This study analyzed how patient age affected the angiogenic properties of adipose-derived mesenchymal stromal cells (ADSCs). ADSCs from aged patients both with and without coronary artery disease acquire aging characteristics, and their angiogenic potential declines because of decreasing proangiogenic factor secretion. This could restrict the effectiveness of autologous cell therapy with ADSCs in aged patients.
Keywords: Adipose-derived stromal cells, Aging, Cell therapy, Therapeutic angiogenesis, Urokinase receptor, Coronary artery disease
Abstract
Tissue regeneration is impaired in aged individuals. Adipose-derived mesenchymal stromal cells (ADSCs), a promising source for cell therapy, were shown to secrete various angiogenic factors and improve vascularization of ischemic tissues. We analyzed how patient age affected the angiogenic properties of ADSCs. ADSCs were isolated from subcutaneous fat tissue of patients with coronary artery disease (CAD; n = 64, 43–77 years old) and without CAD (n = 31, 2–82 years old). ADSC phenotype characterized by flow cytometry was CD90+/CD73+/CD105+/CD45−/CD31− for all samples, and these cells were capable of adipogenic and osteogenic differentiation. ADSCs from aged patients had shorter telomeres (quantitative reverse transcription polymerase chain reaction) and a tendency to attenuated telomerase activity. ADSC-conditioned media (ADSC-CM) stimulated capillary-like tube formation by endothelial cells (EA.hy926), and this effect significantly decreased with the age of patients both with and without CAD. Angiogenic factors (vascular endothelial growth factor, placental growth factor, hepatocyte growth factor, angiopoetin-1, and angiogenin) in ADSC-CM measured by enzyme-linked immunosorbent assay significantly decreased with patient age, whereas levels of antiangiogenic factors thrombospondin-1 and endostatin did not. Expression of angiogenic factors in ADSCs did not change with patient age (real-time polymerase chain reaction); however, gene expression of factors related to extracellular proteolysis (urokinase and its receptor, plasminogen activator inhibitor-1) and urokinase-type plasminogen activator receptor surface expression increased in ADSCs from aged patients with CAD. ADSCs from aged patients both with and without CAD acquire aging characteristics, and their angiogenic potential declines because of decreasing proangiogenic factor secretion. This could restrict the effectiveness of autologous cell therapy with ADSCs in aged patients.
Introduction
Cardiovascular diseases, including coronary artery disease (CAD), are the most frequent causes of mortality in most of countries, despite the prominent progress in conservative and surgical approaches to the stimulation of vascularization. Therapeutic angiogenesis based on the injection of gene constructs with growth factors or stem/progenitor cells into ischemic tissues provides an attractive, novel option to treat such diseases. Multipotent mesenchymal stromal cells (MSCs), derived from bone marrow or adipose tissue, are considered one of the most promising therapeutic agents for tissue regeneration because of their proliferation and differentiation potential, ability to stimulate angiogenesis, and immunologic privilege. Adipose tissue is an ideal source for MSCs because it is largely dispensable, and adipose-derived MSCs (ADSCs) are easily accessible in great amounts with minimal invasiveness compared with bone marrow-derived MSCs [1]. It was shown that local and systemic transplantation of ADSCs in animal models of hind limb ischemia and myocardial infarction (MI) led to an increase in the number of new blood vessels and improved blood perfusion within damaged tissue [2–9]. In attempts to explain the ability of ADSCs to stimulate angiogenesis, different mechanisms are considered. First, these cells produce multiple angiogenic factors that activate migration and proliferation of endothelial cells and their progenitors for new vessel formation [2, 6, 9–11]. Second, ADSCs secrete plasminogen activators and matrix proteases that initiate extracellular matrix (ECM) remodeling. It is required for the migration of cells forming the vessel wall and the release of angiogenic factors sequestered in ECM [12]. Third, ADSCs might differentiate into smooth muscle cells and endothelial cells and stabilize newly formed vessels by functioning as pericytes [3, 13–15]. It is in line with the paradigm that MSCs localize in perivascular space within all tissues and play an important role in vascular network development and remodeling, both in normal and pathological conditions [16].
Although ADSCs have already been used in several clinical trials of cell therapy for myocardial ischemia (e.g., ADVANCE, PRECISE, MyStromalCell Trial) [1, 17–20], their properties in patients with cardiovascular diseases are poorly investigated. Most of the data regarding ADSC regenerative potential were obtained from cells delivered from relatively healthy young donors; however, it was known that aging and disease itself may negatively affect MSC activities [21–26], including proliferation and differentiation potential [27–30] as well as angiogenic properties [29, 31]. Because MSCs are considered to be components of the vessel wall and take part in its reparation after injury, their cellular modification due to aging can be an important pathogenic factor of age-related diseases such as atherosclerosis, diabetes, and arterial hypertension [32]. Impairment of MSC angiogenic properties with age may cause lower effectiveness of autologous cell therapy in aged patients with CAD and chronic hind limb ischemia, the most feasible candidates for therapeutic angiogenesis using stem/progenitor cells.
The aim of the current study was to investigate how patient age affects the properties of ADSCs, with special emphasis on their ability to stimulate angiogenesis. We analyzed angiogenic properties of ADSCs in the cohorts of patients both with CAD and without cardiovascular pathology. The obtained results provide new insights into molecular mechanisms underlying the age-related decline of the therapeutic potential of stem/progenitor cells. Our findings are necessary to increase the effectiveness of autologous cell therapy and to develop novel approaches to the stimulation of endogenous regenerative processes.
Materials and Methods
Patients
Subcutaneous adipose tissue samples (0.5–5 ml) were obtained from patients during various surgical procedures. Thirty-one patients without established cardiovascular diseases were included in the first group. They underwent surgery because of general surgical pathology (appendicitis, hernia), traumas, or hip and knee replacement. The second group comprised 64 patients with CAD (obliterating coronary stenosis confirmed by angiography, stable angina of New York Heart Association functional class II–IV) who underwent coronary artery bypass surgery at the Department of Cardiovascular Surgery of the Russian Cardiology Research and Production Complex.
Exclusion criteria for both groups were autoimmune pathologies, cancer (including past history), acute or chronic inflammatory disease, acute MI in the previous month, heart failure (New York Heart Association functional class III–IV), decompensated diabetes mellitus (HbA1c ≥8%), long-term hormone or antibiotic therapy, anemia (Hb ≤10 g/dl) and hematological disorders, or stroke or craniocerebral injury in the previous 12 months.
Additional special exclusion criteria for the first group (patients without CAD) were any evidence of CAD (based on standard clinical and instrumental examination) including MI or myocarditis, clinical signs of angina or heart failure, arrhythmias such as paroxysmal or permanent form of atrial fibrillation, frequent premature ventricular complexes, paroxysmal ventricular tachycardia, left bundle branch block, clinical manifestation of systemic atherosclerosis, severe dyslipidemia, severe arterial hypertension, valvular diseases, or pulmonary hypertension.
All procedures performed with tissue samples were approved by the ethics committee of the Russian Cardiology Research and Production Complex of the Russian Ministry of Health. Written informed consent for harvesting and using adipose tissue samples for research purposes was obtained from each patient.
Patients in both groups were distributed into cohorts by age according to World Health Organization classification (1963). Age cohort characteristics of patients with and without CAD are presented in Table 1.
Table 1.
Characteristics of patients involved in the study
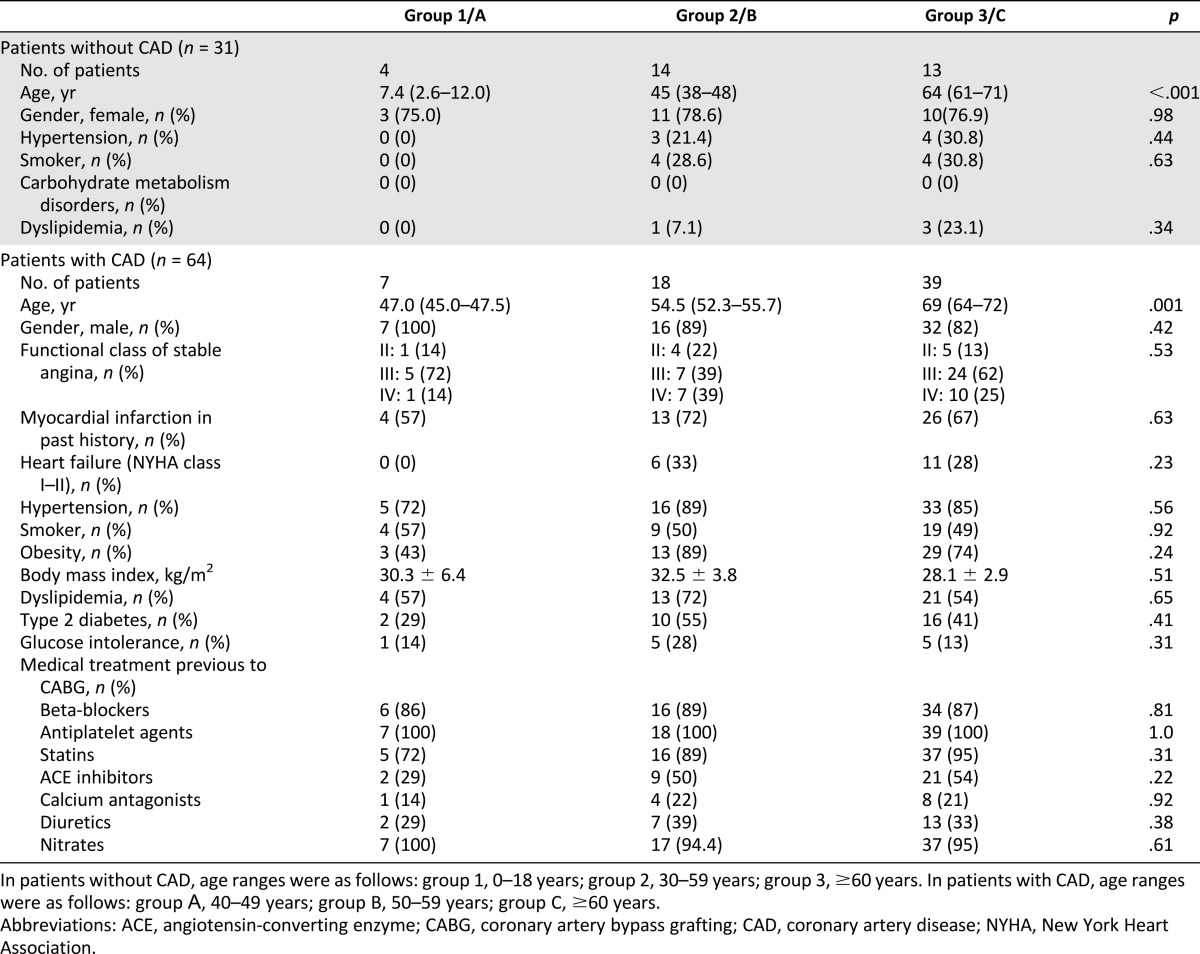
In both groups of patients, there were no statistically significant differences between age cohorts with regard to clinical characteristics, but it should be noted that groups of patients with and without CAD were disparate because of significant differences in gender, age, and other criteria. Consequently, we analyzed age-associated changes of ADSC properties within each group of patients, without comparing them.
Isolation of ADSCs
Subcutaneous adipose tissue samples (0.5–5 ml) were homogenized and digested by collagenase I (200 U/ml; Worthington Biochemical, Lakewood, NJ, http://www.worthington-biochem.com) and dispase (40 U/ml; Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) solution under agitation for 30–40 minutes at 37°C. Then tissue was centrifuged at 200g for 7 minutes, and the supernatant was discarded. The pellet containing ADSCs was lysed to destroy erythrocytes, filtered through a sieve (BD Falcon Cell Strainer, 100 μm; BD Biosciences, San Diego, CA, http://www.bdbiosciences.com) and centrifuged at 200g for 5 minutes. The final pellet was resuspended in culture medium. The cells were cultured in standard conditions (5% carbon dioxide; 37°С) in Advance Stem Cell Basal Medium (ASCBM; HyClone, Logan, UT, http://www.hyclone.com) with 10% Advance Stem Cell Growth Supplement (HyClone), 100 U/ml penicillin/streptomycin, and 100 U/ml fungisone (HyClone). At 24 hours after isolation, nonattached cells were washed off, then medium was changed every 3–4 days. Cell yield was 4–7 × 104 of attached cells per milliliter of tissue. At the second passage, cells (70%–80% confluent) were washed in supplement-free ASCBM overnight, and then the medium was changed and ADSCs were incubated in supplement-free ASCBM for 48 hours. Conditioned medium was collected, supplemented with Protease Inhibitor Cocktail (1:500; Sigma-Aldrich), and frozen in aliquots at −70°С. ADSCs were harvested using HQtase (HyClone), counted, washed in phosphate buffered saline, and frozen in pellet at −70°С.
Analysis of Angiogenic Factors Accumulation in ADSC-Conditioned Medium by Enzyme-Linked Immunosorbent Assay
Vascular endothelial growth factor (VEGF), placental growth factor (PlGF), hepatocyte growth factor (HGF), angiopoetin 1 (ANGPT1), angiogenin (ANG), thrombospondin-1 (THBS1), endostatin (ENDS), and plasminogen activator inhibitor 1 (PAI-1) were measured in ADSC-CM using enzyme-linked immunosorbent assay (R&D Systems Inc., Minneapolis, MN, http://www.rndsystems.com) according to manufacturer instructions. The level of each protein was normalized to cell counts for each sample of conditioned medium.
In Vitro Tube Formation Assay
Effect of ADSC-CM on capillary-like tube formation by endothelial cells EA.hy926 on Matrigel (BD Biosciences) in vitro [33] was evaluated.
Data Analysis and Statistics
Statistical analysis was performed using SigmaStat 9.0 software (Systat Software Inc., Richmond, CA, http://www.systat.com). Values are expressed as mean ± SD for normally distributed data and as median and percentiles (25%–75%) for non-normal data. If normality of data were confirmed (according to the Kolmogorov-Smirnov test and Shapiro-Wilk’s W test), comparison of independent groups was performed by Student’s t test; if the data were not confirmed or if size of the analyzed sample was <10 cases, comparison was performed by Mann-Whitney U criteria. Multiple comparisons were made using one-way ANOVA for normally distributed data and otherwise by Kruskall-Wallis test. For comparison of nominal variable distribution, the chi-square method was used. Correlation analysis was performed using Pearson correlation if both analyzed samples were normally distributed, otherwise the Spearman correlation was applied. Statistical significance was defined as a p value <.05, and all reported statistical tests were two-tailed. Several graphs were generated using Statistica 6.0 software (StatSoft, Tulsa, OK, http://www.statsoft.com).
More detailed methods are presented in the supplemental online data.
Results
ADSCs From Patients With Different Pathologies Keep MSC Characteristics During Chronological Aging
ADSCs isolated from patients of different ages and cultured to the second passage, as described previously, comprised a relatively homogenous population of cells with a fibroblast-like shape. These cells met all minimal criteria for defining MSCs according to the International Society for Cellular Therapy position statement [34]. ADSCs were positive for CD73 (>85%), CD90 (>95%), and CD105 (>95%), with no or low expression of CD14 (<10%), CD19 (<10%), CD34 (<5%), CD45 (<1%), and CD79 (<10%). These cells also expressed pericyte markers like NG2 and PDGFRB, which agreed with a concept of this type of progenitor as perivascular cells. ADSCs isolated from patients of different ages did not differ by immunophenotype.
A defining characteristic of MSCs is an ability to differentiate into adipocytes, osteoblasts, and chondroblasts. ADSCs from each group of patients demonstrated similar abilities for bone mineralization and neutral lipid accumulation in the appropriate culture medium and conditions, thus confirming their multipotency (data not shown).
ADSC From Aged Patients Acquire the Characteristics of Aging
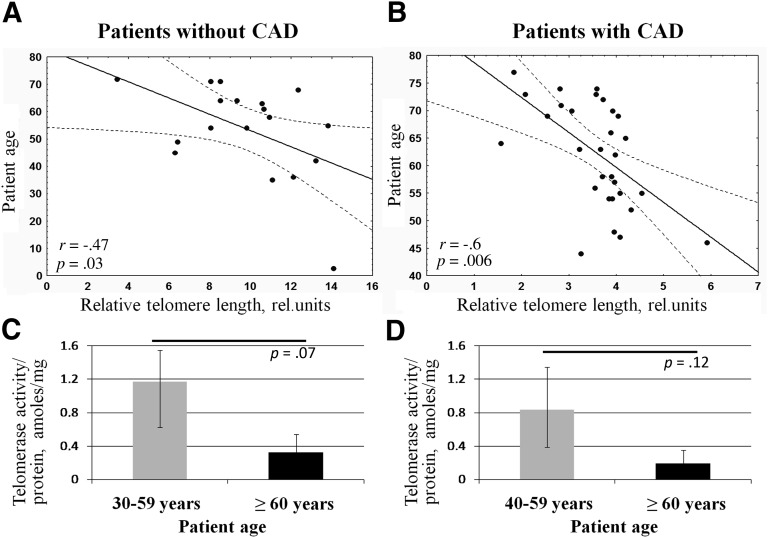
Chronological aging is associated with several cell “biomarkers” such as telomere shortening, attenuated telomerase activity, and increased expression of cell cycle inhibitors leading to the decrease of proliferation activity [21, 35, 36]. We evaluated relative telomere length (as previously described [37]) and telomerase activity of ADSCs from patients with and without CAD. We observed significant negative correlation between relative telomere length and age of patients without cardiovascular pathology (r = −.47, p = .03). In cells isolated from patients with CAD, the observed correlation was even more prominent (r = −.6, p = .006) (Fig. 1A, 1B).
Figure 1.
Adipose-derived mesenchymal stromal cells (ADSCs) from older patients exhibit the characteristics of aging. (A, B): Negative correlation between relative telomere length in ADSCs and age of patients without cardiovascular pathology (n = 18) (A) and with CAD (n = 31) (B). (C, D): Telomerase activity in ADSCs obtained from patients without cardiovascular pathology (C) and with CAD (D). Data are shown as median and percentiles (25%–75%). Abbreviations: amoles, attomoles; CAD, coronary artery disease; rel., related.
Telomerase activity in ADSCs obtained from patients with and without CAD was approximately fivefold lower than in HeLa cells, which served as a control in this test. Measurement of telomerase activity in ADSCs did not reveal significant changes between age subgroups or any significant correlation between telomerase activity and patient age, probably because of high variability between samples. Among patients without cardiovascular pathologies, average telomerase activity tended to decrease in ADSCs from patients older than 60 years compared with patients 30–59 years old (3.5-fold lower, p = .07) (Fig. 1C). For patients with CAD, the trend was similar but the differences between age subgroups were nonsignificant (Fig. 1D). In addition, no significant association was detected between telomerase activity and relative telomere length in both groups of patients.
ADSCs From Aged Patients Have Impaired Angiogenic Potential
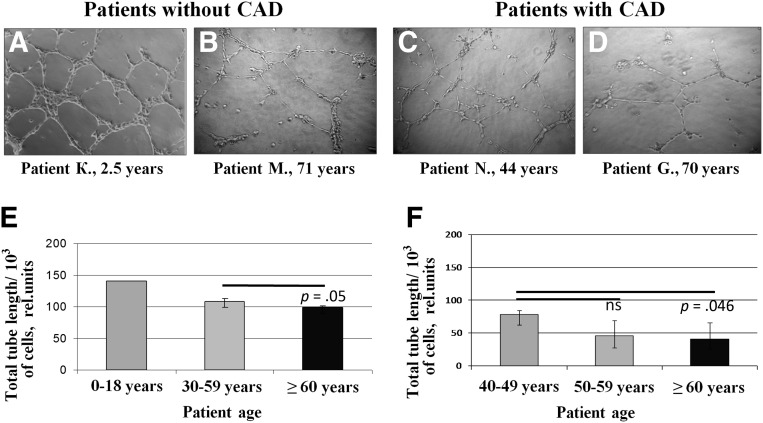
Considering that the ability of ADSCs to stimulate blood vessel growth is mainly mediated by paracrine mechanisms through the production of various angiogenic factors, we analyzed angiogenic activity of summary products secreted by ADSCs on the model of capillary-like tube formation by endothelial cells on Matrigel. ADSC-CM stimulated formation of capillary-like tubes, but this effect essentially decreased with the ages of patients both with and without CAD (Fig. 2). Impairment of angiogenic activity of ADSCs from older patients was confirmed by the significant negative correlation between total tube length and patient age in both groups (r = −.68, p = .01 for patients without CAD; r = −.38, p = .02 for patients with CAD).
Figure 2.
Angiogenic activity of summary products secreted by adipose-derived mesenchymal stromal cells (ADSCs) decreases with patient age. (A–D): Representative microphotographs of capillary-like tubes formed by endothelial cells EA.hy926 on Matrigel in the presence of ADSC-conditioned media. Total tube length measurement for ADSCs from patients without CAD (E) and with CAD (F). For group 1 (patients without CAD, 2–12 years), the presented result was obtained from one ADSC sample (patient K, 2.5 years old). Data are shown as median and percentiles (25%–75%). Magnification, ×10. Abbreviations: CAD, coronary artery disease; ns, not significant; rel., related.
To reveal which angiogenic factors are involved in ADSC angiogenic activity decreasing with age, we analyzed gene expression of various angiogenesis-related factors in ADSCs by real-time polymerase chain reaction. According to the results of correlation analysis, we did not observe significant age-associated changes in gene expression of VEGF, PlGF, HGF, basic fibroblast growth factor, ANGPT1, and ANG (data not shown).
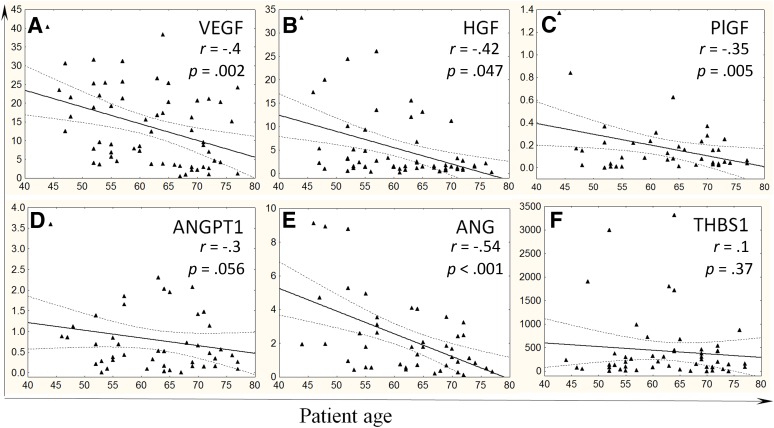
Considering that the level of secreted factors is more essential for paracrine effects of ADSCs on angiogenesis, we evaluated accumulation of key proangiogenic factors in the conditioned medium from ADSCs. We found that ADSCs obtained from older patients with CAD secreted significantly fewer amounts of VEGF, HGF, and ANG, and similar changes were observed for ANGPT1 as a tendency (p = .07) (Table 2). Correlation analysis also showed statistically significant negative correlation between the level of proangiogenic factors (VEGF, PlGF, HGF, ANG) and the age of patients with CAD (Fig. 3A–3E). Because chronic ischemic heart disease itself may negatively affect ADSC properties, we analyzed whether ADSC ability to secrete angiogenic factors declined with the age of patients without cardiovascular pathologies. We found that the level of proangiogenic factors secreted by ADSCs from patients without CAD also negatively correlated with age: VEGF: r = −.42, p = .02; PlGF: r = −.59, p = .01; HGF: r = −.59, p = .03; ANG: r = −.66, p = .005.
Table 2.
Accumulation of angiogenic factors in adipose-derived mesenchymal stromal cell-conditioned media (nanograms per million cells) measured by enzyme-linked immunosorbent assay
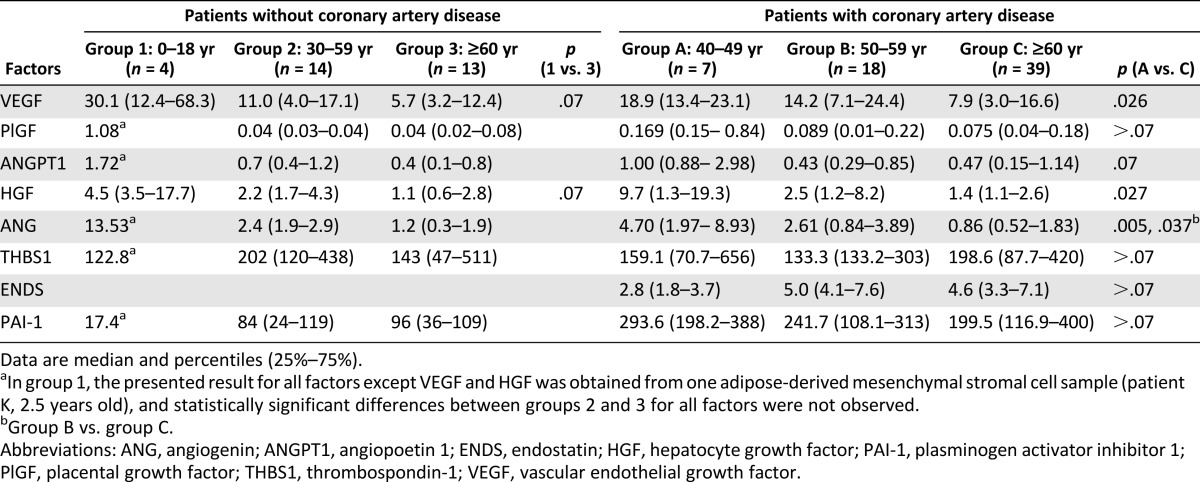
Figure 3.
Proangiogenic growth factor production by adipose-derived mesenchymal stromal cells (ADSCs) from patients with coronary artery disease declines with age. Correlation between patient age and accumulation of VEGF (A), HGF (B), PlGF (C), ANGPT1 (D), ANG (E) as proangiogenic factors, and THBS1 (F) as an antiangiogenic factor in ADSC-conditioned media measured by enzyme-linked immunosorbent assay. y-Axis: Protein content in ADSC-conditioned medium, nanograms per million cells. Abbreviations: ANG, angiogenin; ANGPT1, angiopoetin 1; HGF, hepatocyte growth factor; PlGF, placental growth factor; THBS1, thrombospondin-1; VEGF, vascular endothelial growth factor.
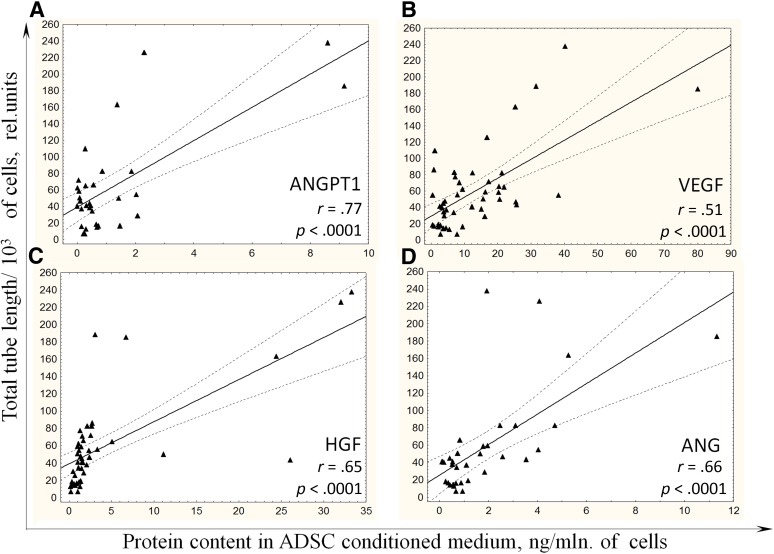
Interestingly, the most prominent correlation between total tube length as an indicator of ADSC angiogenic activity and the level of factor was observed for ANG in the group of patients without CAD (r = .66, p = .05) and for ANGPT1 in the group of patients with CAD (r = .77, p < .0001) (Fig. 4A). In addition, levels of other proangiogenic factors secreted by ADSCs obtained from patients with CAD significantly correlated with total tube length (VEGF: r = .51, p < .0001; HGF: r = .65, p < .0001; ANG: r = .66, p < .0001) (Fig. 4B–4D).
Figure 4.
Angiogenic activity of ADSCs from patients with coronary artery disease correlates with level of proangiogenic growth factors in conditioned medium. Correlations between the levels of ANGPT1 (A), VEGF (B), HGF (C), and ANG (D) in ADSC-conditioned media and angiogenic activity of summary products secreted by ADSCs from patients with coronary artery disease are presented. Abbreviations: ADSC, adipose-derived mesenchymal stromal cell; HGF, hepatocyte growth factor; rel., related; mln, million; VEGF, vascular endothelial growth factor.
Because angiogenesis is regulated by the balance between pro- and antiangiogenic mediators, we proposed that some antiangiogenic factors could contribute to the age-associated impairment of ADSC angiogenic activity. We analyzed gene expression of THBS1 and ENDS in ADSCs as well as their secretion by the cells to the conditioned medium (Table 2). There were no statistically significant correlations between the production of these factors by ADSCs and the age of patients both with and without CAD, at least for THBS1 (Fig. 3F).
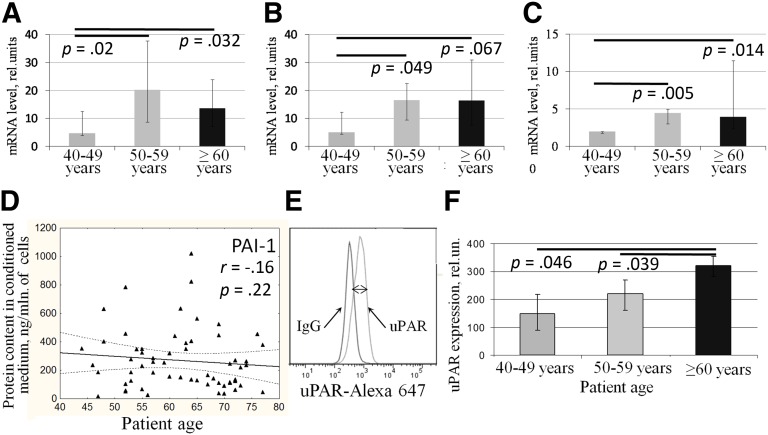
ECM remodeling and directed migration of vascular cells are also crucial for successful angiogenesis. These processes are controlled by several factors including urokinase-type plasminogen activator (uPA) and its receptor (uPAR), PAI-1, and matrix metalloproteases (MMP). We found that gene expression of uPA, uPAR, and PAI-1 significantly increased in ADSCs from aged patients with CAD (Fig. 5A–5C) but not in patients without cardiovascular pathologies (data not shown). We failed to show that ADSCs from older patients with CAD produced more PAI-1 to conditioned medium than ADSCs from younger patients (Fig. 5D); however, we confirmed age-associated increase of uPAR expression on the surface of ADSCs from patients with CAD by flow cytometry (Fig. 5E, 5F). Surprisingly, we found that level of PAI-1 in ADSC-CM positively correlated with the angiogenic activity of ADSCs measured as total tube length (r = .44, p = .003).
Figure 5.
Expression of extracellular proteolysis factors is increased in adipose-derived mesenchymal stromal cells (ADSCs) obtained from aged patients with coronary artery disease (CAD). (A–C): PAI-1 (A), urokinase-type plasminogen activator, (B), and uPAR (C) gene expression in ADSCs obtained from patients with CAD. (D): Correlation between age of patients and accumulation of PAI-1 in ADSC-conditioned media measured by enzyme-linked immunosorbent assay. (E): uPAR expression on the surface of ADSCs measured by flow cytometry as difference between average fluorescence intensity of ADSCs stained with specific and isotype control antibodies (two-sided arrow). (F): Measurement of uPAR expression on the surface of ADSCs from patients with CAD. Data are shown as median and percentiles (25%–75%). Abbreviations: mln, million; rel., related; uPAR, urokinase-type plasminogen activator receptor.
Discussion
Stem/progenitor cells mediate lifelong physiological renewal and regeneration of tissues. Attenuated regeneration potential of aged organisms might be caused by age-associated changes of stem/progenitor cell activity. It was demonstrated that both intrinsic and extrinsic mechanisms are involved in the normal and pathological aging of stem/progenitor cells including MSCs. Bone marrow-derived MSCs from older donors were shown to have worse proliferation and differentiation capacity [21, 22, 27, 38] and were less effective for tissue repair after ischemic injury (e.g., on the animal model of MI) [25, 38]. In contrast to bone marrow-derived MSCs, the number of ADSCs in fat tissue evaluated by flow cytometry does not decrease with age [39, 40], but their clonogenic and proliferation capacity declines [28–30, 39, 41], as does production of VEGF by aged ADSCs [31].
We have previously shown that ADSCs isolated from old mice (18 months), along with acquiring the properties of aged cells (shorter telomeres, higher rate of apoptotic cells, less proliferative capacity, enhanced oxidative damage), have an impaired ability to stimulate blood vessel growth in in vitro and in vivo models of angiogenesis compared with ADSCs from young animals (1–2 months) [42]. In the current study, we analyzed age-associated changes in human ADSCs obtained from both patients without cardiovascular pathology and patients with CAD as a cohort of the most obvious candidates for cell therapy. At the present stage of the study, we were not able to compare these two groups because of significant differences in age and gender. We investigated how ADSC properties change with age within every group.
ADSCs were characterized by flow cytometry for the presence of commonly identified cell-surface markers for MSCs and were consistent with the commonly accepted profile [34]. There were no discernible age-associated changes in MSC marker profiles and ADSC differentiation potential, which correlated with results of Pandey et al. [43].
We found that ADSCs from older patients had some cell characteristics of aging. One was relative telomere length indicating the number of cell divisions. Telomere shortening is considered to be the main causal mechanism for replicative cell senescence, and age-associated telomere damage, diminution of telomere “capping” function, and associated p53 activation have emerged as prime instigators of tissue stem/progenitor cell functional decline [36]. We showed that ADSCs from older patients both with and without CAD had shorter telomeres compared with younger patients and thus confirmed that ADSCs did age during life.
Telomerase is a specific enzyme responsible for maintenance of telomere length, but it also has some telomere-independent functions. Telomerase activity was shown to be the highest in stem and tumor cells and was detected to a certain degree in many kinds of progenitor cells. Its activity is repressed as stem cells start to differentiate [35]. We detected telomerase activity in ADSCs but did not observe its significant reduction with age despite the telomere shortening in ADSCs from older patients. Perhaps there are several subpopulations of ADSCs that vary in telomerase activity, and their balance changes primarily under the influence of aging and pathology. Thus a small subpopulation of cells with relatively high telomerase activity could persist among ADSCs from older patients and garble the summary measurement.
A key mechanism of ADSC therapeutic action is the production of various paracrine factors stimulating angiogenesis and activating endogenous reparation in damaged tissues [2, 4–6, 10, 11, 17, 18]. The role of specific angiogenic factors secreted by ADSCs in realizing their angiogenic potential is poorly studied. To our knowledge, we are first to show that production of different proangiogenic factors such as VEGF, PlGF, HGF, ANGPT1, and ANG by ADSCs from patients both with and without CAD decreases with age, causing reduction of angiogenic activity. It should be noted that age-associated differences in gene expression of proangiogenic factors were not found, indicating that post-transcriptional mechanisms could underlie the decrease of angiogenic factors secretion by ADSCs from older patients, such as regulation by microRNA [24, 30, 43] and age-associated protein misfolding.
The most significant correlation with age of patients in both groups was observed for ANG, which belongs to the family of ribonucleases with specific biological functions and has angiogenic activity. It stimulates endothelial cells proliferation and promotes capillary-like tube formation in vitro [44], which correlates with our data. ANG in complex with actin also induces endothelial and smooth muscle cell invasion by activating cell-associated proteases [45]. It should be noted that the most prominent correlation between ADSC angiogenic activity and level of factor was observed for ANG in the group of patients without CAD but for ANGPT1 in the group of patients with CAD.
ANGPT1, a ligand for the Tie-2 receptor, is a crucial factor for angiogenesis both in normal and pathological conditions because it regulates vessel stabilization, maturation, and remodeling [46, 47]. ANGPT1 has been shown to promote collateral vessel development in tissue ischemia, and coadministration of ANGPT1 and VEGF enhances collateral vessel formation in animal ischemic models [47, 48]. Overexpression in the skin of VEGF alone yielded mice with evidence of leaky vessels, whereas coexpression of ANGPT1 resulted in leakage-resistant vessels [49]. Taken together, the ability of ADSCs to secrete both VEGF and other angiogenic factors including ANG and ANGPT1 provides their angiogenic activity, but production of these factors declines with age, causing the impairment of ADSC angiogenic potential.
Age-associated decrease of ADSC angiogenic activity could also be explained by activation of antiangiogenic factor production by cells from aged patients. One of the potent antiangiogenic factors is ENDS, the C-terminal fragment of collagen XVIII, which significantly modulates the gene expression pattern in endothelial cells and inhibits their proliferation [50]. Another factor, THBS1, inhibits angiogenesis through direct effects on endothelial cell migration, proliferation, survival, and apoptosis and by antagonizing the activity of VEGF [51]. However, we could not find significant age-related changes of THBS1 and ENDS gene expression or secretion by ADSCs, so it is unlikely that they could cause the impairment of ADSC angiogenic properties with age.
In analyzing the expression of factors involved in ECM remodeling and vascular cell migration and invasion, we revealed that mRNA levels of uPA, uPAR, and PAI-1 as well as uPAR surface expression were higher in ADSCs from older patients. These results are consistent with those that we previously obtained with ADSCs from young and old mice [42]. Urokinase, a multidomain protein, not only specifically cleaves plasminogen and converts it into plasmin, particularly causing activation of different MMPs, but also initiates intracellular signaling on binding to its receptor on the cell surface and therefore plays multiple roles in vascular remodeling and angiogenesis [52]. It was demonstrated that the uPA system represented an essential regulatory mechanism in growth factor-induced endothelial cell migration and invasion. Consequently, VEGF, FGF2, and HGF induced PI3K-dependent activation of pro-uPA when bound to uPAR, and this led to an increase in cell surface fibrinolytic activity followed by uPAR internalization and redistribution [53]. uPA gene transfer effectively induces functionally significant angiogenesis in models of acute MI and hind limb ischemia [54]. Therefore, we can speculate that activation of the uPA system in ADSCs from older patients might be a compensatory response to the reduction of proangiogenic factor secretion. It was shown that uPA and uPAR downregulation suppresses angiogenesis in endothelial cells partially by downregulation of angiogenin and inhibition of the angiopoietin-1/AKT/FKHR pathway, and treatment of endothelial cells with recombinant uPA increased ANG secretion [55].
PAI-1 controls the activities of uPA/plasmin/MMP proteolytic activities and thus maintains the tissue homeostasis; it also has a great impact in pathogenesis of multiple age-related diseases, such as cardiovascular pathologies and tumors [56, 57]. Increased PAI-1 expression in adipose tissues plays a significant role in cardiovascular diseases and obesity. Insulin as well as transforming growth factor β (TGFb) stimulates PAI-1 in adipocytes in an ERK1/2 MAPK- and PKC-dependent manner [58]. TGFb also increases PAI-1 expression in human lung fibroblasts [59] and human vein endothelial cells [60] through activation of Nox4 and reactive oxygen species production. Because TGFb expression and reactive oxygen species production are activated in aged MSCs [22, 42], it could explain increasing PAI-1 expression with age. Devy et al. demonstrated that PAI-1 could act as an antiangiogenic factor if presented in micromole concentrations, but in fewer concentrations (up to 100 ng/ml), it stimulated angiogenesis and tumorigenesis [61]. In our study in ADSC-CM, PAI-1 presented in concentrations <100 ng/ml, which explained its positive correlation with ADSC angiogenic activity. We did not observe a significant increase in PAI-1 secretion with age. More detailed analysis of clinically different subgroups of patients with CAD is likely necessary to reveal such trends.
Conclusion
We demonstrated that ADSCs from older patients kept MSC characteristics but acquired some properties of senescent cells. Moreover, aging could essentially affect the angiogenic potential of ADSCs obtained from patients both with and without CAD. This could restrict the effectiveness of autologous cell therapy with ADSCs and require testing of cell material before use as well as development of effective approaches to pretreatment or modification of ADSCs from older patients [42, 62, 63] to enhance therapeutic potential.
Supplementary Material
Footnotes
Contributed equally as first authors.
Acknowledgments
This work was supported by the European Union Seventh Framework Programme (FP7/2007-2013) (grant agreement no. 241558) (SICA-HF), the Russian Ministry of Science and Education within the FTP “R&D in Priority Fields of the S&T Complex of Russia 2007-2012” (no. 02.527.11.0007), the Russian Federal Agency of Science and Innovation (nos. 16.512.11.2251 and 11.519.11.6042), and the Russian Foundation for Basic Research (no. 11-04-01850-a). The sponsors were not involved in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. We thank Dr. I.O. Golubev and Dr. V.I. Makunin for valuable help in delivery of fat tissue samples from patients without cardiovascular pathology. We also thank G.V. Sharonov for performing flow cytometry, E.E. Starostina and E.V. Gluhanyuk for their contribution to data collection, and the staff of the Faculty of Medicine of Lomonosov Moscow State University for assistance with this study.
Author Contributions
A.E.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; N.D.: conception and design, collection and/or assembly of data, data analysis and interpretation, final approval of manuscript; N.K.: conception and design, financial support, data analysis and interpretation, final approval of manuscript; T.K.: collection and/or assembly of data, data analysis and interpretation, final approval of manuscript; R.A.: administrative support, provision of study material or patients, final approval of manuscript; V.T. and Y.P.: conception and design, financial support, administrative support, data analysis and interpretation, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
A.E., N.D., N.K., T.K., V.T., and Y.P. have compensated employment from Lomonosov Moscow State University and compensated research funding. R.A. has compensated employment from Russian Cardiology Research and Production Complex.
References
- 1.Gimble JM, Bunnell BA, Chiu ES, et al. Concise review: Adipose-derived stromal vascular fraction cells and stem cells: Let’s not get lost in translation. Stem Cells. 2011;29:749–754. doi: 10.1002/stem.629. [DOI] [PubMed] [Google Scholar]
- 2.Rehman J, Traktuev D, Li J, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 3.Miyahara Y, Nagaya N, Kataoka M, et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006;12:459–465. doi: 10.1038/nm1391. [DOI] [PubMed] [Google Scholar]
- 4.Nakagami H, Morishita R, Maeda K, et al. Adipose tissue-derived stromal cells as a novel option for regenerative cell therapy. J Atheroscler Thromb. 2006;13:77–81. doi: 10.5551/jat.13.77. [DOI] [PubMed] [Google Scholar]
- 5.Traktuev DO, March KL, Tkachuk VA, et al. Adipose tissue stromal cells — multipotent cells with therapeutic potential for stimulation of angiogenesis in tissue ischemia [in Russian] Kardiologiia. 2006;46:53–63. [PubMed] [Google Scholar]
- 6.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang DZ, Gai LY, Liu HW, et al. Transplantation of autologous adipose-derived stem cells ameliorates cardiac function in rabbits with myocardial infarction. Chin Med J (Engl) 2007;120:300–307. [PubMed] [Google Scholar]
- 8.Cai L, Johnstone BH, Cook TG, et al. IFATS collection: Human adipose tissue-derived stem cells induce angiogenesis and nerve sprouting following myocardial infarction, in conjunction with potent preservation of cardiac function. Stem Cells. 2009;27:230–237. doi: 10.1634/stemcells.2008-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kondo K, Shintani S, Shibata R, et al. Implantation of adipose-derived regenerative cells enhances ischemia-induced angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29:61–66. doi: 10.1161/ATVBAHA.108.166496. [DOI] [PubMed] [Google Scholar]
- 10.Rubina K, Kalinina N, Efimenko A, et al. Adipose stromal cells stimulate angiogenesis via promoting progenitor cell differentiation, secretion of angiogenic factors, and enhancing vessel maturation. Tissue Eng Part A. 2009;15:2039–2050. doi: 10.1089/ten.tea.2008.0359. [DOI] [PubMed] [Google Scholar]
- 11.Madonna R, De Caterina R. Adipose tissue: A new source for cardiovascular repair. J Cardiovasc Med (Hagerstown) 2010;11:71–80. doi: 10.2459/JCM.0b013e328330e9be. [DOI] [PubMed] [Google Scholar]
- 12.Kachgal S, Putnam AJ. Mesenchymal stem cells from adipose and bone marrow promote angiogenesis via distinct cytokine and protease expression mechanisms. Angiogenesis. 2011;14:47–59. doi: 10.1007/s10456-010-9194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sumi M, Sata M, Toya N, et al. Transplantation of adipose stromal cells, but not mature adipocytes, augments ischemia-induced angiogenesis. Life Sci. 2007;80:559–565. doi: 10.1016/j.lfs.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 14.Planat-Benard V, Silvestre JS, Cousin B, et al. Plasticity of human adipose lineage cells toward endothelial cells: Physiological and therapeutic perspectives. Circulation. 2004;109:656–663. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 15.Miranville A, Heeschen C, Sengenès C, et al. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349–355. doi: 10.1161/01.CIR.0000135466.16823.D0. [DOI] [PubMed] [Google Scholar]
- 16.Nombela-Arrieta C, Ritz J, Silberstein LE. The elusive nature and function of mesenchymal stem cells. Nat Rev Mol Cell Biol. 2011;12:126–131. doi: 10.1038/nrm3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madonna R, Geng YJ, De Caterina R. Adipose tissue-derived stem cells: Characterization and potential for cardiovascular repair. Arterioscler Thromb Vasc Biol. 2009;29:1723–1729. doi: 10.1161/ATVBAHA.109.187179. [DOI] [PubMed] [Google Scholar]
- 18.Murohara T, Shintani S, Kondo K. Autologous adipose-derived regenerative cells for therapeutic angiogenesis. Curr Pharm Des. 2009;15:2784–2790. doi: 10.2174/138161209788923796. [DOI] [PubMed] [Google Scholar]
- 19.Bailey AM, Kapur S, Katz AJ. Characterization of adipose-derived stem cells: An update. Curr Stem Cell Res Ther. 2010;5:95–102. doi: 10.2174/157488810791268555. [DOI] [PubMed] [Google Scholar]
- 20.Fehrer C, Lepperdinger G. Mesenchymal stem cell aging. Exp Gerontol. 2005;40:926–930. doi: 10.1016/j.exger.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Sethe S, Scutt A, Stolzing A. Aging of mesenchymal stem cells. Ageing Res Rev. 2006;5:91–116. doi: 10.1016/j.arr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Stolzing A, Jones E, McGonagle D, et al. Age-related changes in human bone marrow-derived mesenchymal stem cells: Consequences for cell therapies. Mech Ageing Dev. 2008;129:163–173. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Katsara O, Mahaira LG, Iliopoulou EG, et al. Effects of donor age, gender, and in vitro cellular aging on the phenotypic, functional, and molecular characteristics of mouse bone marrow-derived mesenchymal stem cells. Stem Cells Dev. 2011;20:1549–1561. doi: 10.1089/scd.2010.0280. [DOI] [PubMed] [Google Scholar]
- 24.Kim M, Kim C, Choi YS, et al. Age-related alterations in mesenchymal stem cells related to shift in differentiation from osteogenic to adipogenic potential: Implication to age-associated bone diseases and defects. Mech Ageing Dev. 2012;133:215–225. doi: 10.1016/j.mad.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 25.Khan M, Mohsin S, Khan SN, et al. Repair of senescent myocardium by mesenchymal stem cells is dependent on the age of donor mice. J Cell Mol Med. 2011;15:1515–1527. doi: 10.1111/j.1582-4934.2009.00998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madonna R, Renna FV, Cellini C, et al. Age-dependent impairment of number and angiogenic potential of adipose tissue-derived progenitor cells. Eur J Clin Invest. 2011;41:126–133. doi: 10.1111/j.1365-2362.2010.02384.x. [DOI] [PubMed] [Google Scholar]
- 27.Sun Y, Li W, Lu Z, et al. Rescuing replication and osteogenesis of aged mesenchymal stem cells by exposure to a young extracellular matrix. FASEB J. 2011;25:1474–1485. doi: 10.1096/fj.10-161497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu M, Kohan E, Bradley J, et al. The effect of age on osteogenic, adipogenic and proliferative potential of female adipose-derived stem cells. J Tissue Eng Regen Med. 2009;3:290–301. doi: 10.1002/term.165. [DOI] [PubMed] [Google Scholar]
- 29.Huang SC, Wu TC, Yu HC, et al. Mechanical strain modulates age-related changes in the proliferation and differentiation of mouse adipose-derived stromal cells. BMC Cell Biol. 2010;11:18. doi: 10.1186/1471-2121-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alt EU, Senst C, Murthy SN, et al. Aging alters tissue resident mesenchymal stem cell properties. Stem Cell Res (Amst) 2012;8:215–225. doi: 10.1016/j.scr.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 31.El-Ftesi S, Chang EI, Longaker MT, et al. Aging and diabetes impair the neovascular potential of adipose-derived stromal cells. Plast Reconstr Surg. 2009;123:475–485. doi: 10.1097/PRS.0b013e3181954d08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams AR, Hare JM. Mesenchymal stem cells: Biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res. 2011;109:923–940. doi: 10.1161/CIRCRESAHA.111.243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aranda E, Owen GI. A semi-quantitative assay to screen for angiogenic compounds and compounds with angiogenic potential using the EA.hy926 endothelial cell line. Biol Res. 2009;42:377–389. [PubMed] [Google Scholar]
- 34.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 35.Flores I, Blasco MA. The role of telomeres and telomerase in stem cell aging. FEBS Lett. 2010;584:3826–3830. doi: 10.1016/j.febslet.2010.07.042. [DOI] [PubMed] [Google Scholar]
- 36.Sahin E, Depinho RA. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature. 2010;464:520–528. doi: 10.1038/nature08982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fan M, Chen W, Liu W, et al. The effect of age on the efficacy of human mesenchymal stem cell transplantation after a myocardial infarction. Rejuvenation Res. 2010;13:429–438. doi: 10.1089/rej.2009.0986. [DOI] [PubMed] [Google Scholar]
- 39.de Girolamo L, Lopa S, Arrigoni E, et al. Human adipose-derived stem cells isolated from young and elderly women: Their differentiation potential and scaffold interaction during in vitro osteoblastic differentiation. Cytotherapy. 2009;11:793–803. doi: 10.3109/14653240903079393. [DOI] [PubMed] [Google Scholar]
- 40.Harris LJ, Zhang P, Abdollahi H, et al. Availability of adipose-derived stem cells in patients undergoing vascular surgical procedures. J Surg Res. 2010;163:e105–e112. doi: 10.1016/j.jss.2010.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khan WS, Adesida AB, Tew SR, et al. The epitope characterisation and the osteogenic differentiation potential of human fat pad-derived stem cells is maintained with ageing in later life. Injury. 2009;40:150–157. doi: 10.1016/j.injury.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 42.Efimenko A, Starostina E, Kalinina N, et al. Angiogenic properties of aged adipose derived mesenchymal stem cells after hypoxic conditioning. J Transl Med. 2011;9:10–22. doi: 10.1186/1479-5876-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pandey AC, Semon JA, Kaushal D, et al. MicroRNA profiling reveals age-dependent differential expression of nuclear factor κB and mitogen-activated protein kinase in adipose and bone marrow-derived human mesenchymal stem cells. Stem Cell Res Ther. 2011;2:49. doi: 10.1186/scrt90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jimi S, Ito K, Kohno K, et al. Modulation by bovine angiogenin of tubular morphogenesis and expression of plasminogen activator in bovine endothelial cells. Biochem Biophys Res Commun. 1995;211:476–483. doi: 10.1006/bbrc.1995.1838. [DOI] [PubMed] [Google Scholar]
- 45.Gao X, Xu Z. Mechanisms of action of angiogenin. Acta Biochim Biophys Sin (Shanghai) 2008;40:619–624. doi: 10.1111/j.1745-7270.2008.00442.x. [DOI] [PubMed] [Google Scholar]
- 46.Distler JH, Hirth A, Kurowska-Stolarska M, et al. Angiogenic and angiostatic factors in the molecular control of angiogenesis. Q J Nucl Med. 2003;47:149–161. [PubMed] [Google Scholar]
- 47.Reiss Y. Angiopoietins. Recent Results Cancer Res. 2010;180:3–13. doi: 10.1007/978-3-540-78281-0_2. [DOI] [PubMed] [Google Scholar]
- 48.Chae JK, Kim I, Lim ST, et al. Coadministration of angiopoietin-1 and vascular endothelial growth factor enhances collateral vascularization. Arterioscler Thromb Vasc Biol. 2000;20:2573–2578. doi: 10.1161/01.atv.20.12.2573. [DOI] [PubMed] [Google Scholar]
- 49.Thurston G, Suri C, Smith K, et al. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286:2511–2514. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- 50.Song N, Ding Y, Zhuo W, et al. The nuclear translocation of endostatin is mediated by its receptor nucleolin in endothelial cells. Angiogenesis. 2012;15:697–711. doi: 10.1007/s10456-012-9284-y. [DOI] [PubMed] [Google Scholar]
- 51.Lawler PR, Lawler J. Molecular basis for the regulation of angiogenesis by thrombospondin-1 and -2. Cold Spring Harb Perspect Med. 2012;2:a006627. doi: 10.1101/cshperspect.a006627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parfyonova YV, Plekhanova OS, Tkachuk VA. Plasminogen activators in vascular remodeling and angiogenesis. Biochemistry (Mosc) 2002;67:119–134. doi: 10.1023/a:1013964517211. [DOI] [PubMed] [Google Scholar]
- 53.Poettler M, Unseld M, Mihaly-Bison J, et al. The urokinase receptor (CD87) represents a central mediator of growth factor-induced endothelial cell migration. Thromb Haemost. 2012;108:357–366. doi: 10.1160/TH11-12-0868. [DOI] [PubMed] [Google Scholar]
- 54.Traktuev DO, Tsokolaeva ZI, Shevelev AA, et al. Urokinase gene transfer augments angiogenesis in ischemic skeletal and myocardial muscle. Mol Ther. 2007;15:1939–1946. doi: 10.1038/sj.mt.6300262. [DOI] [PubMed] [Google Scholar]
- 55.Raghu H, Lakka SS, Gondi CS, et al. Suppression of uPA and uPAR attenuates angiogenin mediated angiogenesis in endothelial and glioblastoma cell lines. PLoS One. 2010;5:e12458. doi: 10.1371/journal.pone.0012458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cesari M, Pahor M, Incalzi RA. Plasminogen activator inhibitor-1 (PAI-1): A key factor linking fibrinolysis and age-related subclinical and clinical conditions. Cardiovasc Ther. 2010;28:e72–e91. doi: 10.1111/j.1755-5922.2010.00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ghosh AK, Vaughan DE. PAI-1 in tissue fibrosis. J Cell Physiol. 2012;227:493–507. doi: 10.1002/jcp.22783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pandey M, Loskutoff DJ, Samad F. Molecular mechanisms of tumor necrosis factor-alpha-mediated plasminogen activator inhibitor-1 expression in adipocytes. FASEB J. 2005;19:1317–1319. doi: 10.1096/fj.04-3459fje. [DOI] [PubMed] [Google Scholar]
- 59.Liu RM, Choi J, Wu JH, et al. Oxidative modification of nuclear mitogen-activated protein kinase phosphatase 1 is involved in transforming growth factor beta1-induced expression of plasminogen activator inhibitor 1 in fibroblasts. J Biol Chem. 2010;285:16239–16247. doi: 10.1074/jbc.M110.111732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jaulmes A, Sansilvestri-Morel P, Rolland-Valognes G, et al. Nox4 mediates the expression of plasminogen activator inhibitor-1 via p38 MAPK pathway in cultured human endothelial cells. Thromb Res. 2009;124:439–446. doi: 10.1016/j.thromres.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 61.Devy L, Blacher S, Grignet-Debrus C, et al. The pro- or antiangiogenic effect of plasminogen activator inhibitor 1 is dose dependent. FASEB J. 2002;16:147–154. doi: 10.1096/fj.01-0552com. [DOI] [PubMed] [Google Scholar]
- 62.Herrmann JL, Abarbanell AM, Weil BR, et al. Optimizing stem cell function for the treatment of ischemic heart disease. J Surg Res. 2011;166:138–145. doi: 10.1016/j.jss.2010.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shevchenko EK, Makarevich PI, Tsokolaeva ZI, et al. Transplantation of modified human adipose derived stromal cells expressing VEGF165 results in more efficient angiogenic response in ischemic skeletal muscle. J Transl Med. 2013;11:138. doi: 10.1186/1479-5876-11-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.