Abstract
Vilazodone is a potent selective serotonin reuptake inhibitor and serotonin 1A receptor partial agonist approved for the treatment of major depressive disorder in adults. To assess the efficacy of vilazodone across a range of symptoms and severities of depression, data from two phase III, 8-week, randomized, double-blind, placebo-controlled trials were pooled for analysis. Overall improvement in depressive symptoms measured using the Montgomery–Åsberg Depression Rating Scale (MADRS) and the 17-item Hamilton Depression Rating Scale was statistically significant (P<0.05) for vilazodone treatment compared with placebo as early as Week 1 and continued throughout double-blind treatment. Vilazodone treatment compared with placebo showed significant improvement on all 10 individual MADRS symptom items at end of treatment (P<0.01). Rates of response and remission were significantly greater in the vilazodone group relative to the placebo group, with numbers needed to treat ranging from eight to nine for response and 12–17 for remission. Between-group treatment differences in MADRS and the other outcome measures were similar among all depression subgroups, with no consistent pattern associated with depression severity. These findings support the efficacy of vilazodone across a broad range of depressive symptoms and severities for the treatment of major depressive disorder.
Keywords: antidepressant, major depressive disorder, selective serotonin reuptake inhibitors, serotonin 1A receptor agonists, vilazodone
Introduction
Major depressive disorder (MDD) is a chronic and debilitating disorder with high rates of medical and psychiatric comorbidity, functional impairment, and significant personal and societal costs (Greenberg et al., 2003; Egede, 2007; Katon et al., 2007; Daly et al., 2010). The heterogeneous symptoms of MDD include sad mood, loss of interest, sleep disturbance, change in appetite, lack of energy, difficulty concentrating, and psychomotor agitation (American Psychiatric Association, 2000). Depression can be characterized as mild, moderate, or severe on the basis of symptom severity, functional impairment, and level of patient distress. Typically, depression severity in clinical trials is categorized by a cutoff score on a depression rating scale such as the Montgomery–Åsberg Depression Rating Scale (MADRS; Montgomery and Asberg, 1979) or Hamilton Depression Rating Scale (HAMD; Hamilton, 1960); threshold scores greater than 28–30 on MADRS or greater than 25–28 on the 17-item HAMD (HAMD17) are commonly, but somewhat arbitrarily, used to define severe depression (Nemeroff, 2007). Approximately one-third of patients with MDD are severely depressed (Thase, 2000); patients with severe depression tend to have a prolonged course of illness, higher rates of morbidity and mortality, less likelihood of spontaneous remission, and recurrent episodes with early relapse (Thase, 2000; Nemeroff, 2007).
Many patients do not fully respond to initial pharmacologic treatment and experience residual depressive symptoms with a poorer long-term prognosis (Zajecka, 2003). Fewer than half of the patients typically attain response (≥50% improvement) or remission (full resolution of symptoms) during initial treatment. In the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study, a large multicenter study on antidepressant treatments conducted in ‘real-world’ clinical settings, patients who received the selective serotonin reuptake inhibitor (SSRI), citalopram, during the initial treatment step had a 47% response rate and a 33% remission rate after 14 weeks of treatment (Trivedi et al., 2006). In addition, among fully remitted patients, 90% still had at least one residual symptom of depression, with the number of residual symptoms being associated with a higher probability of relapse (Nierenberg et al., 2010). These findings point to the need for additional treatment options that offer broad efficacy across diverse MDD populations.
Initial reports of possible synergy between drugs that affect SSRIs and serotonin 1A (5-HT1A) receptors were published in three case studies on treatment-resistant depression in 1991 (Bakish, 1991). Vilazodone, a potent SSRI and 5-HT1A receptor partial agonist, was approved by the US Food and Drug Administration in 2011 for the treatment of MDD in adults. Preclinical evidence has suggested that the partial agonism of vilazodone at the 5-HT1A receptor may increase endogenous serotonin levels more than an SSRI alone (Hughes et al., 2005). The efficacy of vilazodone for the treatment of MDD was shown in two phase III, 8-week, randomized, double-blind, placebo-controlled trials (NCT00683592 and NCT00285376; Rickels et al., 2009; Khan et al., 2011). In both trials, significant improvement versus placebo was observed on the primary efficacy measure, MADRS total score change from baseline to the end of treatment. Vilazodone was generally well tolerated in both studies; diarrhea and nausea, the most common adverse events, were predominantly mild or moderate in intensity, tended to occur early and were transient, and resulted in few discontinuations from treatment (Liebowitz et al., 2011).
Improvement was also observed in the individual trials by significant differences in favor of vilazodone versus placebo on the HAMD17, the Hamilton Anxiety Rating Scale (HAMA; Hamilton, 1959), and the Clinical Global Impressions-Severity (CGI-S) and Clinical Global Impressions-Improvement (CGI-I) Scales (Guy, 1976). The long-term safety and tolerability of vilazodone were supported by a 1-year open-label study (NCT00644358; Robinson et al., 2011). To assess the efficacy of vilazodone across a range of depressive symptoms and severities, data from the two phase III, 8-week, randomized, double-blind, placebo-controlled trials were combined and analyzed.
Methods
Data from two 8-week, randomized, double-blind, placebo-controlled, multicenter studies (Rickels et al., 2009; Khan et al., 2011) on the use of 40 mg/day vilazodone for the treatment of MDD in adults were pooled for this analysis. The studies by Rickels et al. (2009) and Khan et al. (2011) were conducted at 10 and 15 US sites, respectively, in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The final study protocols were approved by appropriate ethics committees; all patients provided written informed consent. Details of study designs and statistical methods have been reported previously (Rickels et al., 2009; Khan et al., 2011). After washout and screening, patients were randomized (1 : 1) to receive vilazodone or placebo for 8 weeks of double-blind treatment. The dosage of vilazodone was increased to the 40-mg target dose (given once daily with food) on a fixed-dose schedule of 10 mg for 7 days, followed by 20 mg for 7 days, and 40 mg for the remainder of the studies. Efficacy was assessed at baseline (Week 0) and at Weeks 1, 2, 4, 6, and 8/end of treatment (EOT).
Inclusion and exclusion criteria
Eligible patients were 18–70 years of age, with a diagnosis of single-episode or recurrent MDD according to the Diagnostic and Statistical Manual of Mental Disorders, 4th ed., text revision criteria (American Psychiatric Association, 2000); the diagnosis was confirmed by Mini-International Neuropsychiatric Interview (MINI; Sheehan et al., 1998). Patients had a major depressive episode at least 4 weeks, but less than 2 years, in duration, a HAMD17 score of 22 or higher, and a HAMD17 item 1 (depressed mood) score of 2 or higher.
Patients were excluded if they had an Axis I disorder other than MDD; patients with generalized anxiety disorder, social phobia, or simple phobia were allowed for inclusion. Additional exclusion criteria included a history of schizophrenia, schizoaffective disorder, and bipolar I or II disorder; substance abuse (prior 3 months) or dependence (prior 6 months); or MDD with postpartum onset, psychotic features, or a seasonal pattern. Patients with significant comorbid medical or physical conditions that might interfere with trial participation were also excluded at the investigator’s discretion.
Efficacy assessments
Post-hoc analyses of pooled data included evaluation of change from baseline to EOT in MADRS total and individual symptom ratings, and in HAMD17, HAMA, CGI-S, and CGI-I scores at each study visit. EOT response (MADRS or HAMD17≥50% decrease from baseline) and remission (MADRS total score≤10 or HAMD score≤7) were assessed and the respective numbers needed to treat (NNTs) were calculated.
Statistical analyses
The intent-to-treat (ITT) population consisted of all patients in the safety population (randomized patients receiving study drug) who underwent postbaseline MADRS assessment. Patients were stratified by baseline MADRS scores into three severity subgroups: moderate (MADRS<30), moderate-to-severe (30≤MADRS<35), and severe (MADRS≥35) depression subgroups.
All analyses were based on the ITT dataset using the last observation carried forward approach to impute missing data. Treatment group differences were based on the least squares mean difference and 95% confidence intervals. Continuous efficacy variables were analyzed using an analysis of covariance model with treatment group and pooled study center as factors, and the corresponding baseline efficacy score as a covariate (baseline CGI-S score was used as an explanatory variable for the analysis of the CGI-I score). Categorical efficacy variables were analyzed using a logistic regression model with treatment group and study ID as explanatory variables. All statistical comparisons were two sided and generated nominal P-values without adjustment for multiplicity.
Results
Patient disposition
A total of 891 patients were randomized to receive vilazodone (n=445) or placebo (n=446). The safety population consisted of 869 patients receiving the study drug (placebo=433; vilazodone=436); 863 of these patients (placebo=432; vilazodone=431) underwent postbaseline MADRS assessment (ITT population). Overall, 349 (80.6%) patients in the placebo group and 345 (79.1%) patients in the vilazodone group completed treatment.
Baseline demographics and clinical characteristics
Patient baseline demographics and clinical characteristics are presented in Table 1. Approximately one-third of patients were experiencing a first lifetime MDD episode and most patients had a current depressive episode of 12 months or less. The mean baseline MADRS score was 31.4, indicative of a patient population with baseline depression symptoms that were in the moderate-to-severe depression range.
Table 1.
Pooled baseline demographics and disease characteristics
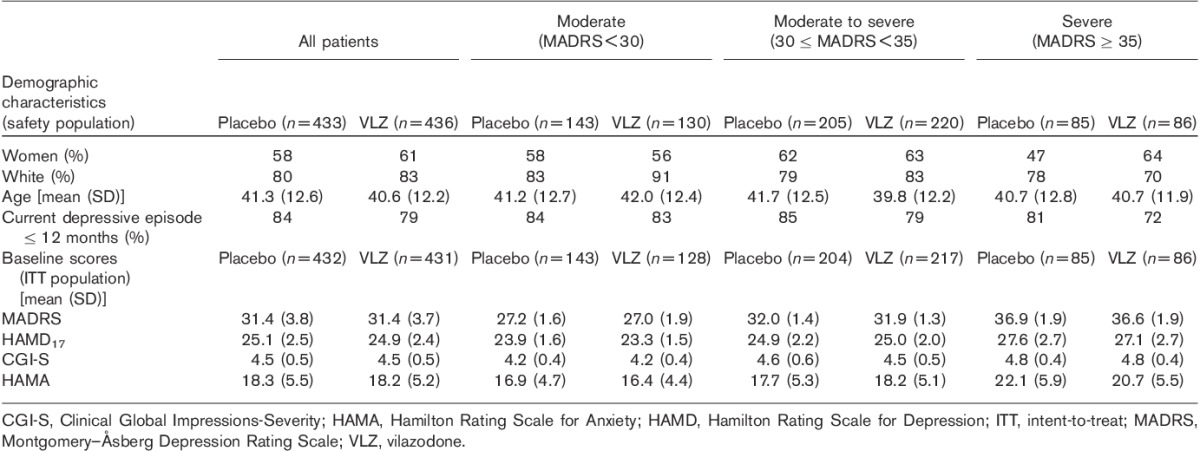
At baseline, 31% of patients had moderate depression (MADRS<30), 49% had moderate-to-severe depression (30≤MADRS<35), and 20% had severe depression (MADRS≥35). Baseline demographic characteristics were similar across depression severity subgroups. A slightly higher percentage of patients with a current MDD episode duration of greater than 12 months were in the severe depression subgroup (23%) compared with the moderate (17%) or moderate-to-severe (18%) depression subgroups. The mean MADRS, HAMD17, CGI-S, and HAMA scores generally increased with increasing depression severity (Table 1).
Efficacy
In the pooled analysis, least squares mean improvement from baseline to EOT in MADRS was significantly greater (P<0.0001) for patients treated with vilazodone compared with placebo (Table 2). Statistically significant differences favoring vilazodone over placebo were evident at the earliest measurement after baseline (Week 1) and continued throughout double-blind treatment (P<0.01, all weeks).
Table 2.
Change from baseline to end of treatment in efficacy assessmentsa
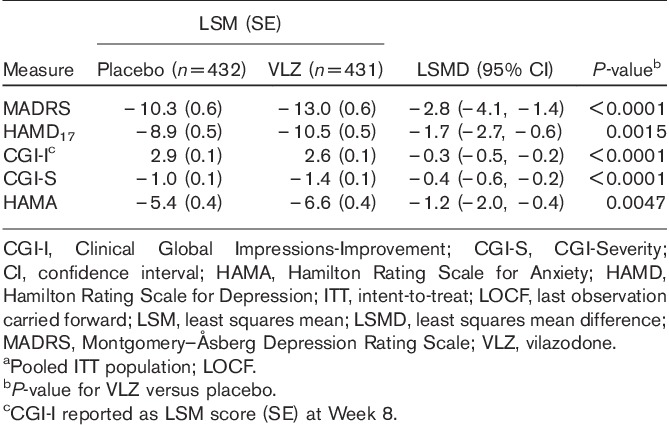
Significant treatment differences from baseline to EOT favoring vilazodone over placebo were also observed for HAMD17 (P<0.05) and CGI-S (P<0.01; Table 2); separation from placebo occurred as early as Week 1 and continued throughout treatment. Improvement in HAMA scores was also significantly greater (P<0.01) for vilazodone versus placebo beginning at Week 6 until EOT. CGI-I favored vilazodone over placebo at all postbaseline visits (P<0.01) and at EOT (P<0.0001; Table 2).
Montgomery–Åsberg Depression Rating Scale individual depressive symptoms analysis
Analyses of MADRS single items examined treatment effects of vilazodone on individual symptoms of depression. Vilazodone treatment compared with placebo treatment resulted in significant improvement (P<0.01) in each of the 10 MADRS symptoms of depression (Fig. 1).
Fig. 1.
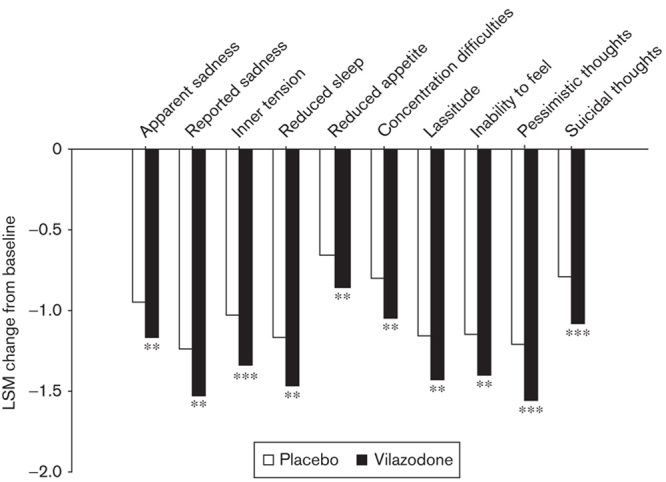
Least squares mean change in MADRS single items at the end of treatment. Pooled ITT population; LOCF. **P<0.01 versus placebo; ***P<0.001 versus placebo. ITT, intent-to-treat; LOCF, last observation carried forward; LSM, least squares mean; MADRS, Montgomery–Åsberg Depression Rating Scale.
Depression severity subgroup analyses
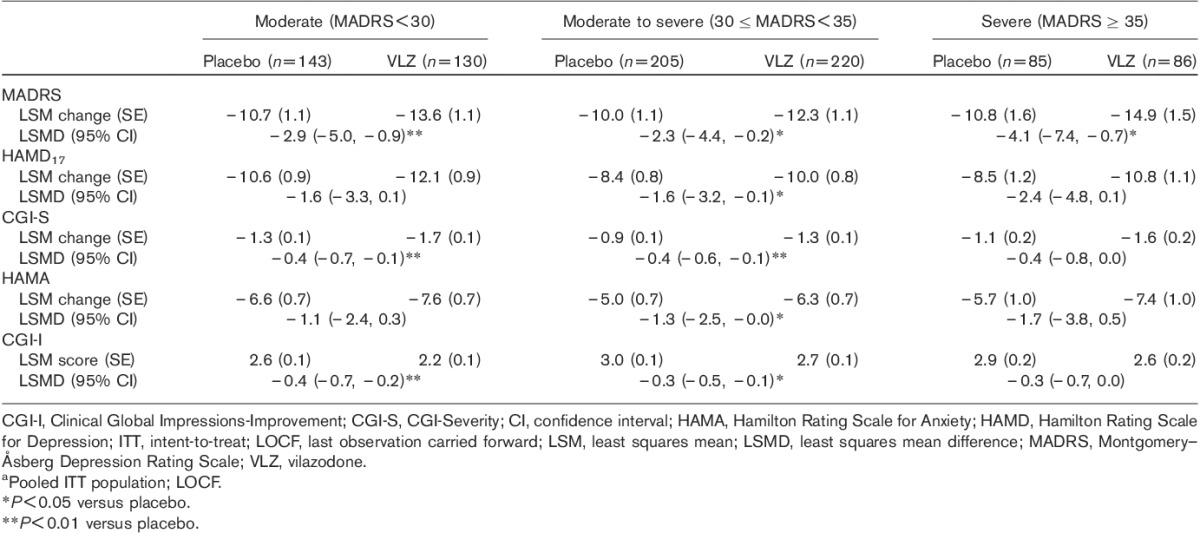
For each depression severity subgroup, MADRS total score improvement from baseline to EOT was significantly greater for vilazodone treatment relative to placebo, with no apparent trend toward greater improvement with increasing baseline depression severity (Table 3). With regard to the secondary efficacy measures, the pattern of improvement indicated by between-group differences across severity subgroups for vilazodone relative to placebo was similar to that observed for the primary MADRS outcome measure (Table 3).
Table 3.
Additional efficacy assessments by baseline depression severity subgroup at the end of treatmenta
Clinical relevance
Vilazodone-treated patients compared with placebo-treated patients had significantly higher response and remission rates at EOT on the basis of both MADRS and HAMD17 criteria (Fig. 2). The NNTs (95% confidence interval) for response were eight (5–17) for MADRS and nine (6–21) for HAMD17; for remission, the NNTs were 12 (7–37) for MADRS and 17 (9–295) for HAMD17.
Fig. 2.
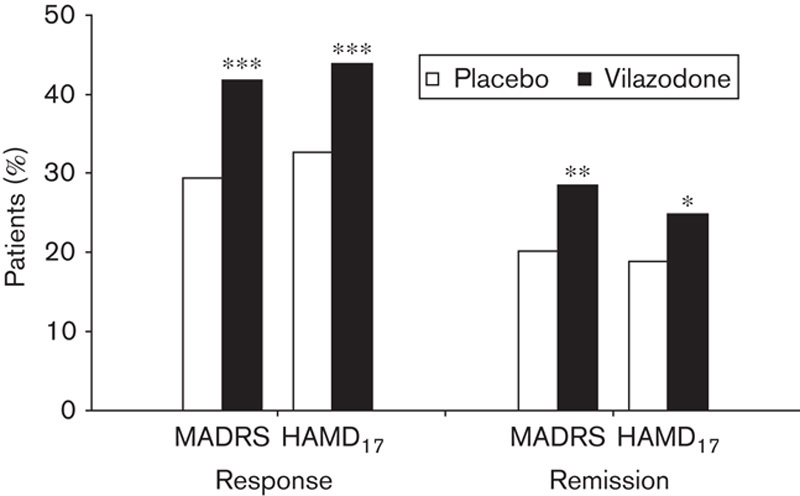
Response and remission rates at the end of treatment. Pooled ITT population; LOCF. *P<0.05, **P<0.01, ***P<0.001. Response is defined as a decrease of 50% or greater from baseline to end of treatment in MADRS or HAMD17 total score. Remission is defined as an end-of-treatment MADRS total score≤10 or HAMD17 score≤7. HAMD17, 17-item Hamilton Depression Rating Scale; ITT, intent-to-treat; LOCF, last observation carried forward; MADRS, Montgomery–Åsberg Depression Rating Scale.
Discussion
Findings from the pooled analyses of data from two positive, 8-week, placebo-controlled, randomized, double-blind trials show a consistent pattern of efficacy for 40 mg vilazodone compared with placebo across a range of depression symptoms and severities. The 2.8-point mean difference in MADRS score at EOT favoring vilazodone over placebo is indicative of clinically relevant improvement in depression (Montgomery and Moller, 2009). Statistically significant and clinically relevant improvements in MADRS total score at Week 8 were observed with vilazodone compared with placebo regardless of the severity of baseline depression symptoms. The MADRS treatment advantage for vilazodone versus placebo (least squares mean difference) was greater than 2 for all severity subgroups, supporting the clinical relevance of treatment across the range of depression severities (Montgomery and Moller, 2009). Between-group treatment differences were generally similar for other efficacy outcome measures across the range of depression severity subsets, with no apparent trends among subgroups. Although a prior meta-analysis has suggested that antidepressant treatment is most efficacious in patients with the most severe symptoms (Kirsch et al., 2008), the results of these analyses support the therapeutic benefit of vilazodone across a spectrum of depression severities. A retrospective analysis of randomized placebo-controlled trials conducted in 2012 concurred with our results, finding significant antidepressant efficacy for patients with mild–moderate MDD (Stewart et al., 2012).
Depression symptom improvement, as measured by both MADRS total score and HAMD17, was apparent for vilazodone treatment compared with placebo by the first postbaseline assessment (Week 1) and continued throughout treatment across a range of depression severities. Patients who experience clinically meaningful improvement (i.e. ≥20% improvement from baseline) in depressive symptoms in the first 2 weeks of treatment have a greater likelihood of stable treatment response and remission of symptoms (Szegedi et al., 2009). Early improvement (e.g. within 2 weeks) may enhance treatment compliance and lessen relapse or recurrence (Demyttenaere et al., 2001; Taylor et al., 2006; Szegedi et al., 2009). The results of these analyses are interesting considering that vilazodone treatment compared with placebo treatment led to a statistically significant improvement in depressive symptoms as early as the first week of treatment despite the fact that patients did not attain full therapeutic dosage of vilazodone until Week 3 of the 8-week double-blind treatment period.
Vilazodone treatment compared with placebo treatment led to a statistically significant improvement in all 10 individual MADRS symptom items. The improvement in the ‘suicidal thoughts’ item was unanticipated because patients with suicidal ideation, recent suicidal attempt, or suicidal risk were excluded from the trials. Efficacy across a broad range of depression symptoms may lessen the likelihood of residual symptoms, which is associated with a risk for relapse, greater psychosocial impairment, and reduced quality of life (Menza et al., 2003).
Vilazodone compared with placebo also significantly improved overall anxiety symptoms on the basis of HAMA scores. It is possible that the combined property of serotonin reuptake inhibition and 5-HT1A partial agonism of vilazodone leads to a broader antidepressant efficacy with enhanced anxiolytic efficacy in MDD patients compared with serotonin reuptake inhibition alone (Blier and Ward, 2003; Sussman, 2003; Papakostas et al., 2007).
The clinical relevance of improvement in depression symptoms with vilazodone was shown by an absolute treatment difference of greater than 10% compared with placebo on all response rate criteria (Montgomery and Moller, 2009). NNT values for response of eight and nine further suggest the clinical relevance of these data as an NNT of less than 10 represents the threshold for demonstrating clinically relevant response to antidepressant treatment (Cipriani et al., 2006).
Remission to an asymptomatic state is considered by many to be the ultimate standard of efficacy for antidepressant drugs in depressive disorders. In these analyses, absolute differences in remission rates for vilazodone versus placebo ranged from 6 to 9%, corresponding to NNTs of 12–17 for remission. A recent review of vilazodone that used a MADRS total score of less than 10 as the remission criterion reported remission rates of 25.4% for vilazodone and 18.1% for placebo, with an NNT of 14 (Citrome, 2012). MADRS remission rates in our analysis (MADRS total score≤10), 28.6% for vilazodone and 20.1% for placebo (NNT=12), were slightly higher than the rates observed in the study by Citrome (2012) (MADRS total score<10). This may reflect the slightly less stringent but more common definition of remission used in our analysis (Zimmerman et al., 2004), in which patients with a MADRS total score of 10, as opposed to a maximum score of 9, are categorized as remitters. In addition, it is possible that the 8-week duration of the trials may have been insufficient for some patients to attain remission of symptoms, and more patients might have remitted with longer treatment; this may be especially true for the more severely depressed patients with high baseline MADRS scores (Schatzberg, 1999).
The two clinical trials with almost identical study designs and patient populations facilitate the pooling of data for increased statistical power to assess additional treatment effects of clinical interest, and provide more precise estimates with large enough sample sizes for subgroup analyses. Limitations of these analyses include lack of head-to-head comparison with other antidepressants and more stringent entry criteria that may limit generalizability. In addition, the cutoff score used to denote severe depression in this analysis (MADRS≥35) is more stringent than the score often used in clinical trials (MADRS≥30; Nemeroff, 2007), resulting in a sample size that may be insufficient to detect significant treatment differences in the subgroup designated as severely depressed. Finally, these analyses were carried out post hoc and did not correct for multiple comparisons.
Vilazodone treatment was significantly superior to placebo treatment on all efficacy outcome measures, with consistent efficacy across a range of depression severities in MDD patients. Clinically relevant advantages of vilazodone treatment over placebo treatment were evident in overall measures of depression, individual depressive symptoms, and antidepressant response and remission rates.
Acknowledgements
This work was supported by Forest Research Institute, Jersey City, NJ, USA. The authors thank Prescott Medical Communications Group, Chicago, IL, USA, for editorial assistance and technical writing.
Conflicts of interest
Dr Khan is the Medical Director of the Northwest Clinical Research Center (NWCRC). NWCRC was an investigative site for the vilazodone phase III studies randomizing 241 patients. He has served as an uncompensated advisor to Forest Research and is not compensated for his role as author of medical manuscripts. Dr Khan is also the Medical Director of Columbia Northwest Pharmaceuticals, which owns intellectual property rights for potential therapies for central nervous system disorders and other medical conditions. Dr Sambunaris has received consultant and speaking fees from Forest Research Institute Inc., as well as Takeda Pharmaceuticals and Mylan Pharmaceuticals. Angelo Sambunaris has received research support from Alkermes, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, BrainCells, CeNeRx, Cephalon, Forest Pharmaceuticals, GlaxoSmithKline, Jazz, Johnson & Johnson Labopharm, Lilly, Lundbeck, MediciNova, Merck, Neurocrine, Novartis, Otsuka, Pfizer, Roche, Sanofi-Aventis, Sepracor/Sunovion, Shire, and Takeda. He has also served as a speaker for Forest Pharmaceuticals and as a consultant for Forest, Mylan, and Takeda. He was also a principal investigator in both vilazodone phase III studies. Dr Edwards is a full-time employee of Forest Laboratories Inc. Dr Ruth is an employee of Prescott Medical Communications Group, a contractor for Forest Research Institute. Dr Robinson has served as a consultant to Forest Laboratories, PGxHealth, Dey Pharmaceuticals, Mylan Pharmaceuticals, and Ironwood Pharmaceuticals. This study was funded by PGxHealth LLC, a subsidiary of Clinical Data Inc. (acquired by Forest Laboratories Inc.). PGxHealth LLC and Forest Laboratories Inc. were involved in the study design; collection, analysis, and interpretation of data; writing of the report; and decision to submit the paper for publication. Editorial assistance was provided by Prescott Medical Communications and was funded by Forest Laboratories Inc.
Footnotes
Correspondence to Arif Khan, Northwest Clinical Research Center, 1900 116th Avenue NE #112, Bellevue, WA 98004, USA Tel: +1 425 453 0404; fax: +1 425 453 1033; e-mail: akhan@nwcrc.net
References
- American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders 2000:Fourth edition, text revisionWashington, DC:American Psychiatric Association [Google Scholar]
- Bakish D.Fluoxetine potentiation by buspirone: three case histories.Can J Psychiatry 1991;36:749–750 [DOI] [PubMed] [Google Scholar]
- Blier P, Ward NM.Is there a role for 5-HT1A agonists in the treatment of depression?Biol Psychiatry 2003;53:193–203 [DOI] [PubMed] [Google Scholar]
- Cipriani A, Barbui C, Brambilla P, Furukawa TA, Hotopf M, Geddes JR.Are all antidepressants really the same? The case of fluoxetine: a systematic review.J Clin Psychiatry 2006;67:850–864 [DOI] [PubMed] [Google Scholar]
- Citrome L.Vilazodone for major depressive disorder: a systematic review of the efficacy and safety profile for this newly approved antidepressant – what is the number needed to treat, number needed to harm and likelihood to be helped or harmed?Int J Clin Pract 2012;66:356–368 [DOI] [PubMed] [Google Scholar]
- Daly EJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Gaynes BN, Warden D, et al. Health-related quality of life in depression: a STAR*D report.Ann Clin Psychiatry 2010;22:43–55 [PubMed] [Google Scholar]
- Demyttenaere K, Enzlin P, Dewe W, Boulanger B, De Bie J, De Troyer W, Mesters P.Compliance with antidepressants in a primary care setting, 2: the influence of gender and type of impairment.J Clin Psychiatry 2001;62Suppl 2234–37 [PubMed] [Google Scholar]
- Egede LE.Major depression in individuals with chronic medical disorders: prevalence, correlates and association with health resource utilization, lost productivity and functional disability.Gen Hosp Psychiatry 2007;29:409–416 [DOI] [PubMed] [Google Scholar]
- Greenberg PE, Kessler RC, Birnbaum HG, Leong SA, Lowe SW, Berglund PA, Corey-Lisle PK.The economic burden of depression in the United States: how did it change between 1990 and 2000?J Clin Psychiatry 2003;64:1465–1475 [DOI] [PubMed] [Google Scholar]
- Guy W.The Clinician Global Severity and Impression Scales.ECDEU Assessment Manual for Psychopharmacology. US Department of Health, Education and Welfare publication (ADM), National Institute of Mental Health 1976Rockville, MD:National Institute of Mental Health. 218–222 [Google Scholar]
- Hamilton M.The assessment of anxiety states by rating.Br J Med Psychol 1959;32:50–55 [DOI] [PubMed] [Google Scholar]
- Hamilton M.A rating scale for depression.J Neurol Neurosurg Psychiatry 1960;23:56–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes ZA, Starr KR, Langmead CJ, Hill M, Bartoszyk GD, Hagan JJ, et al. Neurochemical evaluation of the novel 5-HT1A receptor partial agonist/serotonin reuptake inhibitor, vilazodone.Eur J Pharmacol 2005;510:49–57 [DOI] [PubMed] [Google Scholar]
- Katon W, Lin EH, Kroenke K.The association of depression and anxiety with medical symptom burden in patients with chronic medical illness.Gen Hosp Psychiatry 2007;29:147–155 [DOI] [PubMed] [Google Scholar]
- Khan A, Cutler AJ, Kajdasz DK, Gallipoli S, Athanasiou M, Robinson DS, et al. A randomized, double-blind, placebo-controlled, 8-week study of vilazodone, a serotonergic agent for the treatment of major depressive disorder.J Clin Psychiatry 2011;72:441–447 [DOI] [PubMed] [Google Scholar]
- Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT.Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration.PLoS Med 2008;5:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebowitz M, Croft HA, Kajdasz DK, Whalen H, Gallipoli S, Athanasiou M, Reed CR.The safety and tolerability profile of vilazodone, a novel antidepressant for the treatment of major depressive disorder.Psychopharmacol Bull 2011;44:15–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menza M, Marin H, Opper RS.Residual symptoms in depression: can treatment be symptom-specific?J Clin Psychiatry 2003;64:516–523 [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M.A new depression scale designed to be sensitive to change.Br J Psychiatry 1979;134:382–389 [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Moller HJ.Is the significant superiority of escitalopram compared with other antidepressants clinically relevant?Int Clin Psychopharmacol 2009;24:111–118 [DOI] [PubMed] [Google Scholar]
- Nemeroff CB.The burden of severe depression: a review of diagnostic challenges and treatment alternatives.J Psychiatr Res 2007;41:189–206 [DOI] [PubMed] [Google Scholar]
- Nierenberg AA, Husain MM, Trivedi MH, Fava M, Warden D, Wisniewski SR, et al. Residual symptoms after remission of major depressive disorder with citalopram and risk of relapse: a STAR*D report.Psychol Med 2010;40:41–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papakostas GI, Thase ME, Fava M, Nelson JC, Shelton RC.Are antidepressant drugs that combine serotonergic and noradrenergic mechanisms of action more effective than the selective serotonin reuptake inhibitors in treating major depressive disorder? A meta-analysis of studies of newer agents.Biol Psychiatry 2007;62:1217–1227 [DOI] [PubMed] [Google Scholar]
- Rickels K, Athanasiou M, Robinson DS, Gibertini M, Whalen H, Reed CR.Evidence for efficacy and tolerability of vilazodone in the treatment of major depressive disorder: a randomized, double-blind, placebo-controlled trial.J Clin Psychiatry 2009;70:326–333 [DOI] [PubMed] [Google Scholar]
- Robinson DS, Kajdasz DK, Gallipoli S, Whalen H, Wamil A, Reed CR.A 1-year, open-label study assessing the safety and tolerability of vilazodone in patients with major depressive disorder.J Clin Psychopharmacol 2011;31:643–646 [DOI] [PubMed] [Google Scholar]
- Schatzberg AF.Antidepressant effectiveness in severe depression and melancholia.J Clin Psychiatry 1999;60Suppl 414–21Discussion 22 [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10.J Clin Psychiatry 1998;59Suppl 2022–33Quiz 34–57 [PubMed] [Google Scholar]
- Stewart JA, Deliyannides DA, Hellerstein DJ, McGrath PJ, Stewart JW.Can people with nonsevere major depression benefit from antidepressant medication?J Clin Psychiatry 2012;73:518–525 [DOI] [PubMed] [Google Scholar]
- Sussman N.Depression and painful physical symptoms.Prim Care Companion J Clin Psychiatry 2003;5:19–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szegedi A, Jansen WT, van Willigenburg AP, van der Meulen E, Stassen HH, Thase ME.Early improvement in the first 2 weeks as a predictor of treatment outcome in patients with major depressive disorder: a meta-analysis including 6562 patients.J Clin Psychiatry 2009;70:344–353 [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Freemantle N, Geddes JR, Bhagwagar Z.Early onset of selective serotonin reuptake inhibitor antidepressant action: systematic review and meta-analysis.Arch Gen Psychiatry 2006;63:1217–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thase ME.Treatment of severe depression.J Clin Psychiatry 2000;61Suppl 117–25 [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice.Am J Psychiatry 2006;163:28–40 [DOI] [PubMed] [Google Scholar]
- Zajecka JM.Treating depression to remission.J Clin Psychiatry 2003;64Suppl 157–12 [PubMed] [Google Scholar]
- Zimmerman M, Posternak MA, Chelminski I.Derivation of a definition of remission on the Montgomery–Asberg Depression Rating Scale corresponding to the definition of remission on the Hamilton rating scale for depression.J Psychiatr Res 2004;38:577–582 [DOI] [PubMed] [Google Scholar]


