Abstract
Axonal transport is the lifeline of axons and synapses. After synthesis in neuronal cell-bodies, proteins are conveyed into axons in two distinct rate-classes – fast and slow axonal transport. While fast transport delivers vesicular cargoes, slow transport carries cytoskeletal and cytosolic (or soluble) proteins that have critical roles in neuronal structure and function. While significant progress has been made in dissecting the molecular mechanisms of fast vesicle-transport; mechanisms of slow axonal transport are still unclear. Why is this so? Historically, advances in the axonal transport field have mirrored innovations that allow visualization of the phenomena; and slow-transport cargoes are not as readily seen as motile vesicles. However, new ways of imaging slow transport have re-energized the field, and several such cargoes can now be visualized in living axons. This review first summarizes classic studies that characterized all transport components, and then discusses recent technical and conceptual advances in slow axonal transport that have provided insights into some long-standing mysteries.
Keywords: Cytosolic proteins, soluble proteins, slow axonal transport, transport packets, cargo complexes, diffusion
The word “axonal transport” typically invokes an image of tiny vesicles moving up and down axons – a view reinforced by ‘YouTube’ videos. Though evocative, this is a limited portrait of axonal transport. Hidden from this picture, a deluge of cytoskeletal and soluble proteins are also moving along these very same axons, and their role in maintaining axonal and synaptic function is no less important than their vesicular counterparts. Known as slow axonal transport, these cargoes include microtubules, neurofilaments, actin/actin-related proteins, metabolic enzymes, chaperones, various soluble synaptic proteins involved in exo/endocytosis, and even molecular motors like dynein and myosin – making slow transport indispensable for neuronal form and function. For decades, slow axonal transport has been recognized and studied, but has remained difficult to visualize directly. However, significant advances in recent years have begun to reveal some of its secrets. This review first summarizes experiments that defined slow axonal transport and then outlines insights from recent studies that have visualized the phenomenon and uncovered new mechanistic details in the process.
What is Fast and Slow Axonal Transport?
Our understanding of overall axonal transport is largely derived from classic pulse-chase studies, where newly-synthesized perikaryal proteins are tagged by radiolabeled amino-acids, and their movement into the axon is analyzed by autoradiography (see fig. 1A and Roy et al., 2005). Although the bulk of these studies were done over 30 years ago, they characterized the phenomenon in-vivo and provide a template for interpreting contemporary experiments. Key insights from these studies in a variety of organisms (mice, rats, guinea pigs, rabbits, Aplysia and others) are as follows. After perikaryal synthesis, (1) a population is conveyed rapidly at rates of ~ 50–200 mm/day (fast axonal transport); composed of membranous cargoes (fig. 1B). (2) A distinct pool is conveyed at much lower overall rates of ~ 0.2–10 mm/day (slow axonal transport); composed of cytoskeletal proteins (e.g. tubulin, neurofilaments protein, actin/actin-associated proteins, spectrin) as well as hundreds of soluble or cytosolic proteins (fig. 1C). Examples of the latter include metabolic enzymes (e.g. phosphofructokinase, creatine kinase, aldolase, enolase, GAPDH, SOD-1), heat shock proteins (e.g. hsp-70, hsc-73, CCT), proteins involved in synaptic homeostasis (e.g. synapsin, α-synuclein, clathrin, calmodulin, CamK), motor proteins (dynein, dynactin, myosin), and several other cytosolic proteins like ubiquitin, cyclophilin, annexin; as well as many that are not yet identified. In general, tubulin and neurofilament proteins move the slowest – at rates of ~ 0.2–1 mm/day (called “Slow Component-a” or SCa) – whereas actin/actin-associated proteins and cytosolic/soluble proteins move a little faster, at rates of ~ 1–10 mm/day (called “Slow Component-b” or SCb) (Baitinger and Willard, 1987; Black and Lasek, 1980; Brady et al., 1981; Bray et al., 1992; Dillman et al., 1996b; Jensen et al., 1999; Li et al., 2004; Ma et al., 2000; Nixon et al., 1990; Sekimoto et al., 1991; Willard et al., 1974; Yuan et al., 1999). Although soluble/cytosolic molecules have inherent diffusive properties, the coordinated movement of these proteins over large distances in axons is incompatible with free diffusion. In accordance with physical laws, free diffusion of radiolabel exponentially decays over time, and cannot explain any form of slow transport (Koike and Matsumoto, 1985). Many other properties of cytosolic slow axonal transport are also incompatible with diffusion (for instance motor-dependence, see below).
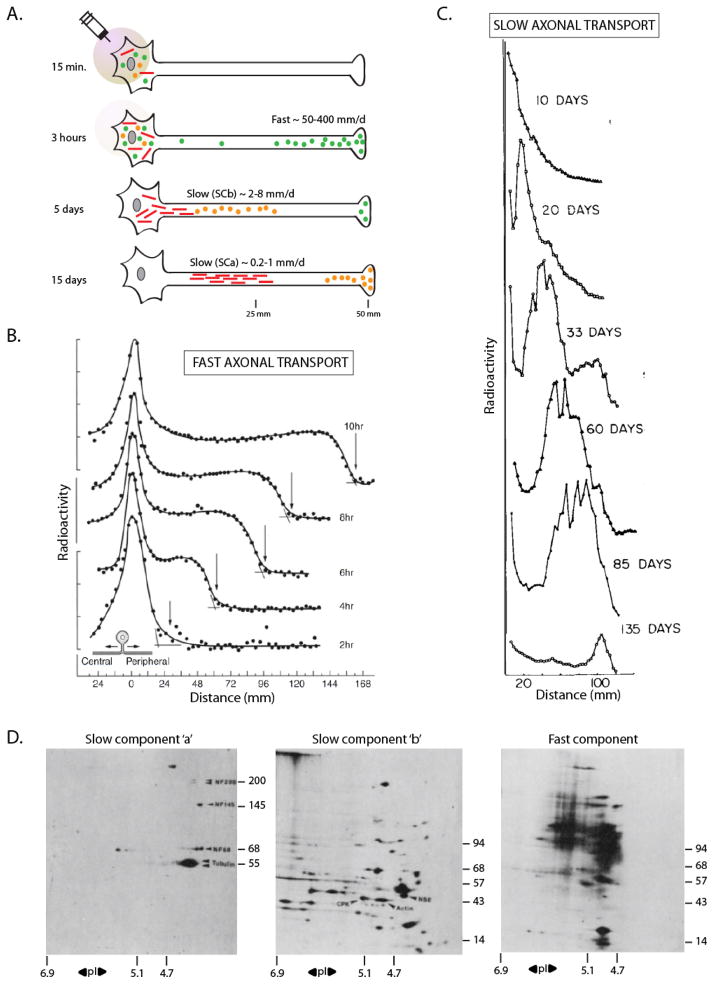
Figure 1. The pulse-chase radiolabeling paradigm to study axonal transport.
(A) Radiolabeled amino acids injected in the vicinity of neuronal cell-bodies of an adult animal are incorporated by newly synthesized proteins, and then transported into axons and distal synapses by endogenous processes. The movement of these proteins is then inferred by analyzing sequential axonal segments over incremental time-periods (for specifics of methods, see figure 2 of Roy et al., 2005). After labeling, a population of proteins (green circles) is rapidly conveyed into axons at rates of 50–400 mm/day (‘fast component’, vesicular cargoes). A second pool enters the axons at velocities that are several orders of magnitude lower at 0.2–8 mm/day (‘slow component’). The slow component can be further resolved into two largely distinct ‘peaks’ composed of cytosolic/soluble cargoes (‘Slow Component-b’ or SCb – orange circles) or the major cytoskeletal cargoes (‘Slow Component-a’ or SCa – red bars).
(B) Kinetics of fast axonal transport in cat sensory axons accessed by pulse-chase radiolabeling. Note the rapid movement of the radiolabeled wave-front along the peripheral axon over 10 hours (~ 4.5 mm/s). Also note the broad plateau behind the advancing ‘front’ suggesting deposition of cargoes (vesicles) during transit.
(C) Kinetics of slow axonal transport in rat motor neurons accessed by pulse-chase radiolabeling. Note the extremely slow movement of the slow component wave-front (~ 100 mm in over 100 days).
(D) 2-D PAGE analysis of the three rate components. Transported radiolabeled proteins from mouse or guinea pig optic axons were separated by mass/charge, and analyzed by autoradiography. Some individual protein ‘spots’ are identified by arrowheads on the gels – neurofilaments (NF) and tubulin (SCa, left); creatine phospho-kinase (CPK), actin and non-specific enolase (NSE, SCb, middle). Note the unique overall composition of the three rate-classes. Isoelectric points are on the x-axis and molecular weights are on the y-axis. Figure (B) adapted from Ochs et al., 1981; figure (C) adapted from Hoffman and Lasek, 1975; figure (D) adapted from Brady and Lasek, 1982 – all with permission.
Cargoes moving in Fast and Slow Axonal Transport
While radiolabeling studies provided a list of proteins moving in the two components, they could not visualize the transport directly, and cargo-structures responsible for fast and slow transport remained unknown. Fast motile structures resembling vesicles were reported in early microscopic studies (reviewed in Grafstein and Forman, 1980), and with advances in transmitted-light microscopy and video-imaging in the 80’s, rapidly-moving vesicles were unequivocally seen in extruded squid axons (Allen et al., 1982; Brady et al., 1982). With subsequent discovery of the motor protein kinesin (Brady, 1985; Vale et al., 1985), it became obvious that the plethora of mobile vesicles was the visual correlate of ‘fast’ radiolabel movement. Investigations spanning the next few decades – and continuing to this day – have provided numerous mechanistic insights into vesicle-transport and this process is understood in detail (Twelvetrees et al., 2012). But while tubulo-vesicular profiles of single vesicles could be pinpointed by their phase-dense silhouettes – or later, by live-imaging of GFP/RFP-tagged membrane-spanning proteins – similar protocols could not be immediately applied to slow component cargoes. The main reason was that the distribution of cytoskeletal and cytosolic proteins is typically continuous along axons, precluding visualization of individual moving structures. Thus in the absence of concrete visual evidence, the cargo-structures conveyed in slow axonal transport was a subject of much debate throughout the 90’s. In particular, the form in which cytoskeletal elements – actin, tubulin and neurofilament protein – were conveyed was a source of contention, with some investigators favoring polymeric form and others a less-defined monomeric/oligomeric form (Vallee and Bloom, 1991; Baas and Brown, 1997; Bray, 1997; Hirokawa et al., 1997).
Axonal transport of Cytoskeletal Polymers: the ‘Stop and Go’ model
The debate over whether cytoskeletal proteins could be transported as polymers or subunits showed its first signs of resolution in 2000, when moving neurofilament polymers were seen in axons (Roy et al., 2000; Wang et al., 2000). Though the neurofilament array in most axons is continuous as mentioned above, it is naturally sparse in very thin axons of some cultured neurons, with ‘gaps’ in distribution where there are no neurofilaments at all. When GFP-tagged neurofilaments were visualized in such axons by live imaging, single neurofilaments were seen to move in the ‘gaps’. Studies over the last decade have resolved many mechanistic aspects of neurofilament transport (Li et al., 2012), including ultrastructural demonstration that these moving assemblies are indeed single neurofilaments (Yan and Brown, 2005); essentially settling reasonable doubt. But more importantly, seeing neurofilaments move revealed a long-standing secret of slow axonal transport.
Surprising at the time of initial discovery, neurofilaments moved rapidly with instantaneous velocities similar to moving vesicles. But compared to vesicles, neurofilament movement was very infrequent, and moreover neurofilaments often paused during transit, unlike the persistent kinetics of motile vesicles. Thus though a single neurofilament moved rapidly, the majority of neurofilaments in an axon (>90%) were paused at any given time (but could potentially move again). This infrequent and intermittent transport behavior of individual neurofilaments would, over time, expectedly result in an overall slow movement of the entire population. Christened the ‘Stop and Go’ model, a substantial amount of evidence supports this concept (Brown et al., 2005; Li et al., 2012). An additional appeal of this model is the implication that the same kinds of “fast” motor proteins could drive both fast and slow axonal transport. This is important, as candidate “slow” motors, capable of producing the velocities of SCa or SCb by continuous engagement, were conspicuously absent from the avalanche of motor protein superfamilies discovered in the 1990s (Miki et al., 2001).
Similarly, structures resembling short microtubules also move infrequently in cultured neurons (Wang and Brown, 2002). This movement is motor-dependent (He et al., 2005), suggesting that microtubules may also be transported in a “stop and go” manner (reviewed in Falnikar and Baas, 2009). Microtubule-movement in other cell-types is well-established, providing a precedent (Keating et al., 1997; Jolly et al., 2010). However unlike neurofilament transport where the evidence is compelling, some aspects of tubulin transport are still ambiguous. For example, it is not clear if microtubules also pause frequently, or how individual polymer movements seen in imaging experiments give rise to the overall slow transport of tubulin seen in radiolabeling studies. Also unlike neurofilaments that are almost entirely present as polymers in steady-state, soluble pools of tubulin also exist in axons, and it has been proposed that soluble tubulin is transported (Galbraith et al., 1999; Terada et al., 2000).
Actin, the third major cytoskeletal protein in neurons, is also conveyed in slow axonal transport; largely in SCb (Willard et al., 1979; Black and Lasek, 1979; Bray et al., 1992). Actin is a key protein in cellular homeostasis, involved in numerous physiologic events in neurons – structural, growth-cone/axon extension, axonal branching, cell-signaling, etc. – yet surprisingly, the mechanistic details of actin transport are almost entirely unknown. The reasons for this gap in knowledge is probably due to the technical difficulties of visualizing cytoskeletal transport as outlined above. Another unclear aspect is that cytoskeletal proteins can be dynamic – with subunits being added and removed from existing polymers – and prevailing models of axonal transport either do not take this into account, or assume a steady-state polymeric state. Though the latter is largely true for neurofilaments – validating the polymer-transport model in this case – tubulin, and in particular actin, are unstable polymers (Mitchison and Kirschner, 1984; Vavylonis et al., 2005) and the interplay between dynamic instability and axonal transport may be an important aspect of its transport behavior in axons.
Cargoes of soluble/cytosolic proteins: Insights from radiolabeling studies
Besides cytoskeletal proteins, cytosolic (or soluble) proteins are also conveyed in slow axonal transport – specifically in SCb. What are the mechanisms conveying these proteins? A look into radiolabeling studies offers three general insights. 1) Though the bulk of any given cytosolic protein is conveyed in SCb, small amounts of radiolabel (10–15%) are also seen in the fast component (Baitinger and Willard, 1987; Garner and Lasek, 1982; Jensen et al., 1999; Lasek et al., 1984; Lund and McQuarrie, 2001, 2002; Paggi and Petrucci, 1992; Petrucci et al., 1991). Although this ‘fast-pool’ has been largely ignored, it may have important implications in understanding SCb transport, as discussed later. 2) A large number of proteins (over 200 at least) move exclusively in SCb (see 2-D gels in fig. 1D). Interestingly, the radiolabeled SCb pool entering synapses via axonal transport is quite large – about three-fold greater than proteins conveyed by fast transport – and many are enriched at synapses (fig. 2A). 3) While cargo-composition of SCa and fast-component is largely thematic (cytoskeletal or vesicular cargoes respectively); composition of SCb is disconcertingly varied – a potpourri of proteins (peripheral synaptic proteins, chaperones, metabolic enzymes, actin/actin-binding proteins, motors, etc.).
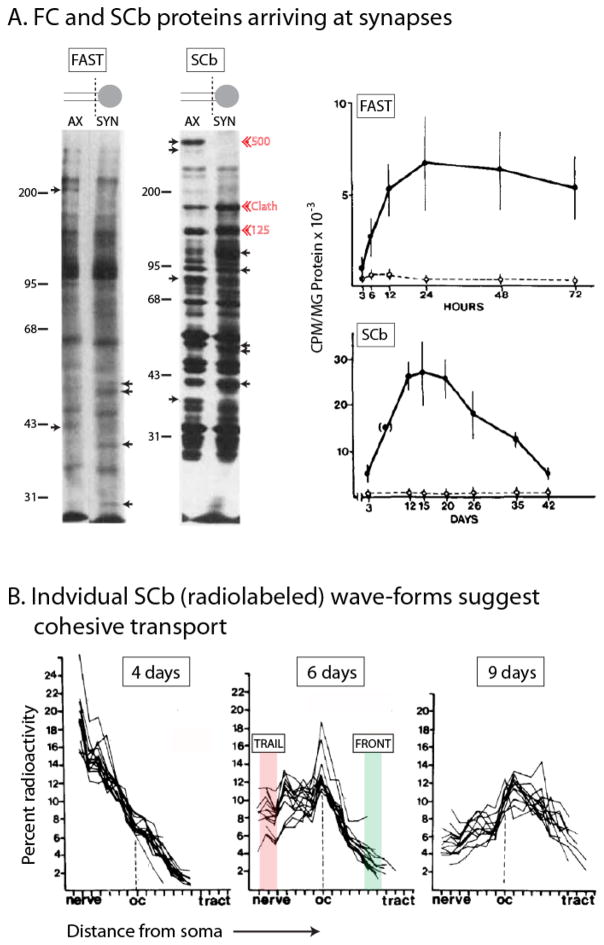
Figure 2. A detailed look at the overall kinetics of cytosolic/soluble cargoes moving in SCb.
(A) After somatic pulse-chase radiolabeling, terminal axon (“AX”) or synaptosomal (“SYN’) preparations from guinea pig retinal ganglion cells were analyzed at various time-points to document the ingress of labeled proteins into distal axons (left lanes) and synapses (right lanes). Small arrows point to proteins that are selectively enriched in axons or synapses. Red arrowheads highlight clathrin and two other major unidentified SCb proteins (125 and 500 kDa). In bottom panel, note that the total pool of transported SCb proteins is about three-fold larger than proteins conveyed in the fast component (bottom panel). Also note the decay in radiolabeled SCb proteins after entry into synapses, suggesting turnover and/or retrograde transport at synapses. Figure adapted from Garner and Mahler, 1987, with permission.
(B) Overlaid radiolabeled “wave-profiles” of 20 SCb proteins at 4, 6 and 9 days after somatic radiolabeling. Note the striking overlap between different SCb wave-profiles suggesting association with a common “carrier”. Also note the maintenance of the overall coherence of the “fronts” (shaded green) and the “peaks”, even after several days of transit; and also the divergence in the radiolabeled “trails” (shaded pink) suggesting that the deposition of individual moving SCb proteins along the axon was variable. Figures adapted from Garner and Lasek, 1982, with permission.
A look at individual SCb transport-profiles also highlights an intriguing aspect of this rate-class. When individual radiolabeled wave-profiles of multiple SCb proteins are overlaid, there is a striking correspondence in overall waveforms, particularly in their ‘fronts’ and the ‘peaks’ (see fig. 2B and Garner and Lasek, 1982; Lasek et al., 1984). This coherence is maintained even after several days of transit (note overlapping wave-profiles of the same 20 SCb proteins at 4, 6 and 9 days in fig. 2B). Despite the inherently limited resolution of radiolabeling studies, this is a remarkable phenomenon, invoking a model where diverse cytosolic SCb proteins bind to a common “carrier structure” (Garner and Lasek, 1982). An extension of this idea is that the SCb proteins may themselves organize into cargo-complexes, which would then bind to a common moving organelle. Indeed radiolabeling studies suggest that actin is co-transported with several actin-binding proteins (Mills et al., 1996); clathrin is co-transported with clathrin-binding proteins (Black et al., 1991; de Waegh and Brady, 1989); and dynein and dynactin proteins are also co-transported (Dillman et al., 1996a; Susalka et al., 2000). How are these sundry proteins transported in a common rate-class, all of them creeping along the axon at rates of a few millimeters per day?
Visualization of cytosolic/soluble protein transport in axons: a tricky business
It was generally thought that directly visualizing the axonal transport of SCb proteins would resolve mechanistic details of this rate-class, but this has proven to be trickier than expected. One way is to tag cytosolic proteins with GFP and visualize them in axons, using methods akin to those used for membrane-bound proteins. However unlike vesicles that appear as discrete particles, a complication with most GFP-tagged SCb proteins is that they have inherent soluble pools that create a fluorescent background “haze”. Thus observations are invariably limited to thin distal axons, where putative particulate structures can be resolved over the background haze. We used such methods in our early studies on SCb, looking at the axonal transport of moving GFP:α-synuclein particles in thin, distal axons (Roy et al., 2007, 2008). Particles containing α-synuclein moved rapidly with an anterograde bias, but the movements were much more infrequent than fast transport, and the particles also paused during transit. Accordingly, we suggested that cytosolic cargoes in SCb were also transported in a “stop and go” fashion, similar to neurofilaments moving in SCa. Several other groups have reported similar results with α-synuclein (Utton et al., 2005; Yang et al. 2010; Freundt et al. 2012). Though the dynamics of α-synuclein particles in this assay likely represent aspects of its axonal transport in SCb, these methods may not provide a complete picture, as the transport behavior of the population is not analyzed.
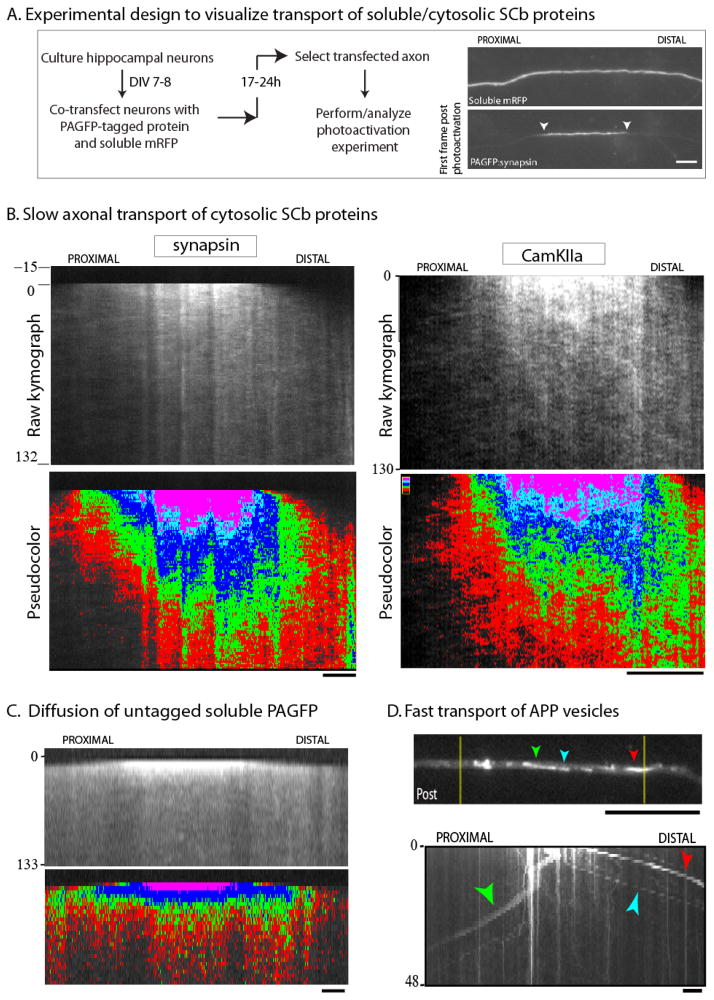
To overcome these limitations, more recently we have used photoactivatable vectors to study SCb transport, analyzing the kinetics of axonal protein populations (Scott et al. 2011). The main advantage of this paradigm is that it allows dynamic visualization of the entire repertoire of soluble molecules in proximal/primary axons, including kinetics of rapidly-mobile/freely-diffusible fractions that could not be observed by our earlier methods. The basic design of these experiments is shown in fig. 3A; detailed protocols are published (Roy et al., 2011). Briefly, cultured neurons are transfected with cytosolic proteins tagged to photoactivatable GFP (PAGFP) and a soluble red marker (to visualize the transfected axon). Thereafter, a discrete region within the axon is photoactivated, and the kinetics of the photoactivated molecules is followed over time by live-imaging (fig. 3A). We found that photoactivated SCb proteins were conveyed in a peculiar manner that superficially resembled diffusion, but was very different from either untagged PAGFP or vesicular proteins. Specifically, cytosolic SCb proteins dispersed as a plume of fluorescence that had a distinct anterograde bias (fig. 3B). However the free diffusion of untagged PAGFP was rapid and unbiased (fig. 3C); and when vesicular proteins were imaged using this paradigm, individual moving vesicles were seen as expected (fig. 3D).
Figure 3. Photoactivation paradigm to visualize the slow axonal transport of cytosolic SCb protein populations.
(A) Cultured neurons are co-transfected with a PAGFP-tagged protein of interest and untagged mRFP (to identify transfected axons). A discrete axonal ROI (~ 20 μm) is photoactivated, and the dispersion of photoactivated molecules is visualized over time (examples of images with PAGFP:synapsin are shown).
(B) Greyscale (above) and pseudo-colored (below) kymographs from two PAGFP-tagged SCb proteins synapsin and CamKIIa, imaged using the paradigm above (distance/time in kymographs is on the x/y axis respectively). Note the anterogradely-biased plume of fluorescence.
(C) Photoactivation of untagged PAGFP leads to a rapid and unbiased diffusion of fluorescence as expected; different from SCb proteins.
(D) Photoactivation of APP – a vesicle-associated fast-component protein – results in the stochastic bidirectional departure of individual vesicles; also different from SCb proteins (colored arrowheads mark the same vesicles in image/kymograph). Scale bar = 5 μm. Figure adapted from Scott et al., 2011 and Tang et al., 2012, with permission.
The estimated rate of biased flow of photoactivated SCb molecules – quantified by measuring the shift in overall fluorescence-peaks over time (for details, see Roy et al. 2011) – was within the range predicted by radiolabeling experiments (albeit slightly lower, ~ 0.01–0.03 μm/sec or 1–3 mm/day), suggesting that this movement represented bonafide SCb transport (Scott et al. 2011). Since cytosolic proteins have inherently diffusible fractions (unlike membrane-anchored proteins); in most cases we also saw small pools that rapidly dispersed bi-directionally, in a manner resembling free diffusion. Note that some fluorescent-tagged cytosolic proteins have very large, rapidly diffusible-pools, and may not be ideal for such analyses (Roy et al. 2011).
Egress of somatically-derived cytosolic proteins into axons reveals mechanistic details of slow transport
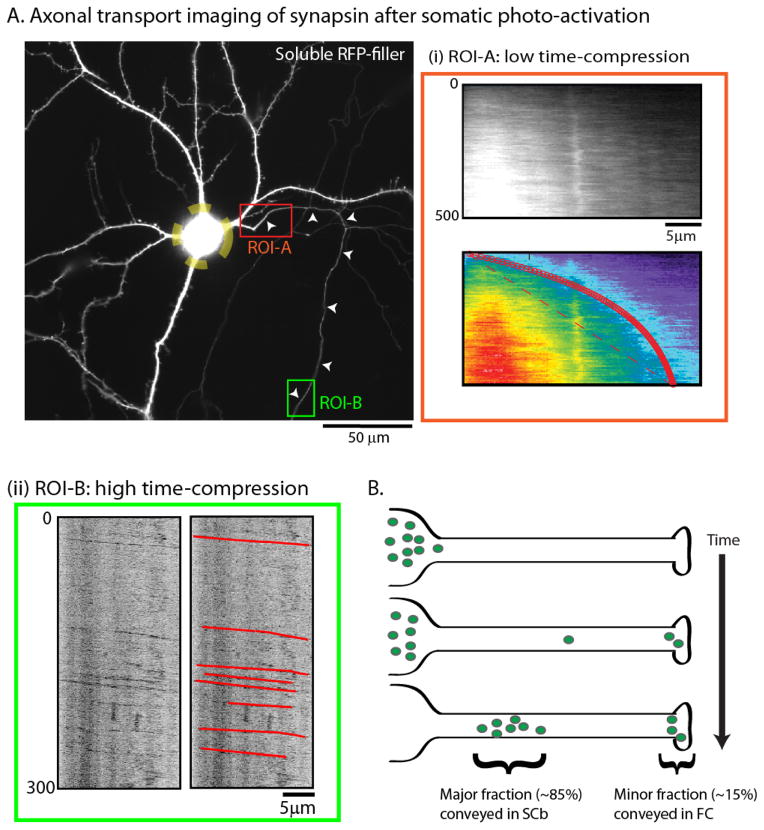
The photoactivation paradigm can also reveal the movement of soluble proteins from soma into axons, thus more closely simulating pulse-chase experiments. In these experiments, PAGFP-tagged SCb proteins at the neuronal soma are photoactivated, and then the ingress of this photoactivated pool into axons is visualized. An example with PAGFP:synapsin is shown in figure 4A (also see Scott et al. 2011). Interestingly, although the bulk of photoactivated synapsin molecules were transported into axons as a slow-moving ‘wave’ as expected [fig. 4A(i)], rapidly-moving synapsin particles were also seen distally [fig. 4A(ii)]. Collectively, these data suggest that while the bulk of synapsin is conveyed as a slowly-biased ‘wave’ (in SCb), a fraction is also transported as persistent particles with kinetics similar to fast transport. The observations are also congruent with radiolabeling experiments where small pools (~10–15%) of synapsin and other SCb proteins move in the fast component (see above). Collectively, the data can be interpreted in the following way. If at any given time, cytosolic molecules in axons are moving both as a slow “wave” as well as fast-moving particles, imaging cytosolic proteins tagged to conventional GFP would highlight the particle-movement, whereas the slow “wave” – at steady-state – would only appear as a diffuse background. However, when SCb proteins are imaged using the photoactivation paradigm where a large protein population is simultaneously photoactivated, the wave-like overall kinetics are highlighted while the fast-moving particles are only occasionally captured (see Tang et al. 2012 and below).
Figure 4. A fraction of SCb proteins are conveyed in the fast component.
(A) To more closely simulate the radiolabeling paradigm, cultured neurons were transfected with GFP:synapsin and soluble mRFP (shown); the neuronal soma was photoactivated (yellow dashed ROI); and the egress of photoactivated molecules into the emergent axon was evaluated over time. While the bulk of synapsin molecules moved slowly into the proximal axon with kinetics expected for slow axonal transport (ROI-A – red box), rapidly-moving particles of synapsin were also seen when the distal axon was imaged after somatic photoactivation (ROI-B – green box).
(B) The “dynamic recruitment” model for SCb transport. After synthesis in the soma, cytosolic molecules intermittently and probabilistically associate with “carriers” moving in fast axonal transport. As such, some molecules remain associated with these carriers for long periods, giving rise to a small population (~ 10–15%) that is rapidly transported to axons and synapses. However the majority of cytosolic molecules are slowly conveyed with kinetics resembling slow axonal transport. An implication of this model is that common transport “carriers” are responsible for conveying both fast component and SCb proteins. Figure adapted from Scott et al., 2011, with permission.
Are cytosolic proteins conveyed as individual molecules, or do they organize into multi-protein complexes? Early biochemical studies suggested that cytosolic SCb proteins in neurons are organized into protein complexes (Lorenz and Willard, 1978), and our biochemical data largely supports this idea. For instance brain cytosolic proteins exist in high-speed pellet fractions where they settle into high-density fractions (Scott et al. 2011). This behavior would not be expected if these proteins were entirely soluble. Moreover upon careful observation, SCb kymographs occasionally have persistent “streaks” of fluorescence – representing fast vectorial motion (Tang et al. 2012) – suggesting that SCb particles accumulate into aggregates or complexes that subsequently associate with fast and persistent motile structures. However the detailed composition of such complexes is as yet unknown.
The search for SCb “carriers” and the ‘dynamic recruitment’ working-model
The slow-moving wave-like kinetics seen in experiments with cultured neurons is likely the visual counterpart of slow axonal transport (SCb) in pulse-chase radiolabeling experiments. How is this slow movement achieved at a molecular level? One possibility is that cytosolic assemblies transiently associate with other persistent cargoes that are continuously moving in fast transport. Due to the transient nature of these associations, the overall movement of the cytosolic population would be much slower than the overall movement of the fast cargoes that the cytosolic particles are associating with. In support of this, closer examination of SCb kymographs from photoactivation experiments reveal occasional persistent vectorial movements that resemble structures moving in fast axonal transport (Tang et al. 2012). One possibility is that SCb molecules transiently associate with moving vesicles, and future studies may clarify this issue.
We propose the following working-model for cytosolic/soluble proteins moving in slow axonal transport (the “dynamic recruitment” model). After synthesis in perikaryal free ribosomes, cytosolic/soluble proteins assemble into multi-protein complexes that can dynamically associate with a mobile “carrier” that is conveyed persistently in fast axonal transport. As the carrier moves out of the cell-body – moving persistently – the soluble/cytosolic protein assemblies are also transported into the axon by virtue of their intermittent associations with the carriers. However, as such associations are dynamic and probabilistic, the overall displacement of the cytosolic population is much slower than the fast-moving carrier, resulting in the slow overall rate seen in the pulse-chase radiolabeling experiments. Moreover, due to the probabilistic nature of such interactions, a small fraction of cytosolic molecules remain associated with mobile vesicles for long periods, and this pool represents the minor “fast-population” observed in previous radiolabeling studies of SCb.
There are obvious parallels of the ‘dynamic recruitment’ model to the ‘stop and go’ model. However unlike neurofilaments that exist as stable polymers, the cytosolic proteins we have reported so far are dynamic (see above and Tang et al., 2012), introducing a variable in the model where the assembly/disassembly kinetics would also be a determinant. An interesting prediction of our model is that SCb proteins that exist in a largely assembled form in axons would exhibit dynamics similar to the ‘stop and go’ motion seen with neurofilaments (as the assembly/disassembly dynamics would only play a minor role in these cases); an idea that can be tested in the future. Given the heterogeneous nature of SCb, it is plausible that many different transport mechanisms at play; nevertheless the above scenario captures the essence of the available radiolabeling and imaging data and offers a working model.
Experiments in other model-systems have also reported an anterogradely-biased wave-like kinetics of soluble/cytosolic proteins. Terada et al. injected a bolus of fluorescently-labeled creatine kinase (a soluble protein conveyed in SCb) into the squid giant axon, and saw a slow anterogradely-biased movement of the labeled protein population at rates reminiscent of SCb (Terada et al., 2000, 2010). Using a drosophila model, Sadananda et al. have also recently shown a kinesin-dependent slow, anterogradely-biased flow of the soluble protein choline acetyltransferase in axons (Sadananda et al. 2012). Thus it appears that the biased flow of cytosolic cargoes in slow axonal transport is a conserved phenomenon, and collective efforts in different model-systems should provide clarity of underlying mechanisms in the future.
Coda
Though slow axonal transport conveys proteins that are critical in maintaining neuronal form and function, and several such proteins also play a role in neurodegeneration – tau, α-synuclein, SOD-1 for instance – our understanding of slow transport pales in comparison to that of its faster counterpart. Though this scientific sluggishness is likely due to the inherent difficulties in visualizing the phenomenon, robust imaging paradigms are now available to directly visualize slow transport. Ongoing advances in development of fluorescent probes, new imaging tools, and innovative ways of integrating these tools will allow us to examine slow axonal transport of a larger repertoire of cargoes with higher fidelity. In the process, they will uncover new mysteries that are undoubtedly hidden within the depths of this historically “unseen” component of axonal transport.
Acknowledgments
Research on slow axonal transport in our laboratory is supported by grants from the NIH (R01NS075233) and the March of Dimes (Basil O’Connor award). I thank Peter Hollenbeck (Purdue) and Bettina Winckler (University of Virginia) for helpful comments on the manuscript, as well as many other laboratories that have generously shared constructs with us. The title is inspired an essay by Freeman Dyson (The Scientist as a Rebel – New York review books).
Abbreviations
- SCa
Slow Component a
- , SCb
Slow Component b
- GFP
green fluorescent protein
- RFP
red fluorescent protein
- GAPDH
glyceraldehyde-3-phosphate dehydrogenease
- SOD
superoxide dismutase
- CCT
chaperonin containing T complex protein-1
- CamK
calcium/calmodulin dependent kinase
Footnotes
Conflict of interest: none
References
- Allen RD, Metuzals J, Tasaki I, Brady ST, Gilbert SP. Fast axonal transport in squid giant axon. Science. 1982;218:1127–1129. doi: 10.1126/science.6183744. [DOI] [PubMed] [Google Scholar]
- Baas PW, Brown A. Slow axonal transport: the polymer transport model. Trends Cell Biol. 1997;7:380–384. doi: 10.1016/S0962-8924(97)01148-3. [DOI] [PubMed] [Google Scholar]
- Baitinger C, Willard M. Axonal transport of synapsin I-like proteins in rabbit retinal ganglion cells. J Neurosci. 1987;7:3723–3735. doi: 10.1523/JNEUROSCI.07-11-03723.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black MM, Lasek RJ. Axonal transport of actin: slow component b is the principal source of actin for the axon. Brain Res. 1979;171:401–13. doi: 10.1016/0006-8993(79)91045-x. [DOI] [PubMed] [Google Scholar]
- Black MM, Chestnut MH, Pleasure IT, Keen JH. Stable clathrin: uncoating protein (hsc70) complexes in intact neurons and their axonal transport. J Neurosci. 1991;11:1163–1172. doi: 10.1523/JNEUROSCI.11-05-01163.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black MM, Lasek RJ. Slow components of axonal transport: two cytoskeletal networks. J Cell Biol. 1980;86:616–623. doi: 10.1083/jcb.86.2.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady ST. A novel brain ATPase with properties expected for the fast axonal transport motor. Nature. 1985;317:73–75. doi: 10.1038/317073a0. [DOI] [PubMed] [Google Scholar]
- Brady ST, Lasek RJ. Axonal transport: a cell-biological method for studying proteins that associate with the cytoskeleton. Methods Cell Biol. 1982;25:365–398. doi: 10.1016/s0091-679x(08)61434-x. [DOI] [PubMed] [Google Scholar]
- Brady ST, Lasek RJ, Allen RD. Fast axonal transport in extruded axoplasm from squid giant axon. Science. 1982;218:1129–1131. doi: 10.1126/science.6183745. [DOI] [PubMed] [Google Scholar]
- Brady ST, Tytell M, Heriot K, Lasek RJ. Axonal transport of calmodulin: a physiologic approach to identification of long-term associations between proteins. J Cell Biol. 1981;89:607–614. doi: 10.1083/jcb.89.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray D. The riddle of slow transport - an introduction. Trends Cell Biol. 1997;7:379. doi: 10.1016/S0962-8924(97)01134-3. [DOI] [PubMed] [Google Scholar]
- Bray JJ, Fernyhough P, Bamburg JR, Bray D. Actin depolymerizing factor is a component of slow axonal transport. J Neurochem. 1992;58:2081–2087. doi: 10.1111/j.1471-4159.1992.tb10949.x. [DOI] [PubMed] [Google Scholar]
- Brown A. Axonal transport of membranous and nonmembranous cargoes: a unified perspective. J Cell Biol. 2003;160:817–821. doi: 10.1083/jcb.200212017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A, Wang L, Jung P. Stochastic simulation of neurofilament transport in axons: the “stop-and-go” hypothesis. Mol Biol Cell. 2005;16:4243–4255. doi: 10.1091/mbc.E05-02-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waegh S, Brady ST. Axonal transport of a clathrin uncoating ATPase (HSC70): a role for HSC70 in the modulation of coated vesicle assembly in vivo. J Neurosci Res. 1989;23:433–440. doi: 10.1002/jnr.490230409. [DOI] [PubMed] [Google Scholar]
- Dillman JF, III, Dabney LP, Karki S, Paschal BM, Holzbaur EL, Pfister KK. Functional analysis of dynactin and cytoplasmic dynein in slow axonal transport. J Neurosci. 1996a;16:6742–6752. doi: 10.1523/JNEUROSCI.16-21-06742.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillman JF, III, Dabney LP, Pfister KK. Cytoplasmic dynein is associated with slow axonal transport. Proc Natl Acad Sci USA. 1996b;93:141–144. doi: 10.1073/pnas.93.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falnikar A, Baas PW. Critical roles for microtubules in axonal development and disease. Results Probl Cell Differ. 2009;48:47–64. doi: 10.1007/400_2009_2. [DOI] [PubMed] [Google Scholar]
- Freundt EC, Maynard N, Clancy EK, Roy S, Bousset L, Sourigues Y, Covert M, Melki R, Kirkegaard K, Brahic M. Neuron-to-neuron transmission of alpha-synuclein fibrils through axonal transport. Ann Neurol. 2012;72:517–524. doi: 10.1002/ana.23747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith JA, Reese TS, Schlief ML, Gallant PE. Slow transport of unpolymerized tubulin and polymerized neurofilament in the squid giant axon. Proc Natl Acad Sci USA. 1999;96:11589–11594. doi: 10.1073/pnas.96.20.11589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner JA, Lasek RJ. Cohesive axonal transport of the slow component b complex of polypeptides. J Neurosci. 1982;2:1824–1835. doi: 10.1523/JNEUROSCI.02-12-01824.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafstein B, Forman DS. Intracellular transport in neurons. Physiol Rev. 1980;60:1167–283. doi: 10.1152/physrev.1980.60.4.1167. [DOI] [PubMed] [Google Scholar]
- He Y, Francis F, Myers KA, Yu W, Black MM, Baas PW. Role of cytoplasmic dynein in the axonal transport of microtubules and neurofilaments. J Cell Biol. 2005;168:697–703. doi: 10.1083/jcb.200407191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N, Funakoshi ST, Takeda S. Slow axonal transport: the subunit transport model. Trends Cell Biol. 1997;7:384–388. doi: 10.1016/S0962-8924(97)01133-1. [DOI] [PubMed] [Google Scholar]
- Hoffman PN, Lasek RJ. The slow component of axonal transport. Identification of major structural polypeptides of the axon and their generality among mammalian neurons. J Cell Biol. 1975;66:351–66. doi: 10.1083/jcb.66.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen PH, Li JY, Dahlstrom A, Dotti CG. Axonal transport of synucleins is mediated by all rate components. Eur J Neurosci. 1999;11:3369–3376. doi: 10.1046/j.1460-9568.1999.00754.x. [DOI] [PubMed] [Google Scholar]
- Jolly AL, Kim H, Srinivasan D, Lakonishok M, Larson AG, Gelfand VI. Kinesin-1 heavy chain mediates microtubule sliding to drive changes in cell shape. Proc Natl Acad Sci U S A. 2010;107:12151–121516. doi: 10.1073/pnas.1004736107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating TJ, Peloquin JG, Rodionov VI, Momcilovic D, Borisy GG. Microtubule release from the centrosome. Proc Natl Acad Sci U S A. 1997;94:5078–5083. doi: 10.1073/pnas.94.10.5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike H, Matsumoto H. Fast axonal transport of membrane protein and intra-axonal diffusion of free leucine in a neuron of Aplysia. Neurosci Res. 1985;2:281–285. doi: 10.1016/0168-0102(85)90006-9. [DOI] [PubMed] [Google Scholar]
- Lasek RJ, Garner JA, Brady ST. Axonal transport of the cytoplasmic matrix. J Cell Biol. 1984;99:212s–221s. doi: 10.1083/jcb.99.1.212s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Hoffman PN, Stirling W, Price DL, Lee MK. Axonal transport of human alpha-synuclein slows with aging but is not affected by familial Parkinson’s disease-linked mutations. J Neurochem. 2004;88:401–410. doi: 10.1046/j.1471-4159.2003.02166.x. [DOI] [PubMed] [Google Scholar]
- Li Y, Jung P, Brown A. Axonal transport of neurofilaments: a single population of intermittently moving polymers. J Neurosci. 2012;32:746–758. doi: 10.1523/JNEUROSCI.4926-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz T, Willard M. Subcellular fractionation of intra-axonally transport polypeptides in the rabbit visual system. Proc Natl Acad Sci USA. 1978;75:505–509. doi: 10.1073/pnas.75.1.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund LM, McQuarrie IG. Calcium/calmodulin-dependent protein kinase IIalpha in optic axons moves with slow axonal transport and undergoes posttranslational modification. Biochem Biophys Res Commun. 2001;289:1157–1161. doi: 10.1006/bbrc.2001.6111. [DOI] [PubMed] [Google Scholar]
- Lund LM, McQuarrie IG. Calcium/calmodulin-dependent protein kinase IIbeta isoform is expressed in motor neurons during axon outgrowth and is part of slow axonal transport. J Neurosci Res. 2002;67:720–728. doi: 10.1002/jnr.10162. [DOI] [PubMed] [Google Scholar]
- Ma D, Himes BT, Shea TB, Fischer I. Axonal transport of microtubule-associated protein 1B (MAP1B) in the sciatic nerve of adult rat: distinct transport rates of different isoforms. J Neurosci. 2000;20:2112–2120. doi: 10.1523/JNEUROSCI.20-06-02112.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KE, Heidemann SR. What is slow axonal transport? Exp Cell Res. 2008;314:1981–1990. doi: 10.1016/j.yexcr.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Mills RG, Minamide LS, Yuan A, Bamburg JR, Bray JJ. Slow axonal transport of soluble actin with actin depolymerizing factor, cofilin, and profilin suggests actin moves in an unassembled form. J Neurochem. 1996;67:1225–1234. doi: 10.1046/j.1471-4159.1996.67031225.x. [DOI] [PubMed] [Google Scholar]
- Mitchison T, Kirschner M. Dynamic instability of microtubule growth. Nature. 1984;312:237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- Miki H, Setou M, Kaneshiro K, Hirokawa N. All kinesin superfamily protein, KIF, genes in mouse and human. Proc Natl Acad Sci U S A. 2001;98:7004–7011. doi: 10.1073/pnas.111145398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon RA, Fischer I, Lewis SE. Synthesis, axonal transport, and turnover of the high molecular weight microtubule-associated protein MAP 1A in mouse retinal ganglion cells: tubulin and MAP 1A display distinct transport kinetics. J Cell Biol. 1990;110:437–448. doi: 10.1083/jcb.110.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs S. Characterization of fast orthograde transport. Neurosci Res Program Bull. 1981;20:19–31. [PubMed] [Google Scholar]
- Paggi P, Petrucci TC. Neuronal compartments and axonal transport of synapsin I. Mol Neurobiol. 1992;6:239–251. doi: 10.1007/BF02780556. [DOI] [PubMed] [Google Scholar]
- Petrucci TC, Macioce P, Paggi P. Axonal transport kinetics and posttranslational modification of synapsin I in mouse retinal ganglion cells. J Neurosci. 1991;11:2938–2946. doi: 10.1523/JNEUROSCI.11-09-02938.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Coffee P, Smith G, Liem RK, Brady ST, Black MM. Neurofilaments are transported rapidly but intermittently in axons: implications for slow axonal transport. J Neurosci. 2000;20:6849–6861. doi: 10.1523/JNEUROSCI.20-18-06849.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Zhang B, Lee VM, Trojanowski JQ. Axonal transport defects: a common theme in neurodegenerative diseases. Acta Neuropathol. 2005;109:5–13. doi: 10.1007/s00401-004-0952-x. [DOI] [PubMed] [Google Scholar]
- Roy S, Winton MJ, Black MM, Trojanowski JQ, Lee VM. Rapid and intermittent cotransport of slow component-b proteins. J Neurosci. 2007;27:3131–3138. doi: 10.1523/JNEUROSCI.4999-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Winton MJ, Black MM, Trojanowski JQ, Lee VM. Cytoskeletal requirements in axonal transport of slow component-b. J Neurosci. 2008;28:5248–5256. doi: 10.1523/JNEUROSCI.0309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Yang G, Tang Y, Scott DA. A simple photoactivation and image analysis module for visualizing and analyzing axonal transport with high temporal resolution. Nat Protoc. 2011;7:62–68. doi: 10.1038/nprot.2011.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadananda A, Hamid R, Doodhi H, Ghosal D, Girotra M, Jana SC, Ray K. Interaction with a kinesin-2 tail propels choline acetyltransferase flow towards synapse. Traffic. 2012;13:979–991. doi: 10.1111/j.1600-0854.2012.01361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DA, Das U, Tang Y, Roy S. Mechanistic logic underlying the axonal transport of cytosolic proteins. Neuron. 2011;70:441–454. doi: 10.1016/j.neuron.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekimoto S, Tashiro T, Komiya Y. Two 68-kDa proteins in slow axonal transport belong to the 70-kDa heat shock protein family and the annexin family. J Neurochem. 1991;56:1774–1782. doi: 10.1111/j.1471-4159.1991.tb02080.x. [DOI] [PubMed] [Google Scholar]
- Susalka SJ, Hancock WO, Pfister KK. Distinct cytoplasmic dynein complexes are transported by different mechanisms in axons. Biochim Biophys Acta. 2000;1496:76–88. doi: 10.1016/s0167-4889(00)00010-0. [DOI] [PubMed] [Google Scholar]
- Tang Y, Das U, Scott DA, Roy S. The slow axonal transport of alpha-synuclein-mechanistic commonalities amongst diverse cytosolic cargoes. Cytoskeleton (Hoboken) 2012;69:506–513. doi: 10.1002/cm.21019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada S, Kinjo M, Aihara M, Takei Y, Hirokawa N. Kinesin-1/Hsc70-dependent mechanism of slow axonal transport and its relation to fast axonal transport. EMBO J. 2010;29:843–854. doi: 10.1038/emboj.2009.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada S, Kinjo M, Hirokawa N. Oligomeric tubulin in large transporting complex is transported via kinesin in squid giant axons. Cell. 2000;103:141–155. doi: 10.1016/s0092-8674(00)00094-5. [DOI] [PubMed] [Google Scholar]
- Tytell M, Black MM, Garner JA, Lasek RJ. Axonal transport: each major rate component reflects the movement of distinct macromolecular complexes. Science. 1981;214:179–181. doi: 10.1126/science.6169148. [DOI] [PubMed] [Google Scholar]
- Twelvetrees A, Hendricks AG, Holzbaur EL. SnapShot: axonal transport. Cell. 2012;149:950–950. doi: 10.1016/j.cell.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Utton MA, Noble WJ, Hill JE, Anderton BH, Hanger DP. Molecular motors implicated in the axonal transport of tau and alpha-synuclein. J Cell Sci. 2005;118:4645–4654. doi: 10.1242/jcs.02558. [DOI] [PubMed] [Google Scholar]
- Vallee RB, Bloom GS. Mechanisms of fast and slow axonal transport. Annu Rev Neurosci. 1991;14:59–92. doi: 10.1146/annurev.ne.14.030191.000423. [DOI] [PubMed] [Google Scholar]
- Vale RD, Schnapp BJ, Reese TS, Sheetz MP. Movement of organelles along filaments dissociated from the axoplasm of the squid giant axon. Cell. 1985;40:449–454. doi: 10.1016/0092-8674(85)90159-x. [DOI] [PubMed] [Google Scholar]
- Vavylonis D, Yang Q, O’Shaughnessy B. Actin polymerization kinetics, cap structure, and fluctuations. Proc Natl Acad Sci U S A. 2005;102:8543–8548. doi: 10.1073/pnas.0501435102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Brown A. Rapid movement of microtubules in axons. Curr Biol. 2002;12:1496–1501. doi: 10.1016/s0960-9822(02)01078-3. [DOI] [PubMed] [Google Scholar]
- Wang L, Ho CL, Sun D, Liem RK, Brown A. Rapid movement of axonal neurofilaments interrupted by prolonged pauses. Nat Cell Biol. 2000;2:137–141. doi: 10.1038/35004008. [DOI] [PubMed] [Google Scholar]
- Willard M, Cowan WM, Vagelos PR. The polypeptide composition of intra-axonally transported proteins: evidence for four transport velocities. Proc Natl Acad Sci U S A. 1974;71:2183–2187. doi: 10.1073/pnas.71.6.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard M, Wiseman M, Levine J, Skene P. Axonal transport of actin in rabbit retinal ganglion cells. J Cell Biol. 1979;81:581–91. doi: 10.1083/jcb.81.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Brown A. Neurofilament polymer transport in axons. J Neurosci. 2005;25:7014–21. doi: 10.1523/JNEUROSCI.2001-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ML, Hasadsri L, Woods WS, George JM. Dynamic transport and localization of alpha-synuclein in primary hippocampal neurons. Mol Neurodegener. 2010;5:9. doi: 10.1186/1750-1326-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan A, Mills RG, Bamburg JR, Bray JJ. Cotransport of glyceraldehyde-3-phosphate dehydrogenase and actin in axons of chicken motoneurons. Cell Mol Neurobiol. 1999;19:733–744. doi: 10.1023/A:1006953022763. [DOI] [PMC free article] [PubMed] [Google Scholar]