fortified with iron only or iron and 1 of 2 amounts of zinc. Am J Clin Nutr. 2005;81:637–47 [DOI] [PubMed] [Google Scholar]
- 22.Hotz C, DeHaene J, Woodhouse LR, Villalpando S, Rivera JA, King JC. Zinc absorption from zinc oxide, zinc sulfate, zinc oxide + EDTA, or sodium-zinc EDTA does not differ when added as fortificants to maize tortillas. J Nutr. 2005;135:1102–5 [DOI] [PubMed] [Google Scholar]
- 23.Avalos Mishaan AM, Zavaleta N, Griffin IJ, Hilmers DC, Hawthorne KM, Abrams SA. Bioavailability of iron and zinc from a multiple micronutrient-fortified beverage. J Pediatr. 2004;145:26–31 [DOI] [PubMed] [Google Scholar]
- 24.Bhutta ZA, Bird SM, Black RE, Brown KH, Gardner JM, Hidayat A, Khatun F, Martorell R, Ninh NX, Penny ME, et al. Therapeutic effects of oral zinc in acute and persistent diarrhea in children in developing countries: pooled analysis of randomized controlled trials. Am J Clin Nutr. 2000;72:1516–22 [DOI] [PubMed] [Google Scholar]
- 25.Vitale G, Barbaro F, Ianiro G, Cesario V, Gasbarrini G, Franceschi F, Gasbarrini A. Nutritional aspects of Helicobacter pylori infection. Minerva Gastroenterol Dietol. 2011;57:369–77 [PubMed] [Google Scholar]
- 26.Sarker SA, Davidsson L, Mahmud H, Walczyk T, Hurrell RF, Gyr N, Fuchs GJ. Helicobacter pylori infection, iron absorption, and gastric acid secretion in Bangladeshi children. Am J Clin Nutr. 2004;80:149–53 [DOI] [PubMed] [Google Scholar]
- 27.Heuberger F, Pantoflickova D, Gassner M, Oneta C, Grehn M, Blum AL, Dorta G. Helicobacter pylori infection in Swiss adolescents: prevalence and risk factors. Eur J Gastroenterol Hepatol. 2003;15:179–83 [DOI] [PubMed] [Google Scholar]
- 28.Morihara M, Aoyagi N, Kaniwa N, Kojima S, Ogata H. Assessment of gastric acidity of Japanese subjects over the last 15 years. Biol Pharm Bull. 2001;24:313–5 [DOI] [PubMed] [Google Scholar]
- 29.Serfaty-Lacrosniere C, Wood RJ, Voytko D, Saltzman JR, Pedrosa M, Sepe TE, Russell RR. Hypochlorhydria from short-term omeprazole treatment does not inhibit intestinal absorption of calcium, phosphorus, magnesium or zinc from food in humans. J Am Coll Nutr. 1995;14:364–8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.