Abstract
Celiac disease is caused by an inappropriate immune response to ingested gluten proteins. As a dietary antigen, gluten undergoes extensive but incomplete proteolytic digestion in the intestinal lumen. The resultant peptide fragments of gluten require deamidation, but not necessarily further intracellular processing for presentation. Recent studies reveal why the disease associated HLA-DQ2 molecule is particularly suited for binding proline-rich gluten peptides. In comparison, DQ8 exhibits different binding characteristics, which may explain the lesser risk for disease in association with this molecule.
Introduction
Celiac disease (CeD) is an intestinal disorder caused by an inappropriate immune response to ingested wheat gluten (consisting of the gliadin and glutenin subcomponents) and related proteins of rye and barley in genetically predisposed individuals. Elimination of these proteins from the diet leads to complete remission. Patients with active CeD have a variable degree of symptoms, ranging from severe malabsorption to no subjective symptoms at all. The diagnosis is made by demonstration of typical intestinal histopathology, but the presence of auto-antibodies to the enzyme transglutaminase 2 and gliadin in gluten-consuming subjects is used as a diagnostic adjunct.
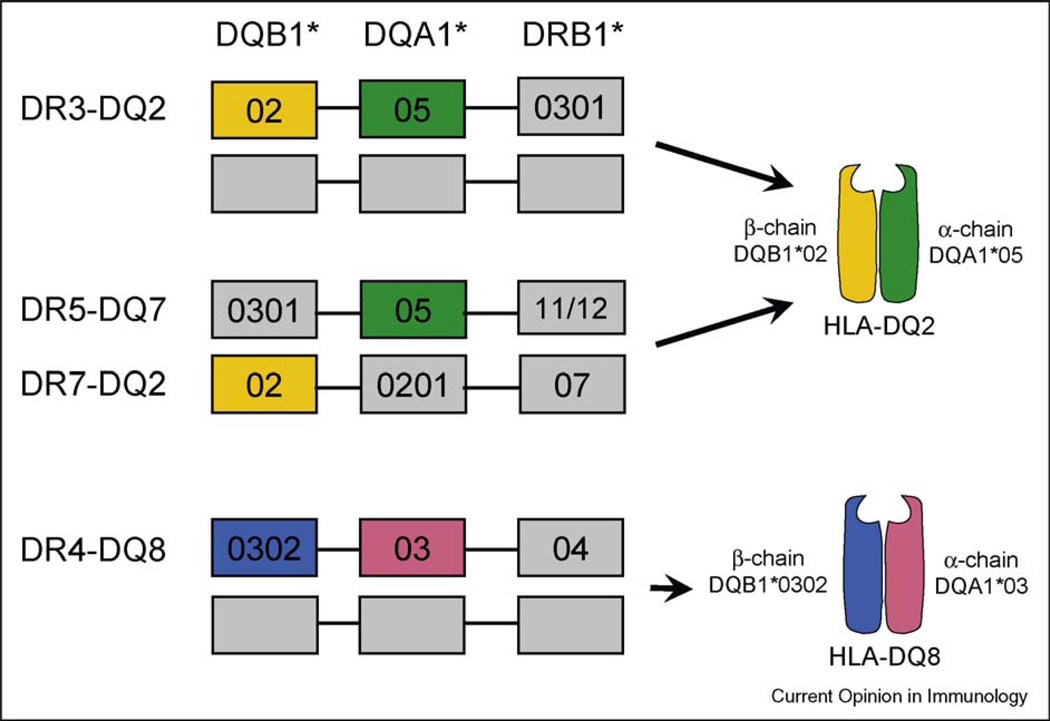
Screening studies indicate that CeD affects about 1% of Caucasian populations [1]. CeD exhibits a very strong HLA association, in which the relative risk of disease development for carriers of certain alleles is increased 30-fold [2], higher than the HLA-association seen in many other auto-immune diseases such as type I diabetes or rheumatoid arthritis [2]. Approximately 90% of celiac patients carry the HLA-DQ2 heterodimer encoded by the DQA1*05 and DQB1*02 genes, carried either in cis on the DR3-DQ2 haplotype common to many autoimmune diseases, or in trans where the α chain is encoded on the DR5-DQ7 haplotype on one chromosome and the β chain on the DR7-DQ2 haplotype on the other chromosome [3]. Most of the patients that are DQ2 negative carry DQ8 (Figure 1). These disease associated DQ2 and DQ8 molecules present gluten peptides or related antigens to disease-specific CD4+ T cells.
Figure 1.
HLA association in celiac disease. A vast majority of celiac patients express the HLA-DQ2 heterodimer encoded by the DQA1*05 and DQB1*02 genes. These two genes are carried either in cis on the DR3-DQ2 haplotype, or in trans in individuals who are DR5-DQ7 and DR7-DQ2 heterozygous. Most DQ2-negative patients express DQ8 encoded on the DR4-DQ8 haplotype.
Luminal pre-processing and transepithelial transport of gluten antigen
Unlike other exogenous proteins that have to be processed intracellularly in antigen presenting cells (APC) before presentation on MHC class II molecules, dietary antigens such as gluten follow a unique antigen processing itinerary. Dietary proteins are subjected to extensive pre-APC processing in the luminal compartment before they encounter APC, which are likely to be localized in the lamina propria, as enterocytes generally do not express HLA-DQ molecules [4,5]. Indeed, the luminal and brush-border enzymes are so efficient that most dietary proteins are broken down to fragments too small to be immunogenic. However, owing to its high proline content, gliadin is remarkably resistant to luminal and brush-border proteolysis and large fragments remain intact after digestion. The most illustrative peptide fragment is the 33mer produced by digestion of certain α-gliadin proteins. This 33mer fragment remains intact even after extended incubation with gastric, pancreatic and intestinal brush-border membrane enzymes [6]. It contains six overlapping copies of three different DQ2-restricted T cell epitopes, and is recognized by T cell lines from nearly all adult CeD patients. Even more intriguingly, this peptide can bind to DQ2 molecules directly on the surface of APC and can thus be presented to T cells without the need for further intracellular processing [7]. The recent finding that DQ2 responds poorly to HLA-DM-mediated peptide editing and that a large fraction of surface-expressed DQ2 molecules are occupied with conventional CLIP peptides (CLIP1) or an atypical invariant chain CLIP fragment (CLIP2 peptide)[8•,9] raise the possibility that surface bound DQ2 may be more susceptible to extracellular peptide exchange. Thus, gliadin can bypass the conventional intracellular processing requirements by being subjected to extensive luminal and brush-border proteolysis and direct extracellular binding of the resultant antigenic peptides to surface DQ2 by displacement of CLIP1/CLIP2 peptides from the binding groove.
Several studies have shown a defective increase in the transepithelial translocation of gliadin peptides in active celiac tissues, which resolve after treatment with gluten-free diet [10–12,13•]. Reduced number of horizontal tight junction (TJ) strands and decreased transepithelial resistance have been reported in active celiac mucosa [14,15], pointing to a more ‘leaky’ gut that permits increased paracellular gliadin transport. In addition, gliadin is also transported to the serosal compartment via the transcellular pathway, and this transcytosis is altered in active celiac tissues. A large fraction of the immunodominant 33mer gliadin peptide was delivered intact to the serosal side in active celiac mucosa compared to complete degradation in controls and biopsies from treated celiac patients [12]. Data from Schumann et al. show that enterocytes take up the 33mer by endocytosis and this epithelial uptake was 10-fold higher in active celiac tissues compared with controls or treated celiac patients [13•]. Interestingly, IFN-γ, a cytokine prevalent in active CeD, weakens the epithelial barrier by triggering internalization of TJ proteins [16] and IFN-γ treatment of the intestinal epithelial cell line Caco-2 increases translocation of the 33mer [13•].
Matysiak-Budnik and et al. have recently suggested a receptor-mediated mechanism for transepithelial gliadin transport [17•]. The transferrin receptor CD71 binds secretory IgA and is overexpressed on the apical surface of enterocytes in active celiac mucosa. Ex vivo transcytosis experiments suggest that CD71 can mediate the transport of IgA-gliadin complexes, a process that can be specifically blocked by IgA or soluble CD71 receptors. In addition to high titres of gliadin-specific IgA in patients with active CeD, gliadin-specific IgG is also present. Given the fact that the neonatal Fc-receptor (FcRn) is expressed in adult human intestinal epithelial cells [18] and mediates apical to basolateral transcytosis of IgG-antigen immunecomplexes [19], FcRn may also transport gliadin antigens across the epithelial barrier by transcytosis of immunecomplexes of anti-gliadin-IgG and gliadin.
Antigen presenting cells
Two groups have investigated and characterized the mucosal dendritic cell (DC) populations in celiac tissues. Both studies found severalfold increases in the number of DC in lamina propria of active celiac mucosa, compared with treated celiac or normal biopsies [20••,21••]. However, the studies differ on which DC subtype dominates, myeloid or plasmacytoid DC. Ráki et al. used immunohistological methods to characterize and enumerate DQ2+ APC in situ in lamina propria of small intestine biopsies. The numbers of macrophages, identified by the CD68 marker, and of CD1c+CD11c+ myeloid DC in active celiac mucosa were similar to those found in healthy tissues. In comparison, the number of CD1c− myeloid DC, the major subset of CD11c+ DC in normal tissues, was increased by about threefold in untreated celiac lesions. Notably, this study found no or very few CD11c−CD123+ plasmacytoid DC in the duodenal mucosa [20••]. By contrast, Di Sabatino et al. identified CD123+ plasmacytoid DC as the major DC population in the small intestinal lamina propria and this subset was largely responsible for the increase of DCs in untreated celiac mucosa [21••]. In the absence of other reports, it is difficult to reconcile these two studies with regard to the phenotype of mucosal DC. On one hand, it appears convincing that Di Sabatino and et al. [21••] have found a substantial number of CD123+ APC in mucosal tissues. On the other hand, Raki et al. [20••] found that CD68+ macrophages and CD11c+ myeloid DC together account for all the DQ2+ APC in situ in mucosal tissue, without any evidence of a CD123+ plasmacytoid DC population. It is difficult to compare these results directly because the studies use different sets of cell surface markers and detection methods with different sensitivities. The first study relied heavily on flow cytometric characterization of cell suspensions isolated from intestinal tissues. However, the chemical and enzymatic manipulations necessary to yield single cell suspensions introduces bias because some cell subsets, in particular CD11c+ cells, are more vulnerable to this procedure and are lost to a greater extent than others. Nevertheless, the fact that these authors found CD123+ cells in situ by immunohistological staining, and that flow cytometric characterization showed that the CD123+ cells are negative for the endothelial cell marker CD31, and positive for the co-stimulatory markers CD80, CD83 and CD86, as well as TLR9, shows convincingly that these cells are indeed APCs.
Despite these inconsistencies, both studies show that the number of APC is increased in tissues affected in active celiac disease, but reverts to normal level upon successful treatment with a gluten-free diet. Presumably, both circulating DCs and monocytes are recruited to the inflamed mucosa during active CeD. Monocytes differentiate in situ into cells with mature phenotypes, either CD68+ macrophage-like cells, or DC-like cells expressing high levels of maturation markers CD80 and CD86. DCs isolated from active celiac tissues are excellent APCs and transcribe higher levels of IFN-α, an important cytokine for celiac pathogenesis [21••]. As discussed above, the precise phenotype of the most prevalent DCs in active celiac mucosa is still under debate. The phenotypic description of DC subsets, and indeed, for the entire monocyte-macrophage-DC cell lineage, also known as the mononuclear phagocyte system, is particularly confusing depending on the combination of surface markers used in each study. Even the concept that dendritic cells merit a separate lineage has been called into question [22]. Nevertheless, it is important to note that functionally, the CD11c+ APC subset is superior in presenting the immunodominant 33mer peptide to T cells than both the DC-SIGN+ macrophages, and the remaining cells depleted of CD11c+ and DC-SIGN+ APCs [20••].
The gluten antigen, enzymatic post-translational modification and HLA binding
Of the gluten proteins, both gliadins (alcohol soluble) divided into α-gliadin, γ-gliadin and ω-gliadin, and the glutenins (alcohol insoluble) can cause CeD. Gluten proteins are rich in glutamine and proline and the gliadins typically contain 35% glutamine and 25% proline.
Gliadin-specific, DQ2-restricted or DQ8-restricted CD4+ T cells can be cultured in vitro from small intestine biopsies from celiac patients, but not controls [23]. The disease associated DQ2 and DQ8 molecules prefer negatively charged amino acid residues in certain binding pockets (P4, P6 and P7 for DQ2; P1 and P9 for DQ8). However, native gluten contains few negatively charged residues. These charges are introduced post-translationally by specific and targeted deamidation, that is, the conversion of glutamine residue to glutamate, catalyzed by the enzyme tissue transglutaminase or transglutaminase 2 (TG2). Deamidation increases the binding of gluten derived peptides to DQ2 and DQ8, and is in many cases essential for T cell recognition. This deamidation-dependent recognition exemplifies the importance of post-translational protein modification in creating novel T cell epitopes [24,25].
Where and when the necessary gluten deamidation takes place is still in question. TG2 is enzymatically active only in the presence of millimolar levels of Ca2+. Upon activation, the enzyme undergoes a dramatic conformational change in which the C-terminal residues are displaced by as much as 120 A ° [26••]. TG2 is a ubiquitous protein found both intra-extracellularly and extracellularly. The intracellular Ca2+ concentration is low and tightly controlled. By contrast, Ca2+ concentration is high in the extracellular environment and thus it has been assumed that extracellular TG2 is enzymatically active. However, a recent study revealed that the majority of extracellular TG2 is inactive, despite an environment conductive to enzyme activation [27••]. However, TG2 can be enzymatically activated by proper injury signals, such as those present in the in vitro wounding model, or in vivo in the acute poly(I:C) injury model [27••]. These new insights in TG2 biology suggest that the post-translational modifications of gluten antigen observed in CeD may be elicited only in the presence of existing tissue damage, such as those caused by infection, or inflammation in the early stages of CeD.
TG2 is expressed on most cell surfaces, notably the surface of monocytes [28] where it is complexed with membrane-bound integrins and matrix fibronectin [29]. The presence of TG2 on the APC surface leads to the tempting idea that this TG2 pool directly participates in the deamidation of gluten peptides, and maybe also facilitates the uptake of gluten epitopes enzymatically complexed to TG2 in a hapten-carrier-like manner. No evidence in support of this notion has been obtained [30], although the function of surface TG2 in tissue resident APC has not been tested. The monoclonal antibody 6B9 [31] used to demonstrate abundant surface expression of TG2 is, in fact, specific for CD44 [32]. Thus, the question remains whether TG2 is present on APC cell surfaces, and if so, whether this pool of TG2 participates in deamidation of gluten peptides or enhanced uptake of gluten antigens.
Since the identification of the first DQ2-restricted gliadin T cell epitope in 1998 [33], more than a dozen celiac-specific T cell epitopes have been identified, mostly from wheat gliadin [34–37], a few from glutenin [38] and oat avenin [39]. The majority of these epitopes are DQ2-restricted, only three DQ8-restricted gluten epitopes having been identified so far [38,40,41••]. Gliadin-derived T cell epitopes tend to cluster in proline-rich regions [35]. There are several reasons for this clustering. Proline contributes to the proteolytic resistance of gliadin peptides, such that proline-rich fragments are more likely to survive the proteolytic environment in the gut. Second, the TG2-mediated deamidation that results in enhanced DQ2 or DQ8 binding and T cell recognition is highly sequence-specific. In this context, the positioning of proline residues in the immediate vicinity is shown to be the most important factor that determines whether a glutamine residue is targeted by TG2 or not [42,43]. In addition, we have found that some gliadin epitopes, notably those most often recognized by celiac lesion T cells, are better substrates for TG2 and are deamidated faster, than the less well-recognized epitopes [44•]. Thus, it appears that the epitope-modifying enzyme TG2 is an important player in shaping gliadin T cell epitope repertoire in CeD.
Proline residues are unusually abundant in gliadin-derived T cell epitopes, with as many as four proline residues within the 9mer core binding region (Table 1). Because proline is an amino acid that cannot participate in essential hydrogen-bonds to the peptide backbone, proline-rich peptides are not particularly suited for binding to MHC class II molecules. DQ2 proves to be an exception, as it readily accepts proline in certain positions, notably in P1, P3, P5 and P8, where proline positioning is not penalized by loss of hydrogen bonds between peptide backbone and conserved MHC residues, as shown by the crystal structure of DQ2 bound with the DQ2-α-I gliadin peptide [6]. Owing to a unique Arg53α deletion, DQ2 is the only DQ molecule known to accept proline at P1 [41••,45]. Nearly half of the DQ2-restricted gliadin T cell epitopes, and all the DQ2-restricted α-gliadin epitopes found within the immunodominant 33mer peptide, have proline in P1. A recent binding study confirmed that DQ2 is the preferred class II molecule by gliadin peptides containing celiac T cell epitopes [46]. In comparison, the minor HLA-susceptibility allele, DQ8, accommodates proline-rich peptides less well, in particular because its inability to accommodate proline in P1 [41••,47•] (Figure 2). Thus, DQ8 cannot present the most immunodominant DQ2-restricted epitopes of α-gliadins and the only identified DQ8-restricted epitope of α-gliadin is not located within proline-rich proteolytic resistant regions of the α-gliadin proteins. Owing to its special ability to accommodate proline-rich peptides and its preference for negatively charged glutamate as anchor residues, the DQ2 molecule is therefore uniquely suited to bind proline-rich and glutamate-rich gliadin fragments produced by luminal digestion and TG2 deamidation. DQ8 shares some of these binding characteristics but is overall less well suited for binding and presentation of gliadin peptides, and therefore this allele confers less CeD risk compared with DQ2.
Table 1.
Alignment of the core region of DQ2-restricted and DQ8-restricted gluten epitopes.
| HLA restriction | Epitope | Peptide-binding register, P1–P9 | Present in the 33mer | Reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||||
| DQ2 | avenin | P | Y | P | E | Q | E | E | P | F | [39] | |
| gliadin-α-I | P | F | P | Q | P | E | L | P | Y | ✓ | [34] | |
| gliadin-α-III | P | Y | P | Q | P | E | L | P | Y | ✓ | [35] | |
| gliadin-α-II | P | Q | P | E | L | P | Y | P | Q | ✓ | [34] | |
| gliadin-γ-I | P | Q | Q | S | F | P | E | Q | Q | [33] | ||
| gliadin-γ-II | I | Q | P | E | Q | P | A | Q | L | [37] | ||
| gliadin-γ-VI | Q | Q | P | F | P | E | Q | P | Q | [36] | ||
| gliadin-γ-IV | S | Q | P | E | Q | E | F | P | Q | [35] | ||
| gliadin-γ-VII | P | Q | P | E | Q | E | F | P | Q | [36] | ||
| gliadin-γ-III | Q | Q | P | E | Q | P | Y | P | Q | [35] | ||
| gliadin-γ-VII | Q | Q | P | E | Q | P | F | P | Q | [36] | ||
| gliadin-α20 | F | R | P | E | Q | P | Y | P | Q | [37] | ||
| glutenin-17 | P | F | S | E | Q | E | Q | P | V | [37] | ||
| DQ8 | gliadin-γ-I | E | Q | P | Q | Q | P | F | P | Q | [41] | |
| gliadin-γ-I | E | Q | P | Q | Q | P | Y | P | E | [41] | ||
| gliadin-α-I | E | G | S | F | Q | P | S | Q | E | [40] | ||
| glutenin | E | G | Y | Y | P | T | S | P | E | [38] | ||
Glutamate residues formed by TG2-mediated deamidation that are important for recognition by DQ2-restricted or DQ8-restricted T cells are shown in bold. Additional glutamine residues also targeted by TG2 are underlined.
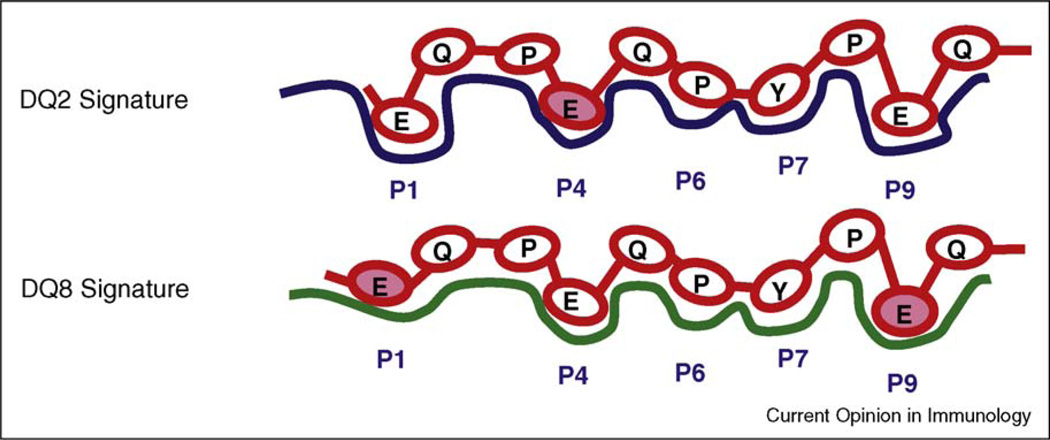
Figure 2.
Peptide binding signatures of DQ2 and DQ8 molecules. The DQ2-restricted γ-III epitope and the DQ8-restricted γ-I epitope recognized by lesion derived T cells of CeD patients share the same 9 amino acid core sequence. This sequence contains three glutamate residues formed by TG2-mediated deamidation in positions P1, P4 and P9. DQ2 prefers negatively charged glutamate residue in P4 (shaded) whereas DQ8 prefers glutamate in P1 and P9 (shaded).
Acknowledgements
We thank Stig Tollefsen for assistance with Figure 2. This work was supported by the Research Council of Norway (to SWQ and LMS), the European Commission (to LMS), the National Institutes of Health grants DK53056, DK51362 and DK44319 (to RSB) and the Harvard Digestive Disease Center (NIH DK34854) (to RSB).
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Maki M, Mustalahti K, Kokkonen J, Kulmala P, Haapalahti M, Karttunen T, Ilonen J, Laurila K, Dahlbom I, Hansson T, et al. Prevalence of celiac disease among children in Finland. N Engl J Med. 2003;348:2517–2524. doi: 10.1056/NEJMoa021687. [DOI] [PubMed] [Google Scholar]
- 2.Thorsby E, Lie BA. HLA associated genetic predisposition to autoimmune diseases: genes involved and possible mechanisms. Transpl Immunol. 2005;14:175–182. doi: 10.1016/j.trim.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 3.Sollid LM, Markussen G, Ek J, Gjerde H, Vartdal F, Thorsby E. Evidence for a primary association of celiac disease to a particular HLA-DQ alpha/beta heterodimer. J Exp Med. 1989;169:345–350. doi: 10.1084/jem.169.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scott H, Sollid LM, Fausa O, Brandtzaeg P, Thorsby E. Expression of major histocompatibility complex class-II subregion products by jejunal epithelium in patients with celiac disease. Scand J Immunol. 1987;26:563–571. doi: 10.1111/j.1365-3083.1987.tb02290.x. [DOI] [PubMed] [Google Scholar]
- 5.Kelly J, Weir DG, Feighery C. Differential expression of HLA-D gene products in the normal and coeliac small bowel. Tissue Antigens. 1988;31:151–160. doi: 10.1111/j.1399-0039.1988.tb02076.x. [DOI] [PubMed] [Google Scholar]
- 6.Shan L, Molberg O, Parrot I, Hausch F, Filiz F, Gray GM, Sollid LM, Khosla C. Structural basis for gluten intolerance in celiac sprue. Science. 2002;297:2275–2279. doi: 10.1126/science.1074129. [DOI] [PubMed] [Google Scholar]
- 7.Qiao SW, Bergseng E, Molberg O, Xia J, Fleckenstein B, Khosla C, Sollid LM. Antigen presentation to celiac lesion-derived T cells of a 33-mer gliadin peptide naturally formed by gastrointestinal digestion. J Immunol. 2004;173:1757–1762. doi: 10.4049/jimmunol.173.3.1757. [DOI] [PubMed] [Google Scholar]
- 8. Fallang LE, Roh S, Holm A, Bergseng E, Yoon T, Fleckenstein B, Bandyopadhyay A, Mellins ED, Sollid LM. Complexes of two cohorts of CLIP peptides and HLA-DQ2 of the autoimmune DR3-DQ2 haplotype are poor substrates for HLA-DM. J Immunol. 2008;181:5451–5461. doi: 10.4049/jimmunol.181.8.5451. This study shows that the DQ2 molecule is associated with unusually high amounts of CLIP1 (conventional CLIP) and CLIP2 (II 96–104) owing to stable DQ2-CLIP complexes and inefficient DQ2 interaction with DM.
- 9.Wiesner M, Stepniak D, de Ru AH, Moustakis AK, Drijfhout JW, Papadopoulos GK, Van Veelen PA, Koning F. Dominance of an alternative CLIP sequence in the celiac disease associated HLA-DQ2 molecule. Immunogenetics. 2008;60:551–555. doi: 10.1007/s00251-008-0310-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friis S, Dabelsteen E, Sjostrom H, Noren O, Jarnum S. Gliadin uptake in human enterocytes. Differences between coeliac patients in remission and control individuals. Gut. 1992;33:1487–1492. doi: 10.1136/gut.33.11.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zimmer KP, Poremba C, Weber P, Ciclitira PJ, Harms E. Translocation of gliadin into HLA-DR antigen containing lysosomes incoeliac diseaseenterocytes. Gut. 1995;36:703–709. doi: 10.1136/gut.36.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matysiak-Budnik T, Candalh C, Dugave C, Namane A, Cellier C, Cerf-Bensussan N, Heyman M. Alterations of the intestinal transport and processing of gliadin peptides in celiac disease. Gastroenterology. 2003;125:696–707. doi: 10.1016/s0016-5085(03)01049-7. [DOI] [PubMed] [Google Scholar]
- 13. Schumann M, Richter JF, Wedell I, Moos V, Zimmermann-Kordmann M, Schneider T, Daum S, Zeitz M, Fromm M, Schulzke JD. Mechanisms of epithelial translocation of the a2-gliadin-33mer in coeliac sprue. Gut. 2008;57:747–754. doi: 10.1136/gut.2007.136366. Demonstrates that the epithelial translocation of the 33mer occurs by transcytosis and is regulated by interferon-γ. Uptake of the 33mer is higher in untreated CeD tissues than in controls.
- 14.Schulzke JD, Schulzke I, Fromm M, Riecken EO. Epithelial barrier and ion transport in coeliac sprue: electrical measurements on intestinal aspiration biopsy specimens. Gut. 1995;37:777–782. doi: 10.1136/gut.37.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schulzke JD, Bentzel CJ, Schulzke I, Riecken EO, Fromm M. Epithelial tight junction structure in the jejunum of children with acute and treated celiac sprue. Pediatr Res. 1998;43:435–441. doi: 10.1203/00006450-199804000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Chiba H, Kojima T, Osanai M, Sawada N. The significance of interferon-γ-triggered internalization of tight-junction proteins in inflammatory bowel disease. Sci STKE. 2006;2006:e1. doi: 10.1126/stke.3162006pe1. [DOI] [PubMed] [Google Scholar]
- 17. Matysiak-Budnik T, Moura IC, Arcos-Fajardo M, Lebreton C, Menard S, Candalh C, Ben-Khalifa K, Dugave C, Tamouza H, van Niel G, et al. Secretory IgA mediates retrotranscytosis of intact gliadin peptides via the transferrin receptor in celiac disease. J Exp Med. 2008;205:143–154. doi: 10.1084/jem.20071204. This paper suggests that the transferrin receptor CD71, a receptor that binds IgA and is overexpressed in active CeD, is responsible for the protected transepithelial transport of gliadin peptides.
- 18.Israel EJ, Taylor S, Wu Z, Mizoguchi E, Blumberg RS, Bhan A, Simister NE. Expression of the neonatal Fc receptor, FcRn, on human intestinal epithelial cells. Immunology. 1997;92:69–74. doi: 10.1046/j.1365-2567.1997.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshida M, Claypool SM, Wagner JS, Mizoguchi E, Mizoguchi A, Roopenian DC, Lencer WI, Blumberg RS. Human neonatal Fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity. 2004;20:769–783. doi: 10.1016/j.immuni.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 20. Raki M, Tollefsen S, Molberg O, Lundin KEA, Sollid LM, Jahnsen FL. A unique dendritic cell subset accumulates in the celiac lesion and efficiently activates gluten-reactive T cells. Gastroenterology. 2006;131:428–438. doi: 10.1053/j.gastro.2006.06.002. This study identifies CD11c+DQ2+CD1c− myeloid DC as the major APC population in intestinal lamina propria and is increased in untreated CeD. These myeloid DCs isolated from intestinal tissues are excellent antigen presenting cells.
- 21. Di Sabatino A, Pickard KM, Gordon JN, Salvati V, Mazzarella G, Beattie RM, Vossenkaemper A, Rovedatti L, Leakey NAB, Croft NM, et al. Evidence for the role of Interferon-alfa production by dendritic cells in the Th1 response in celiac disease. Gastroenterology. 2007;133:1175–1187. doi: 10.1053/j.gastro.2007.08.018. This study identifies CD123+ plasmacytoid DC as the major APC population that is increased in untreated CeD. The CD123+ cells from these patients displayed a more activated phenotype and showed more transcripts of inflammatory cytokines.
- 22.Hume DA. Macrophages as APC and the dendritic cell myth. J Immunol. 2008;181:5829–5835. doi: 10.4049/jimmunol.181.9.5829. [DOI] [PubMed] [Google Scholar]
- 23.Molberg O, Kett K, Scott H, Thorsby E, Sollid LM, Lundin KEA. Gliadin specific, HLA DQ2-restricted T cells are commonly found in small intestinal biopsies from coeliac disease patients, but not from controls. Scand J Immunol. 1997;46:103–108. [PubMed] [Google Scholar]
- 24.Michaelsson E, Malmstrom V, Reis S, Engstrom A, Burkhardt H, Holmdahl R. T cell recognition of carbohydrates on type II collagen. J Exp Med. 1994;180:745–749. doi: 10.1084/jem.180.2.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skipper JC, Hendrickson RC, Gulden PH, Brichard V, Van PA, Chen Y, Shabanowitz J, Wolfel T, Slingluff CL, Jr, Boon T, et al. An HLA-A2-restricted tyrosinase antigen on melanoma cells results from posttranslational modification and suggests a novel pathway for processing of membrane proteins. J Exp Med. 1996;183:527–534. doi: 10.1084/jem.183.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pinkas DM, Strop P, Brunger AT, Khosla C. Transglutaminase 2 undergoes a large conformational change upon activation. PLoS Biol. 2007;5:e327. doi: 10.1371/journal.pbio.0050327. The authors trapped human TG2 in complex with an inhibitor that mimics gluten peptides. The X-ray crystal structure of the inhibitor stabilized TG2 showed an extended conformation dramatically different from earlier transglutaminase structures in which C-terminal residues are displaced by 120 Å.
- 27. Siegel M, Strnad P, Watts RE, Choi K, Jabri B, Omary MB, Khosla C. Extracellular transglutaminase 2 is catalytically inactive, but is transiently activated upon tissue injury. PLoS ONE. 2008;3:e1861. doi: 10.1371/journal.pone.0001861. In contrast to earlier studies of TG2 activity involving tissue homogenization and lysis, this paper studied TG2 cross-linking activity in situ in the tissues. Under normal physiological conditions, the majority of extracellular TG2 is inactive. However, TG2 could be activated by injury signals.
- 28.Akimov SS, Belkin AM. Cell surface tissue transglutaminase is involved in adhesion and migration of monocytic cells on fibronectin. Blood. 2001;98:1567–1576. doi: 10.1182/blood.v98.5.1567. [DOI] [PubMed] [Google Scholar]
- 29.Akimov SS, Krylov D, Fleischman LF, Belkin AM. Tissue transglutaminase is an integrin-binding adhesion coreceptor for fibronectin. J Cell Biol. 2000;148:825–838. doi: 10.1083/jcb.148.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raki M, Schjetne KW, Stamnaes J, Molberg O, Jahnsen FL, Issekutz TB, Bogen B, Sollid LM. Surface expression of transglutaminase 2 by dendritic cells and its potential role for uptake and presentation of gluten peptides to T cells. Scand J Immunol. 2007;65:213–220. doi: 10.1111/j.1365-3083.2006.01881.x. [DOI] [PubMed] [Google Scholar]
- 31.Mohan K, Pinto D, Issekutz TB. Identification of tissue transglutaminase as a novel molecule involved in human CD8+ T cell transendothelial migration. J Immunol. 2003;171:3179–3186. doi: 10.4049/jimmunol.171.6.3179. [DOI] [PubMed] [Google Scholar]
- 32.Stamnaes J, Fleckenstein B, Lund-Johansen F, Sollid LM. The monoclonal antibody 6B9 recognizes CD44 and not cell surface transglutaminase 2. Scand J Immunol. 2008;68:534–542. doi: 10.1111/j.1365-3083.2008.02173.x. [DOI] [PubMed] [Google Scholar]
- 33.Sjostrom H, Lundin KEA, Molberg O, Korner R, McAdam SN, Anthonsen D, Quarsten H, Noren O, Roepstorff P, Thorsby E, Sollid LM. Identification of a gliadin T-cell epitope in coeliac disease: general importance of gliadin deamidation for intestinal T-cell recognition. Scand J Immunol. 1998;48:111–115. doi: 10.1046/j.1365-3083.1998.00397.x. [DOI] [PubMed] [Google Scholar]
- 34.Arentz-Hansen H, Korner R, Molberg O, Quarsten H, Vader W, Kooy YMC, Lundin KEA, Koning F, Roepstorff P, Sollid LM, McAdam SN. The intestinal T cell response to α-gliadin in adult celiac disease is focused on a single deamidated glutamine targeted by tissue transglutaminase. J Exp Med. 2000;191:603–612. doi: 10.1084/jem.191.4.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arentz-Hansen H, McAdam SN, Molberg O, Fleckenstein B, Lundin KE, Jorgensen TJ, Jung G, Roepstorff P, Sollid LM. Celiac lesion T cells recognize epitopes that cluster in regions of gliadins rich in proline residues. Gastroenterology. 2002;123:803–809. doi: 10.1053/gast.2002.35381. [DOI] [PubMed] [Google Scholar]
- 36.Qiao SW, Bergseng E, Molberg O, Jung G, Fleckenstein B, Sollid LM. Refining the rules of gliadin T cell epitope binding to the disease-associated DQ2 molecule in celiac disease: importance of proline spacing and glutamine deamidation. J Immunol. 2005;175:254–261. doi: 10.4049/jimmunol.175.1.254. [DOI] [PubMed] [Google Scholar]
- 37.Vader W, Kooy Y, van Veelen P, de Ru A, Harris D, Benckhuijsen W, Pena S, Mearin L, Drijfhout JW, Koning F. The gluten response in children with celiac disease is directed toward multiple gliadin and glutenin peptides. Gastroenterology. 2002;122:1729–1737. doi: 10.1053/gast.2002.33606. [DOI] [PubMed] [Google Scholar]
- 38.van de Wal Y, Kooy YMC, van Veelen P, Vader W, August SA, Drijfhout JW, Pena SA, Koning F. Glutenin is involved in the gluten-driven mucosal T cell response. Eur J Immunol. 1999;29:3133–3139. doi: 10.1002/(SICI)1521-4141(199910)29:10<3133::AID-IMMU3133>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 39.Arentz-Hansen H, Fleckenstein B, Molberg O, Scott H, Koning F, Jung G, Roepstorff P, Lundin KEA, Sollid LM. The molecular basis for oat intolerance in patients with celiac disease. PLoS Med. 2004;1:e1. doi: 10.1371/journal.pmed.0010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van de Wal Y, Kooy YM, van Veelen PA, Pena SA, Mearin LM, Molberg O, Lundin KEA, Sollid LM, Mutis T, Benckhuijsen WE, et al. Small intestinal T cells of celiac disease patients recognize a natural pepsin fragment of gliadin. Proc Natl Acad Sci U S A. 1998;95:10050–10054. doi: 10.1073/pnas.95.17.10050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tollefsen S, rentz-Hansen H, Fleckenstein B, Molberg O, Raki M, Kwok WW, Jung G, Lundin KEA, Sollid LM. HLA-DQ2 and -DQ8 signatures of gluten T cell epitopes in celiac disease. J Clin Invest. 2006;116:2226–2236. doi: 10.1172/JCI27620. This study shows that although DQ8 molecules present some of the same gliadin-derived peptides as DQ2, DQ8-restricted T cell epitopes have different deamidation requirements (in P1 or P9) thanDQ2 (in P4, P6 or P7).
- 42.Vader LW, de Ru A, van der WY, Kooy YM, Benckhuijsen W, Mearin ML, Drijfhout JW, van Veelen P, Koning F. Specificity of tissue transglutaminase explains cereal toxicity in celiac disease. J Exp Med. 2002;195:643–649. doi: 10.1084/jem.20012028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fleckenstein B, Molberg O, Qiao SW, Schmid DG, der Mulbe F, Elgstoen K, Jung G, Sollid LM. Gliadin T cell epitope selection by tissue transglutaminase in celiac disease. J Biol Chem. 2002;277:34109–34116. doi: 10.1074/jbc.M204521200. [DOI] [PubMed] [Google Scholar]
- 44. Dørum S, Qiao SW, Sollid LM, Fleckenstein B. Quantification of transglutaminase 2-mediated deamidation of gluten peptides by mass spectrometry. J Proteome Res. 2009:8. doi: 10.1021/pr800960n. This paper shows large variations in the rate of TG2-mediated deamidation between different gluten-derived peptides and suggests that the rate of deamidation by TG2 is a factor that influences the T cell response to gluten in CeD.
- 45.Stepniak D, Wiesner M, de Ru AH, Moustakas AK, Drijfhout JW, Papadopoulos GK, van Veelen PA, Koning F. Large-scale characterization of natural ligands explains the unique gluten-binding properties of HLA-DQ2. J Immunol. 2008;180:3268–3278. doi: 10.4049/jimmunol.180.5.3268. [DOI] [PubMed] [Google Scholar]
- 46.Bergseng E, Sidney J, Sette A, Sollid LM. Analysis of the binding of gluten T-cell epitopes to various human leukocyte antigen class II molecules. Hum Immunol. 2008;69:94–100. doi: 10.1016/j.humimm.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 47. Henderson KN, Tye-Din JA, Reid HH, Chen Z, Borg NA, Beissbarth T, Tatham A, Mannering SI, Purcell AW, Dudek NL, et al. A structural and immunological basis for the role of human leukocyte antigen DQ8 in celiac disease. Immunity. 2007;27:23–34. doi: 10.1016/j.immuni.2007.05.015. In this study, this first crystal structure of DQ8 molecule in complex with a gliadin peptide (QQYPSGEGSFQPSQENPQ) was resolved. It showed that the glutamates at P1 and P9 formed buried salt-bridging interactions with DQ8.