Abstract
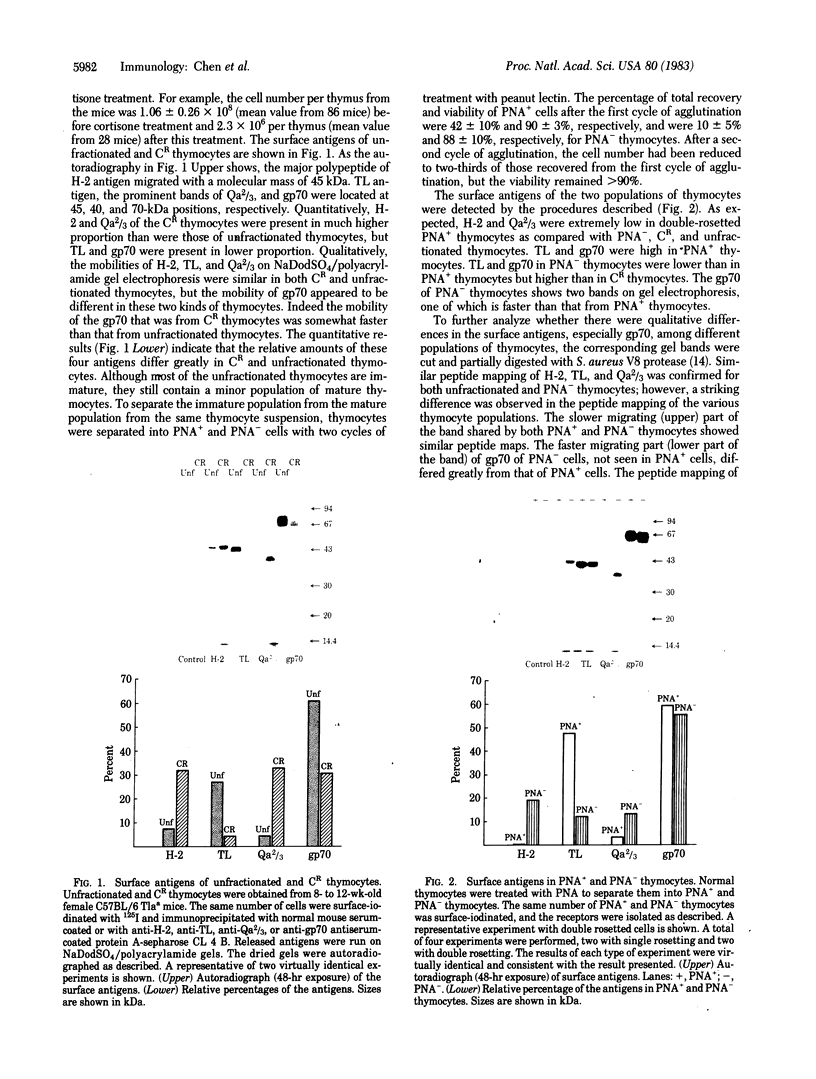
Peanut agglutinin-positive thymocytes, peanut agglutinin-negative thymocytes, cortisone-resistant thymocytes, and unfractionated thymocytes were prepared from congeneic C57BL/6 Tla mice. By using surface iodination and immunoprecipitation of solubilized antigen with specific antisera (e.g., anti-H-2D, anti-TL, anti-Qa2/3, and anti-gp70), the released specific antigens were electrophoresed on polyacrylamide gels, and their radioactivity was measured. The relative percentages of surface antigens H-2D, TL, Qa2/3, and gp70 were 3.2%, 47.5%, 2.5%, and 46.8%, respectively, for peanut agglutinin-positive thymocytes; 31.8%, 4.4%, 32.7%, and 31.1%, respectively, for cortisone-resistant thymocytes; 13.2%, 28.7%, 12.3%, and 45.8%, respectively, for peanut agglutinin-negative thymocytes; and 7.7%, 27.1%, 4.3%, and 60.9%, respectively, for unfractionated thymocytes. After incubation with thymosin fraction V or T-cell growth factor (interleukin II) for 20 hr, the changes in surface antigens of peanut agglutinin-positive thymocytes closely correlated with their normal maturation (i.e., H-2D increases and TL decreases). Thymic factors (e.g., thymosin alpha 1, thymopoietin pentapeptide, facteur thymic serique) had only small or no effects on surface antigens of peanut agglutinin-positive thymocytes. The results suggest that peptides yet to be identified in thymosin fraction V may play an important role in intrathymic evolution and that T-cell growth factor is possibly a peripheral signal derived from activated T cells that modulates T-cell receptors and may be a critical regulator of intrathymic cellular development.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott J., Doyle P. J., Ngiam K., Olson C. L. Ontogeny of murine T lymphocytes. I. Maturation of thymocytes induced in vitro by tumor necrosis factor-positive serum (TNF+)1,2. Cell Immunol. 1981 Jan 1;57(1):237–250. doi: 10.1016/0008-8749(81)90136-2. [DOI] [PubMed] [Google Scholar]
- Boyse E. A., Old L. J. The immunogenetics of differentiation in the mouse. Harvey Lect. 1978;71:23–53. [PubMed] [Google Scholar]
- Cantor H., Weissman I. Development and function of subpopulations of thymocytes and T lymphocytes. Prog Allergy. 1976;20:1–64. [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Goldstein A. L., Low T. L., McAdoo M., McClure J., Thurman G. B., Rossio J., Lai C. Y., Chang D., Wang S. S., Harvey C. Thymosin alpha1: isolation and sequence analysis of an immunologically active thymic polypeptide. Proc Natl Acad Sci U S A. 1977 Feb;74(2):725–729. doi: 10.1073/pnas.74.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein G., Scheid M. P., Boyse E. A., Schlesinger D. H., Van Wauwe J. A synthetic pentapeptide with biological activity characteristic of the thymic hormone thymopoietin. Science. 1979 Jun 22;204(4399):1309–1310. doi: 10.1126/science.451537. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Reisner Y., Linker-Israeli M., Sharon N. Separation of mouse thymocytes into two subpopulations by the use of peanut agglutinin. Cell Immunol. 1976 Jul;25(1):129–134. doi: 10.1016/0008-8749(76)90103-9. [DOI] [PubMed] [Google Scholar]
- Rothenberg E. Expression of differentiation antigens in subpopulations of mouse thymocytes: regulation at the level of de novo synthesis. Cell. 1980 May;20(1):1–9. doi: 10.1016/0092-8674(80)90228-7. [DOI] [PubMed] [Google Scholar]
- Scheid M. P., Goldstein G., Boyse E. A. The generation and regulation of lymphocyte populations: evidence from differentiative induction systems in vitro. J Exp Med. 1978 Jun 1;147(6):1727–1743. doi: 10.1084/jem.147.6.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid M. P., Hoffmann M. K., Komuro K., Hämmerling U., Abbott J., Boyse E. A., Cohen G. H., Hooper J. A., Schulof R. S., Goldstein A. L. Differentiation of T cells induced by preparations from thymus and by nonthymic agents. J Exp Med. 1973 Oct 1;138(4):1027–1032. doi: 10.1084/jem.138.4.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortman K., Von Boehmer H., Lipp J., Hopper K. Subpopulations of T-lymphocytes. Physical separation, functional specialisation and differentiation pathways of sub-sets of thymocytes and thymus-dependent peripheral lymphocytes. Transplant Rev. 1975;25:163–210. [PubMed] [Google Scholar]
- Smith K. A., Baker P. E., Gillis S., Ruscetti F. W. Functional and molecular characteristics of T-cell growth factor. Mol Immunol. 1980 May;17(5):579–589. doi: 10.1016/0161-5890(80)90156-x. [DOI] [PubMed] [Google Scholar]
- Touraine J. L., Hadden J. W., Good R. A. Sequential stages of human T lymphocyte differentiation. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3414–3418. doi: 10.1073/pnas.74.8.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung J. S., Shen F. W., Viamontes G., Palladino M., Fleissner E. The same genetic locus directs differentiation-linked expression of endogenous retrovirus gp70 on thymocytes and spleen cells in the mouse. Immunogenetics. 1982 Jan;15(1):103–108. doi: 10.1007/BF00375507. [DOI] [PubMed] [Google Scholar]
- Tung J. S., Vitetta E. S., Fleissner E., Boyse E. A. Biochemical evidence linking the GIX thymocyte surface antigen to the gp69/71 envelope glycoprotein of murine leukemia virus. J Exp Med. 1975 Jan 1;141(1):198–205. doi: 10.1084/jem.141.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman I. L. Thymus cell migration. J Exp Med. 1967 Aug 1;126(2):291–304. doi: 10.1084/jem.126.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]