Abstract
Curcumin, a plant phenol, has been used for centuries in traditional medicines for its anti-inflammatory and anti-neoplastic properties. The compound is believed to act on a range of proteins involved in cell cycle regulation. In this study, the effect of curcumin on ERK-1/2 pathway protein expression and on proliferation of nasopharyngeal carcinoma cells was investigated. CNE-2Z nasopharyngeal carcinoma cells were cultured with 10, 20, 40, or 80 μM curcumin for 24 h before proliferation was assessed by MTT colorimetry. Cell proliferation was increasingly inhibited as the concentration of curcumin increased (P<0.005). Additionally, Western blotting revealed that expression of p-ERK-1/2, MMP-9, and TIMP-1 was altered following curcumin treatment, also in a dose-dependent manner. Expression of p-ERK-1/2 and MMP-9 decreased, while expression of TIMP-1 increased (P<0.05). Finally, CNE-2Z cells were xenografted under the skin of 18 nude mice. Mice were treated with vehicle only (control), 24 mg/kg curcumin (low-dose group), or 50 mg/kg curcumin (high-dose group) every other day for 40 days beginning 24 h after xenografting. Compared to tumors from the control group, the volume and weight of xenograft tumors was significantly lower in both curcumin groups, with a higher magnitude of difference in the high-dose curcumin group (P<0.05). These results indicate that curcumin treatment can inhibit proliferation of nasopharyngeal carcinoma cells and alter expression of proteins in the ERK-1/2 signaling pathway. Therefore, curcumin warrants further investigation as a potential treatment for nasopharyngeal cancer.
Keywords: Nasopharyngeal carcinoma, curcumin, ERK1/2 signaling pathway, matrix metalloproteinases, proliferation
Introduction
Curcumin, a yellow phenolic pigment extracted from root stocks of rhizoma Curcuma longa, Curcuma aromatica, Curcuma zedoary, and Acorus calamus, has various pharmacological actions and has been used for centuries in traditional Chinese medicine [1]. Recent studies have demonstrated the anti-neoplastic properties of this compound; both in vivo and in vitro, curcumin can inhibit tumor growth, induce apoptosis of tumor cells [2], and reduce invasion and metastasis of tumor cells [3,4]. These activities have generated interest in the potential clinical application of curcumin extract to treat cancer, among other illnesses [5].
The anti-inflammatory and anti-neoplastic properties of curcumin appear to be mediated by its direct and indirect actions on cell cycle regulators [6,7]. Indeed, studies of both curcumin extracts and analogues have demonstrated that these substances bind to a variety of transcription factors, cytokines, growth factors, and other proteins that are involved in cell growth, proliferation, and migration. For example, curcumin affects signaling by interacting with diverse molecules like TNF-a, COX-2, NF-ΚB, and several MMPs (matrix metalloproteinases) [8,9].
One signal transduction pathway of particular interest is the mitogen-activated protein kinase (MAPK) pathway. MAPK is a serine/threonine kinase activated by a variety of stimuli such as cytokines, growth factors, and neurotransmitters. Upon activation, MAPK phosphorylates nuclear transcription factors and other protein kinases, and regulates gene and protein expression in cell division, proliferation, migration, and apoptosis [10,11]. The MAPK pathway contains four subfamilies in mammalian cells: extracellular signal-regulated protein kinase (ERK), c-Jun amino-terminal kinase (JNK/SAPK), p38 MAPK, and ERK5/BMK1 (big MAP kinase 1). Importantly, dysregulation of members of the MAPK family has been implicated in oncogenesis [12]. Signaling through the subfamily ERK-1/2 pathway, which regulates cell proliferation [13] and apoptosis [14], involves a number of downstream targets, including MMPs [15]. Activated MMPs can degrade the extracellular matrix, a phenomenon that can promote tumor metastasis and recurrence [16]. However, MMPs can be inhibited by curcumin [17].
Nasopharyngeal carcinoma is one of the common cancers in Southeast Asia and southern provinces of China [18,19]. To determine whether curcumin administration alters signaling through the ERK-1/2 pathway, expression of ERK-1/2, MMP-9, and TIMP-1 was assessed in nasopharyngeal carcinoma CNE-2Z cells treated with curcumin. CNE-2Z cells were also xenografted to nude mice to determine whether curcumin treatment could prevent or slow the proliferation of cancer cells. The findings of this study imply that curcumin offers a potential avenue for nasopharyngeal cancer therapy and suggests a potential mechanism for its effectiveness.
Materials and methods
Cell line
Nasopharyngeal carcinoma CNE-2Z cells (1×107/mL) (Cell Bank, Chinese Academy of Sciences in Shanghai) were cultured in RPMI-1640 medium containing 10% fetal bovine serum (Gibco, USA) in an incubator (Galaxy S+, England) under 5% CO2. Medium was changed every 2-3 days for passage.
Mice
BALB/C nude mice were obtained from Guangdong Medical Experiment Animal Center. Mice were specific pathogen free, male, and 6 to 8 weeks of age, with a body mass of 18 to 24 g. Drinking water, feed, and caging materials were sterilized.
Tumor xenografts were performed. CNE-2Z cells cultured to 70 to 80% confluency were digested with trypsin containing EDTA (Sigma Chemical, St. Louis, MO) and counted on a cell counter (Beckman, USA). Suspended cells (1×107 cells/mouse) were injected subcutaneously into the back right side of 18 nude mice. Inoculated mice were randomly assigned to 3 groups of 6. The control group received intraperitoneal (i.p.) injection of vehicle alone (0.1 mL/10 g body weight). The low-dose group received i.p. injection of 25 mg/kg curcumin. The high-dose group received i.p. injection of 50 mg/kg curcumin. Treatments were administered beginning 24 h after xenograft and once every other day for 20 total treatments. Tumor xenografts were measured [length (a) and width (b)] using a vernier caliper, and tumor volumes were calculated as V=a*b2/2. Following the final treatment, mice were euthanized and tumors were dissected and weighed. Tumor tissues were fixed with 10% formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin using typical histology methods. Tumor sections were observed under light microscope.
MTT proliferation assay
CNE-2Z cells in logarithmic phase were inoculated on a 96-well plate at 2×104/mL, 150 μL in each well. Following culture for 24 h in RPMI-1640 medium containing 10% fetal calf serum, medium was extracted and cells were treated with 100 μL of curcumin (Sigma, USA) at 10, 20, 40, and 80 μM concentrations, respectively. Three wells were used for each concentration group; no drug was added to the control group. Cells were cultured for 24 h; 20 μL 5 mg/mL MTT solution (Gibco, USA) were added for 4 h at room temperature. Supernatant was removed, and 100 μL dimethyl sulfoxide (DMSO, Gibco, USA) were added to each well, with shaking on an oscillator to dissolve. After 30 min at room temperature, the optical density was determined (OD) on a plate reader (Thermo, UK) using enzyme-linked immunosorbent assay (ELISA) at 490 nm wavelength. OD readings were replicated thrice. The inhibition rate of cell proliferation was determined as a percentage using the following formula: [1-(OD value of experimental group/OD value of control group)]×100%.
Western blotting
CNE-2Z cells treated with 10, 20, 40, or 80 μM curcumin for 24 h were collected and washed with pre-cooled PBS twice. Pre-cooled cell lysis buffer and 1 μM PMSF were added for 30 min, then samples were centrifuged (Microfuge 22R, USA) at 12000 rpm and 4°C for 20 minutes. Supernatant was removed, and samples were stored at -80°C. Protein was quantified using the Bradford method. Proteins were separated by SDS-PAGE electrophoresis (DYY-8C, Beijing Liuyi Instrument Factory) and transferred to PVDF membrane. Blocking buffer (containing 5% skim milk TBST) was incubated with membrane at room temperature for 1 h. Rabbit anti-human ERK-1 and ERK-2 monoclonal antibodies, mouse anti-human p-ERK monoclonal antibody, and sheep polyclonal antibodies against TIMP-1 and MMP-9 (all 1:500, Santa Cruz, USA) were used to probe membrane. Membrane was incubated at 4°C overnight and washed with TBST thrice. HRP-labeled goat anti-rabbit IgG, goat anti-mouse IgG, or rabbit anti-goat IgG secondary antibody (1:5000, Santa Cruz, USA), used to detect respective primary antibody, were incubated with membrane at 37°C for 1 h, and membrane was then washed with TBST. Membrane was exposed to X-ray; films were imaged (GIS-2016, Shanghai Tanon Science & Technology Co. Ltd.) and analyzed (Bio-Rad, USA) to determine protein concentrations relative to β-actin control.
Statistical analysis
Statistical tests were performed using SPSS17.0 statistical package. Measurements are expressed as mean±standard deviation (χ̅±s). Comparisons between groups were performed by univariate analysis of variance and pairwise SNK. Tests were two-sided, with a alpha level of 0.05, and P<0.05 was considered statistically significant.
Results
Curcumin inhibits nasopharyngeal carcinoma cell proliferation in a dose-dependent manner
CNE-2Z nasopharyngeal carcinoma cells treated with varying concentrations of curcumin for 24 h and inhibition of cell proliferation rates were assessed by MTT colorimetry. Inhibition of CNE-2Z proliferation increased with increasing concentrations of curcumin, from about 20% inhibition with 10 μM curcumin to ~67% inhibition with 80 μM curcumin (Figure 1). Significant differences in proliferation inhibition were detected at these varying doses (P<0.005).
Figure 1.
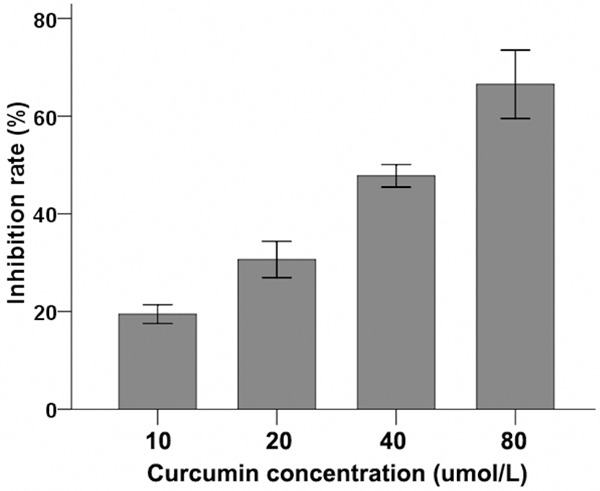
Effect of different concentrations of curcumin on inhibition rate of CNE-2Z cell proliferation. CNE-2Z cells were treated with varying concentrations of curcumin for 24 h, 3 replicates per concentration. After treatment, MTT colorimetry was used to detect the inhibition of cell proliferation. Significant differences were observed in inhibition rates, with inhibition increasing as the dose of curcumin increased (P<0.005).
Curcumin effects expression of ERK-1/2 pathway proteins in CNE-2Z cells
To determine whether curcumin treatment affects expression of proteins in the ERK-1/2 pathway, of CNE-2Z cells cultured with varying doses of curcumin (as above) were also analyzed by Western blot to detect relative expression of ERK-1/2, p-ERK-1/2, TIMP-1, and MMP-9 (Figure 2). No significant difference in relative expression was observed for ERK-1/2 (Figure 3A). Treatment of CNE-2Z cells with 10, 20, 40, and 80 μM curcumin inhibited p-ERK-1/2 expression; inhibition increased with increasing curcumin concentration (Figure 3B, P<0.05). Relative MMP-9 expression also significantly decreased (Figure 3C), while expression of the MMP inhibitor TIMP-1 significantly increased (Figure 3D, P<0.05).
Figure 2.
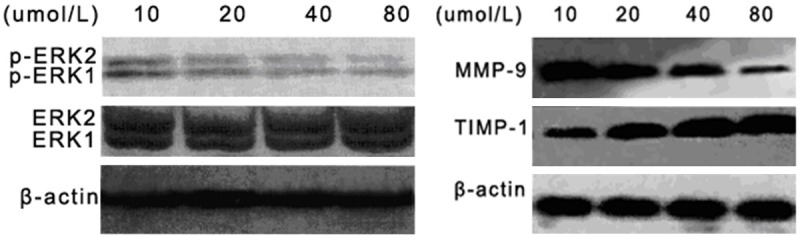
Immunoblot reveals altered expression of p-ERK-1/2, MMP-9, and TIMP-1 in CNE-2Z cells treated with curcumin. Cells were cultured with varying concentrations of curcumin (10 μM, 20 μM, 40 μM, 80 μM) for 24 h, then subjected to Western blot analysis using antibodies against ERK-1, ERK-2, p-ERK1, p-ERK-2, MMP-9, and TIMP-1. Relative protein expression was quantified using β-actin as a control.
Figure 3.
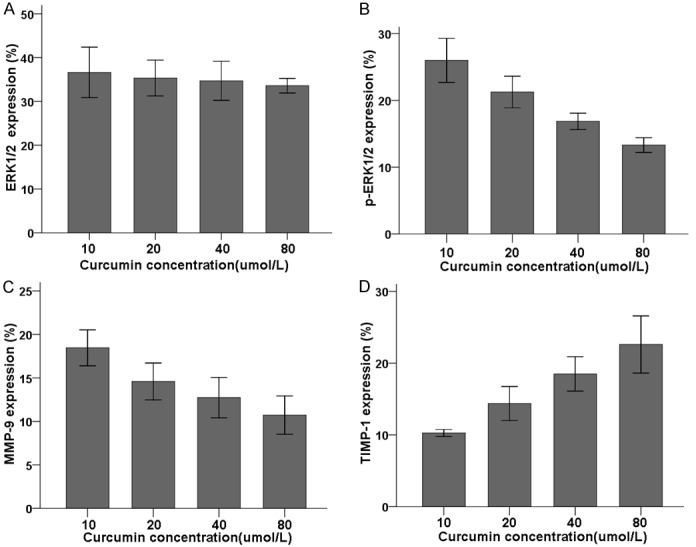
Effect of different concentrations of curcumin on relative expression of ERK-1/2 (A), p-ERK-1/2 (B), MMP-9 (C), and TIMP-1 (D) proteins in CNE-2Z cells. CNE-2Z cells were treated for 24 h with varying concentrations of curcumin, subjected to Western blot, and analyzed for relative expression level compared to β-actin, with 3 replicates per concentration. Relative expression of ERK-1 and ERK-2 (A) were unchanged across curcumin concentrations, but relative expression of the phosphorylated forms of these proteins (B) decreased with increasing curcumin concentration (P<0.05). Relative expression of MMP-9 (C) also decreased with increasing curcumin concentration, while expression of TIMP-1 (D) increased with increasing concentration of curcumin.
Curcumin reduces tumor xenograft size in mice
Volume of tumor xenografts was measured every 10 days after inoculation. Mice were treated with vehicle control, low-dose curcumin, or high-dose curcumin every other day for 40 days. Tumor xenograft volume was significantly smaller in the high-dose (50 mg/kg curcumin) and low-dose (25 mg/kg curcumin) groups compared with the control group (Figure 4); further, tumor volume was significantly smaller in the high-dose group than in the low-dose group (P<0.05). Tumors were dissected and weighed after the 40-day treatment period, and tumors weighed significantly less after high-dose (2.10±0.18 g) and low-dose (3.54±0.56 g) curcumin treatment than after vehicle treatment (4.63±0.73 g; F=32.754, P=0.001). Further, when tumor sections were analyzed under light microscopy, xenograft tumors from mice treated with high-dose curcumin had necrosis in the center and ulceration on the surface (not shown). In contrast, tumor sections from mice in the low-dose group had relatively mild necrosis and ulceration, while tumors from mice in the control group did not display necrosis.
Figure 4.
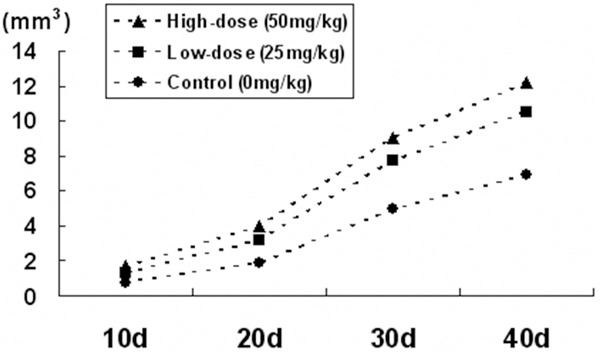
Volume of CNE-2Z tumor xenografts in nude mice treated with vehicle only (Control), with low-dose curcumin (Low-dose), or with high-dose curcumin (High-dose) every other day for 40 days (n=6 per group). Over the study period, tumor volumes were significantly lower in the high-dose group compared to either the control or low-dose groups; tumor volumes in the low-dose group were significantly lower than in the control group.
Discussion
Despite occurring commonly in Southeast Asia and southern provinces of China, nasopharyngeal rarely occurs in Northern China, Europe, and America [18,19]. Occurrence, progression, and metastasis of nasopharyngeal carcinoma is considered a multi-factor, multi-step process, as it shows a continuous quantity differential change in trait variation [20,21]. Importantly, by the time of diagnosis more than 70% of cases have already had cervical lymph node metastasis, and 20-35% of the cases have had distant metastasis; thus, determining the mechanisms behind its invasion and metastasis is important for improving diagnosis and prognosis [22].
In this study, human nasopharyngeal carcinoma CNE-2Z cells treated with curcumin demonstrated inhibited proliferation in a dose-dependent manner; inhibition increased with increasing curcumin concentration. Consistent with this, when CNE-2Z cells were xenografted into nude mice that were subsequently treated with varying concentrations of curcumin, tumor sizes were significantly smaller with increasing concentration of curcumin. Additionally, tumor sections from treated mice displayed necrosis and ulceration, ranging from mild in the low-dose group to severe in the high-dose group. Thus, curcumin treatment appears to slow proliferation of tumors and may also lead to apoptosis of cancer cells.
This inhibition of cell proliferation appears to involve changes in MAPK signaling, particularly in the ERK-1/2 pathway. This signaling pathway plays an important role in the growth, development, proliferation, and malignant transformation of cells, with ERK-1/2 as the key molecules. Indeed, the ERK-1/2 pathway is abnormally activated in a variety of tumors; p-ERK-1/2 can activate other proteins, thereby promoting tumor initiation, invasion, and metastasis [23]. In the current study, curcumin administration inhibited p-ERK-1/2 expression in CNE-2Z cells in a dose-dependent manner, but, interestingly, had no effect on total ERK-1/2 expression. This finding indicates that curcumin can effectively inhibit the ERK-1/2 signaling pathway by (directly or indirectly) altering the phosphorylation of ERK-1/2. This, in turn, may promote changes in expression of downstream proteins like MMP-9 and TIMP-1. MMP-9 degrades extracellular matrix and is implicated in promoting infiltration of cancer cells into the surrounding tissues and playing an important regulatory role in tumor angiogenesis, tumor invasion, and metastasis [24]. In tumor cells, synthesis of MMPs is negatively regulated by the Raf/ERK pathway [25]. TIMPs, on the other hand, are inhibitors of MMPs, and the balance of TIMPs and MMPs is very important in regulating homeostasis of extracellular matrix [25]. In fact, activity and expression of TIMPs are reduced in malignant tumors [15], thus upregulation of MMPs and downregulation of TIMPs are likely important for invasion and metastasis of cancer cells. Here, we have shown that curcumin administration in vitro decreased MMP-9 expression and increased TIMP-1 expression; this effect likely promotes apoptosis of CNE-2Z cells, observed as necrosis and ulceration on the tumor tissue sections.
In summary, curcumin administration affected expression of proteins in the ERK-1/2 pathway, particularly altering the MMP/TIMP ratio. The treatment also inhibited proliferation of CNE-2Z cells in vitro and resulted in smaller tumor size in vivo. These findings indicate a potential mechanism for the anti-cancer activity of curcumin through the ERK-1/2 pathway, and provide a basis for future investigation of curcumin as a treatment for nasopharyngeal carcinoma.
Disclosure of conflict of interest
None.
References
- 1.Asher GN, Spelman K. Clinical utility of curcumin extract. Altern Ther Health Med. 2013;19:20–22. [PubMed] [Google Scholar]
- 2.Choudhuri T, Pal S, Agwarwal ML, Das T, Sa G. Curcumin induces apoptosis in human breast cancer cells through p53-dependent Bax induction. FEBS Lett. 2002;512:334–340. doi: 10.1016/s0014-5793(02)02292-5. [DOI] [PubMed] [Google Scholar]
- 3.Menon LG, Kuttan R, Kuttan G. Anti-metastatic activity of curcumin and catechin. Cancer Lett. 1999;141:159–165. doi: 10.1016/s0304-3835(99)00098-1. [DOI] [PubMed] [Google Scholar]
- 4.Chen HW, Yu SL, Chen JJ, Li HN, Lin YC, Yao PL, Chou HY, Chien CT, Chen WJ, Lee YT, Yang PC. Anti-invasive gene expression profile of curcumin in lung adenocarcinoma based on a high throughput microarray analysis. Mol Pharmacol. 2004;65:99–110. doi: 10.1124/mol.65.1.99. [DOI] [PubMed] [Google Scholar]
- 5.Gupta SC, Kismali G, Aggarwal BB. Curcumin, a component of turmeric: from farm to pharmacy. Biofactors. 2013;39:2–13. doi: 10.1002/biof.1079. [DOI] [PubMed] [Google Scholar]
- 6.Shehzad A, Lee J, Lee YS. Curcumin in various cancers. Biofactors. 2013;39:56–68. doi: 10.1002/biof.1068. [DOI] [PubMed] [Google Scholar]
- 7.Kunnumakkara AB, Anand P, Aggarwal BB. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008;269:199–225. doi: 10.1016/j.canlet.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Gupta SC, Prasad S, Kim JH, Patchva S, Webb LJ, Priyadarsini IK, Aggarwal BB. Multi targeting by curcumin as revealed by molecular interaction studies. Nat Prod Rep. 2011;28:1937–1955. doi: 10.1039/c1np00051a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shehzad A, Lee YS. Molecular mechanisms of curcumin action: signal transduction. Biofactors. 2013;39:27–36. doi: 10.1002/biof.1065. [DOI] [PubMed] [Google Scholar]
- 10.Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta. 2010;1802:396–405. doi: 10.1016/j.bbadis.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Blüthgen N, Legewie S. Systems analysis of MAPK signal transduction. Essays Biochem. 2008;45:95–107. doi: 10.1042/BSE0450095. [DOI] [PubMed] [Google Scholar]
- 12.Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta. 2010;1802:396–405. doi: 10.1016/j.bbadis.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- 14.Cho HS, Chang SH, Chung YS, Shin JY, Park SJ, Lee ES, Hwang SK, Kwon JT, Tehrani AM, Woo M, Noh MS, Hanifah H, Jin H, Xu CX, Cho MH. Synergistic effect of ERK inhibition on tetrandrine-induced apoptosis in A549 human lung carcinoma cells. J Vet Sci. 2009;10:23–28. doi: 10.4142/jvs.2009.10.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rao JS. Molecular mechanisms of glioma invasiveness: the role of proteases. Nat Rev Cancer. 2003;3:489–501. doi: 10.1038/nrc1121. [DOI] [PubMed] [Google Scholar]
- 16.Shuman Moss LA, Jensen-Taubman S, Stetler-Stevenson WG. Matrix metalloproteinases: changing roles in tumor progression and metastasis. Am J Pathol. 2012;181:1895–1899. doi: 10.1016/j.ajpath.2012.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Subramaniam D, Ponnurangam S, Ramamoorthy P, Standing D, Battafarano RJ, Anant S, Sharma P. Curcumin induces cell death in esophageal cancer cells through modulating Notch signaling. PLoS One. 2012;7:e30590. doi: 10.1371/journal.pone.0030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chou J, Lin YC, Kim J, You L, Xu Z, He B, Jablons DM. Nasopharyngeal carcinoma--review of the molecular mechanisms of tumorigenesis. Head Neck. 2008;30:946–63. doi: 10.1002/hed.20833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao Y, DePinho RA, Ernst M, Vousden K. Cancer research: past, present and future. Nat Rev Cancer. 2011;11:749–754. doi: 10.1038/nrc3138. [DOI] [PubMed] [Google Scholar]
- 20.Jia WH, Qin HD. Non-viral environmental risk factors for nasopharyngeal carcinoma: A systematic review. Semin Cancer Biol. 2012;22:117–126. doi: 10.1016/j.semcancer.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 21.Hildesheim A, Wang CP. Genetic predisposition factors and nasopharyngeal carcinoma risk: A review of epidemiological association studies, 2000-2011: Rosetta stone for NPC: Genetics, viral infection, and other environmental factors. Semin Cancer Biol. 2012;22:107–116. doi: 10.1016/j.semcancer.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tao Y, Bidault F, Bosq J, Bourhis J. Distant metastasis of undifferentiated carcinoma of nasopharyngeal type. Onkologie. 2008;31:574–575. doi: 10.1159/000164934. [DOI] [PubMed] [Google Scholar]
- 23.Mccubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M, Tafuri A, Stivala F, Libra M, Basecke J, Evangelisti C, Martelli AM, Franklin RA. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773:1263–1284. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Urbanski SJ, Edwards DR, Maitland A, Leco KJ, Watson A, Kossakowska AE. Expression of metalloproteinases and their inhibitors in primary pulmonary carcinomas. Br J Cancer. 1992;66:1188–1194. doi: 10.1038/bjc.1992.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang D, Bar Eli M, Meloche S, Brodt P. Dual regulation of MMP-2 expression by the type 1 insulin-like growth factor receptor: the phosphatidylinositol 3-kinase/Akt and Raf/ERK pathways transmit opposing signals. J Biol Chem. 2004;279:19683–19690. doi: 10.1074/jbc.M313145200. [DOI] [PubMed] [Google Scholar]


