This paper highlights the role that hypothyroidism can play in the diagnosis, treatment, and recovery of cancer. Timely diagnosis and treatment is pertinent to minimize the complications of hypothyroidism, to optimize cancer therapy, and to minimize recurrence. The authors discuss newer therapies associated with hypothyroidism and the negative impact that hypothyroidism can have in patients with a malignancy.
Keywords: Hypothyroidism, Malignancy, Chemotherapy, Optimal therapy, Review
Learning Objectives
Describe the impact of hypothyroidism in patients with cancer.
Identify options for managing hypothyroidism in patients with cancer.
Abstract
Hypothyroidism is a common disease that is easily treated in the majority of cases, when readily diagnosed; however, presentation of an aggregate of its symptoms is often clinically overlooked or attributed to another disease and can potentially be lethal. Already prevalent in older women, its occurrence in younger patients is rising as a result of radiation therapy, radioactive iodine therapy, and newer antineoplastic agents used to manage various malignancies. The presence of nonspecific constitutional symptoms and neuropsychiatric complaints in cancer patients can be attributed to a myriad of other diagnoses and therapies. Thyroid dysfunction can be easily overlooked in cancer patients because of the complexity of cancer’s clinical picture, particularly in the pediatric population. Underdiagnosis can have important consequences for the management of both hypothyroidism and the malignancy. At minimum, quality of life is adversely affected. Untreated hypothyroidism can lead to heart failure, psychosis, and coma and can reduce the effectiveness of potentially life-saving cancer therapies, whereas iatrogenic causes can provoke atrial fibrillation and osteoporosis. Consequently, the diagnosis and treatment of hypothyroidism in cancer patients are pertinent. We summarize the history, epidemiology, pathophysiology, clinical diagnosis, and management of hypothyroidism in cancer patients.
Implications for Practice:
Clinicians should be aware of the role that hypothyroidism can play in the diagnosis, treatment, and recovery of cancer. Because the myriad of symptoms associated with hypothyroidism can easily be attributed to the initial malignancy, to chemotherapy or radiation therapy, or to cancer recurrence, it is easy to miss the diagnosis of hypothyroidism. Timely diagnosis and treatment is necessary to minimize the complications of hypothyroidism, to optimize cancer therapy, and to minimize recurrence. We highlight newer therapies associated with hypothyroidism and the negative impact that hypothyroidism can have in patients with a malignancy.
Introduction
Hypothyroidism is the most common hormone deficiency. The severity of hypothyroidism varies significantly, and it has a variety of end organ effects. Because of both the nonspecific symptoms of hypothyroidism and the similar symptoms and morbidities associated with malignancies and their treatment, hypothyroidism can often go undiagnosed and untreated in patients with cancer. Failure to adequately manage both overt and subclinical hypothyroidism can have serious consequences, hence the recognition of its presence is crucial for the successful treatment of cancer patients. Hypothyroidism is commonly noted in older women because of the prevalence of autoimmune thyroiditis. Younger women and men are now being diagnosed secondary to other important causes, including previous thyroid, brain, and spinal cord surgery and irradiation and medications. Hypothyroidism is easily treated with thyroxine (T4) replacement. Unfortunately, suboptimal dosing is common. This review summarizes the current understanding of the history, epidemiology, pathophysiology, and clinical diagnosis and management of hypothyroidism.
History
Gull initially described previously healthy women who acquired clinical features of cretinism in 1874, and the term “myxedema” was coined by Ord in 1878 to describe a syndrome in women with coarse features, dry skin, mental dullness, hypothermia, and edema [1]. At the same time, Kocher and Reverdin independently described development of a cretin-like state after thyroid resection, termed “cachexia strumipriva” [1]. Autoimmune thyroiditis was not described until 1912, when Hashimoto noted women with struma lymphomatosa, goiters that appeared to turn into lymphoid tissue [2]. It was not until 1956 when Campbell et al. noted the presence of circulating thyroid antibodies in association with autoimmune thyroiditis [3]. Initially treated with sheep thyroid extract, thyroid hormone was initially crystallized by Kendall in 1914 [4], with Harington and Barger synthesizing it in 1927 [5]. Discovered in 1952 by Gross and Pitt-Rivers [6], tri-iodothyronine (T3) was not found to be endogenously generated from T4 until more than a decade later, when described by Braverman et al. [7]. Finally, the diagnosis of hypothyroidism became possible when Mayberry et al. described the use of thyrotropin (TSH) immunoassays in 1971 [8].
Definitions
Hypothyroidism is an underactive thyroid gland resulting in retardation of growth and mental development, that occurs when (a) the gland fails to produce enough T4 to meet the body’s needs, (b) the body fails to convert a sufficient amount of T4 to T3 in peripheral tissues, or (c) the nervous system fails to stimulate the thyroid gland. This insufficient amount of hormone slows life-sustaining body processes, damages organs and tissues throughout the body, and can result in life-threatening complications. Hypothyroidism is classified based on the timing of its presentation, the level of endocrine dysfunction, and its severity. In primary hypothyroidism, the serum TSH level is elevated, and the distinction between overt and mild (subclinical) disease is determined biochemically by noting the free T4 concentration in the serum. Central hypothyroidism is a reduction in circulating thyroid hormone resulting from inadequate stimulation of a normal thyroid gland by TSH. It is considered to be secondary if pituitary disease is present. Myxedema refers to patients with overt hypothyroidism that is severe and/or complicated. Cretinism occurs in untreated congenital hypothyroidism when the patient presents with mental retardation, short stature, deafness, and facial deformities.
Epidemiology
Hypothyroidism is a common disorder, especially in women, with the incidence increasing with age. Primary disease is more common than secondary (1,000:1), hence serum TSH levels have been used to estimate its prevalence in different populations. The Whickham study reported high serum TSH levels in 7.5% of women and 2.8% of men in the United Kingdom [9], and the Third National Health and Nutrition Examination Survey found 4.6% of Americans with elevated levels, with only 0.3% having overt hypothyroidism and 4.3% having subclinical disease [10]. Traditionally noted to be more prevalent in whites, compared with Hispanics and blacks, controversies surrounding possible ethnic differences in TSH levels and its diagnostic reference range warrant evaluation in all patients in whom there is suspicion. Patient populations with a higher risk than the general population of developing hypothyroidism include patients with a family history of autoimmune thyroid disorders; postpartum women; patients with a history of previous head and neck or thyroid irradiation or surgery; those with other autoimmune endocrine disorders; and patients treated with irradiation and certain chemotherapy drugs for cranial and spinal, thyroid, and gastrointestinal cancers [11–18].
Etiology
Autoimmune Thyroiditis
Hashimoto’s thyroiditis is the most prevalent acquired thyroid disorder and the most common cause of hypothyroidism in the U.S. Present in 4%–9.5% of the adult population, it is seven times more common in women, with the incidence increasing during middle age [9, 10, 19, 20]. Pathogenesis is supported by histologic evidence of lymphocytic infiltration of the thyroid gland and fibrous replacement of the thyroid parenchymal tissue, resulting in gradual gland destruction. Complement activation and antibody-dependent cell cytotoxicity result from the presence of thyroid peroxidase antibodies [21]. Despite their prevalence in patients with hypothyroidism, little evidence shows that these antibodies play a vital role in the pathogenesis of Hashimoto’s thyroiditis. The current belief is that T-cell-mediated cytotoxicity and apoptosis pathways have more of an influence on the outcome of this disease. The affected thyrocytes express major histocompatibility complex class II proteins necessary for CD4 T lymphocytes and activated CD4 T cells specific for thyroid antigens. The genetic predisposition results from an autosomal dominant inheritance of the autoantibodies [22]. Depending on the variant present, the gland can be nonpalpable, diffusely enlarged with a firm consistency, irregularly contoured, hard and markedly enlarged, or even tender and painful [23–25]. Clinically, these patients are hypothyroid or euthyroid or have transient thyrotoxicosis followed by hypothyroidism (Hashitoxicosis). Autoimmune hypothyroidism can arise in conjunction with other endocrine deficiencies, including hypoparathyroidism, adrenal insufficiency, diabetes mellitus, chronic mucocutaneous candidiasis, primary ovarian failure, or other autoimmune diseases including Sjogren’s syndrome, pernicious anemia, vitiligo, atrophic gastritis, and systemic sclerosis [26].
Acquired Primary Hypothyroidism
In other parts of the world, iodine deficiency remains an important cause of hypothyroidism. Other common causes include thyroid resection, radioiodine therapy, and drugs such as lithium, thionamide, interferon, and rifampicin. Although hypothyroidism is inevitable after total thyroidectomy for malignancy or Graves’ disease, hypothyroidism can also occur after lobectomy or a Sistrunk procedure for benign thyroid nodules or thyroglossal duct remnant [27, 28]. External beam radiotherapy for head and neck malignancy, radioactive iodine therapy for thyrotoxicosis, environmental radioiodine exposure, and experimental use of radioiodinated immunoglobulins for cancer treatment all have hypothyroidism as a complication [16, 29–31]. Exposure to other toxins, including rescorcinol and polybrominated and polychlorinated biphenyls, has also been reported to cause hypothyroidism. Last, patients with hemochromatosis are at risk from iron infiltration of the thyroid gland [32].
Hypothyroidism Resulting From Medications
Several medications have been linked to interference with thyroid hormone production or to provoking autoimmunity to the thyroid (Tables 1 and 2). Pharmacologic quantities of iodine achieved in patients treated with amiodarone, Lugol solution, saturated solution of potassium iodide, and denileukin diftitox can inhibit thyroid hormone production and cause hypothyroidism. Amiodarone-induced hypothyroidism occurs in 15%–20% of patients treated with amiodarone [33]. Amiodarone usually causes thyroid hormone discharge from the damaged gland in patients with chronic autoimmune thyroiditis and results in both hypo- and hyperthyroidism. The effects of amiodarone are usually transient, hence withdrawal is not necessary.
Table 1.
Pharmacologic agents that affect thyroid function
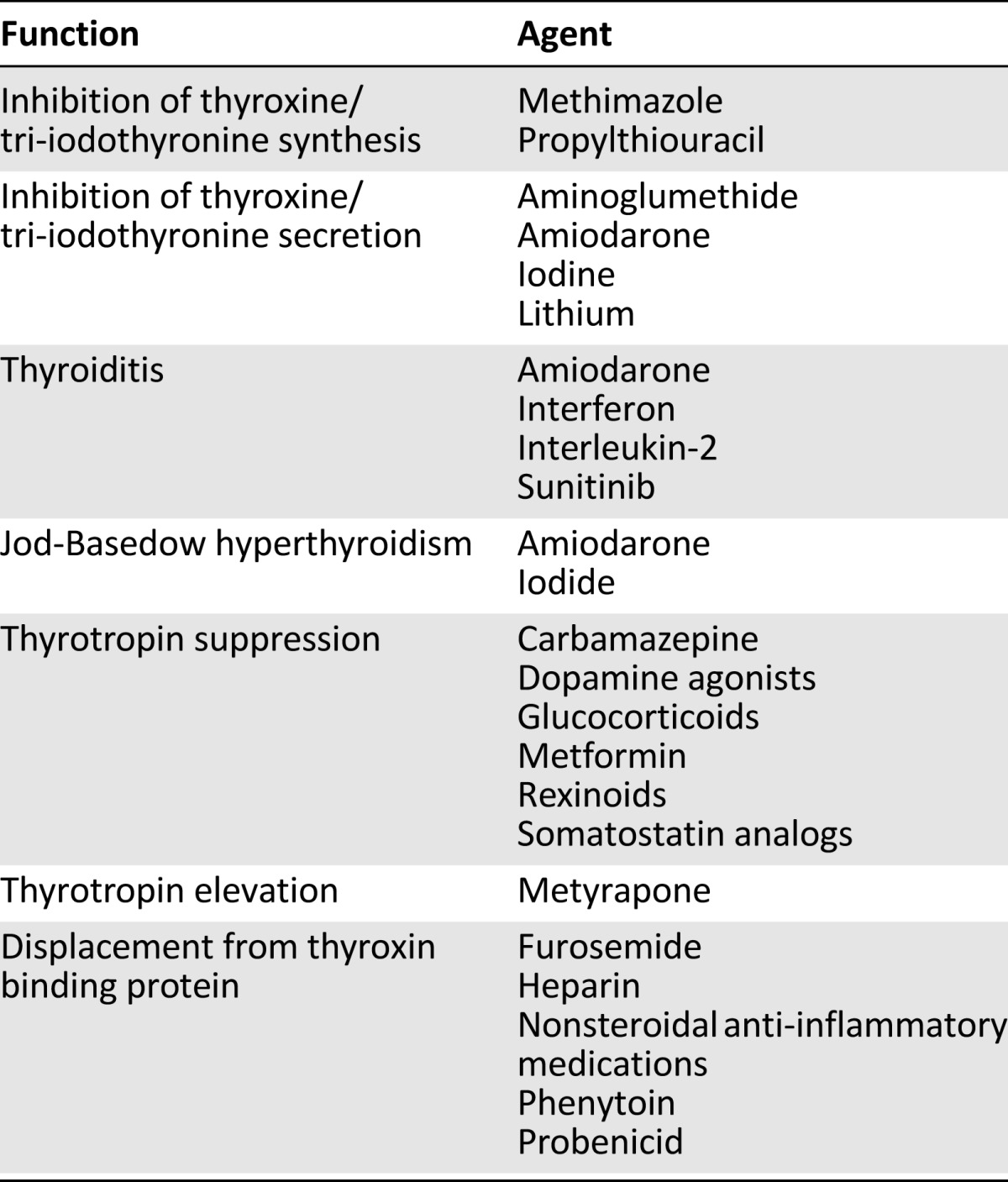
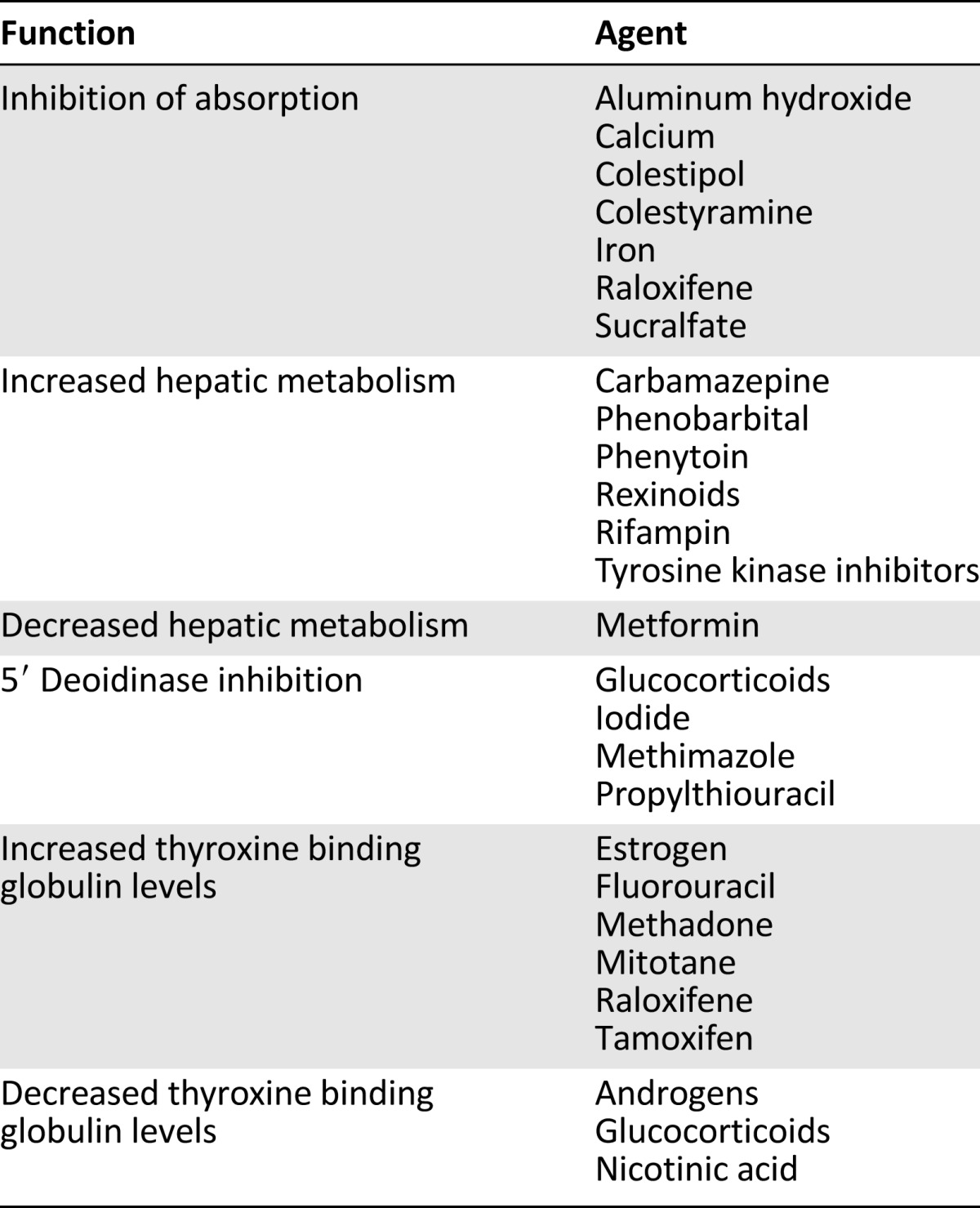
Table 2.
Medications that affect serum levothyroxine levels
Improved cancer survival and clinical response to treatment with interleukin-2 has been noted in patients with melanoma, renal cell carcinoma, gliomas, and indolent breast cancer. In these instances, the presence of hypothyroidism is a marker of remission, improved treatment response, and decreased tumor growth.
Hypothyroidism Resulting From Radiation
Thyroid iatrogenic sequelae can occur after the treatment of cancers, most notably pediatric malignancies. A late side effect of curative radiotherapy in the head and neck region is hypothyroidism. The pathophysiology of radiation-induced thyroid damage is multifactorial. Radiation inhibits follicular epithelial function and progressively alters the endothelium, resulting in cell degeneration and necrosis, follicular disruption and vascular degeneration and thrombosis, acute and chronic inflammation, fibrous organization, and partial epithelial regeneration [34, 35]. The cytotoxic β radiation released during iodine 131 isotopic decay directly damages the thyrocytes and small thyroid vessels and leads to atherosclerosis in larger vessels. Even though radiotherapy can result in several thyroid dysfunctions, primary hypothyroidism is the most common, occurring an average of 2–7 years after treatment, in a dose-dependent manner [16, 36, 37]. Several studies have demonstrated that the risk is proportionate to the dose of radiation, with neck, mantle, C2–T2 spine, brain stem, Waldeyer’s ring and neck, supraclavicular and nasopharyngeal regions, and total body irradiation carrying the highest risk [38–42]. The development of hypothyroidism in older breast cancer survivors is fairly common because a portion of the thyroid gland can be included in the treatment field; however, supraclavicular irradiation does not amplify risks. The incidence of hypothyroidism is as high as 30%–50% in patients treated with radiation for a head and neck malignancy or Hodgkin’s disease [40, 41, 43, 44].
Hypothyroidism Related to Chemotherapy
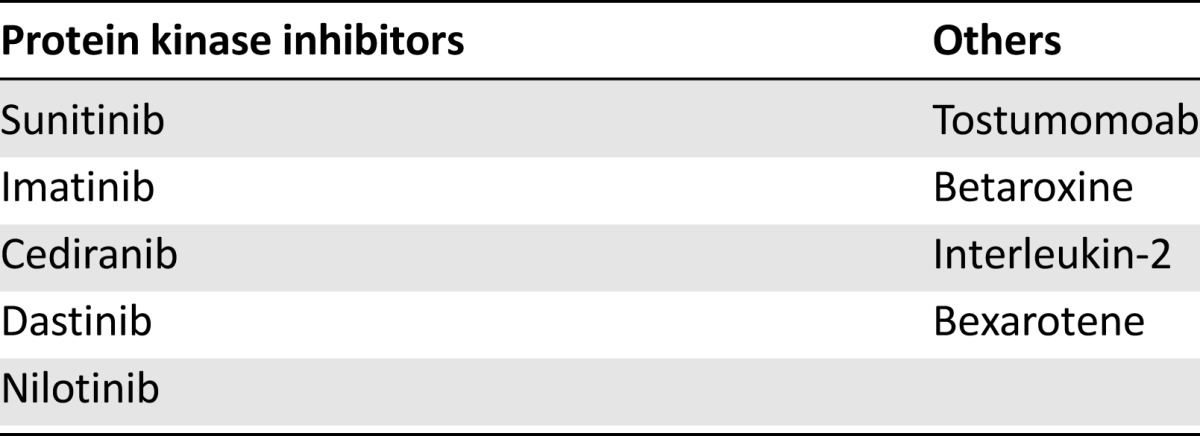
Some malignancies known to be resistant to radiation and traditional chemotherapeutic agents have exhibited improved survival and tumor burden with newer therapies, such as specific tyrosine kinase inhibitors. These new pharmacologic agents have proven beneficial in metastatic melanoma, renal cell carcinoma, and advanced gastrointestinal stromal tumors; however, cases of both transient and profound hypothyroidism have been reported in 25%–70% of patients [45–48] (Table 3). Sunitinib (sunitinib malate, Sutent; Pfizer, New York, NY, http://www.pfizer.com), the standard of care in first-line treatment of advanced renal cell carcinoma, is also approved for the treatment of imatinib-refractory gastrointestinal stromal tumors. Reported rates of hypothyroidism range from 4% to 27%; however, the exact mechanisms remain unclear [49–51]. The strongest possibility is its antiangiogenic effect by inhibition of vascular endothelial growth factor signaling and/or impaired blood flow by reduced vascularity and capillary regression. In the presence of levothyroxine therapy, imatinib elevates TSH levels by increasing the deiodination and conjugation of T3 and T4, increasing their renal clearance and causing clinical hypothyroidism. The symptoms of overt hypothyroidism and the return of TSH to reference range in patients treated with imatinib and levothyroxine after thyroidectomy for medullary thyroid cancer resolved within weeks of imatinib withdrawal [52]. Although not as prevalent, other tyrosine kinase inhibitors and vascular endothelial growth factor receptor blockers have demonstrated a high incidence of clinical hypothyroidism. Forty-five percent of patients treated with cediranib for solid tumors developed hypothyroidism, whereas patients with chronic myelogenous leukemia had reported incidence of hypothyroidism of 13% and 22%, respectively, after being treated with imatinib, dastinib, and nilotinib [53, 54].
Table 3.
Chemotherapeutic agents associated with hypothyroidism
Improved cancer survival and clinical response to treatment with interleukin-2 has been noted in patients with melanoma, renal cell carcinoma, gliomas, and indolent breast cancer [55–58]. In these instances, the presence of hypothyroidism is a marker of remission, improved treatment response, and decreased tumor growth. The positive aspect is treatment of the malignancy, but consequences of the resultant hypothyroidism include difficulty in distinguishing the origin of the symptoms and, if hypothyroidism goes unrecognized and untreated, can be life threatening; myxedema coma and cardiac compromise can occur, as has been shown in association with sunitinib [59–61]. Other medications being investigated for use in lung, breast and thyroid malignancies, T-cell lymphoma, non-Hodgkin’s lymphoma, pheochromocytoma, and carcinoid and neuroblastoma have also demonstrated a significant incidence of hypothyroidism. Bexarotene, a selective retinoid X receptor agonist, alters cell growth and differentiation as well as apoptosis by forming heterodimers with the thyroid hormone receptor inside the nucleus. A reported 40%–100% of patients have TSH suppression within 8 hours of treatment, without thyroid stimulation [62], whereas the CD20 antibody tositumomab results in a later occurrence (6–24 months after therapy) when combined with iodine 131 [63–65].
Transient Hypothyroidism
Subacute (de Quervain’s) thyroiditis and postpartum thyroiditis usually result in transient hypothyroidism. Treatment with certain medications, including amiodarone and sunitinib, can also result in transient episodes. Seventy-five percent to 85% of patients with de Quervain’s and postpartum thyroiditis regain normal thyroid function with supportive therapy, without thyroid hormone supplementation treatment [1].
Central (Secondary) Hypothyroidism
Central hypothyroidism is acquired when diseases interfere with hypothalamic TSH-releasing hormone (TRH) production or its delivery by the pituitary stalk to the anterior pituitary gland or with pituitary TSH production. Radiotherapy, surgery, and pituitary adenomas are the most common causes [66].
Radiation therapy for cranial and spinal malignancies, head and neck cancers, and lymphomas attenuate the production of TRH or TSH. Germinomas, gliomas, and meningiomas can impinge on the hypothalamus, whereas craniopharyngiomas and chordomas impinge on the pituitary stalk in the suprasellar region. Trauma resulting in transection of the pituitary stalk or hemorrhage can interrupt TRH delivery, whereas sarcoidosis, hemochromatosis, and Langerhans’ cell histiocytosis can impair hypothalamic TRH production [67–69]. Other rare entities that can affect pituitary thyrotrope function include lymphocytic hypophysitis, infection, metastases, apoplectic infarction, and the retinoid X receptor-selective ligand betaroxine [70]. Traditional chemotherapy administered without radiation for the treatment of leukemia and lymphoma rarely results in thyroid disorders; however, in a small pediatric series, Baronio et al. noted the presence of TRH suppression after chemotherapy treatment for acute lymphoblastic leukemia, with 33% of the patients having a concomitant decrease in free T4 [71].
Traditional chemotherapy administered without radiation for the treatment of leukemia and lymphoma rarely results in thyroid disorders; however, in a small pediatric series, Baronio et al. noted the presence of TRH suppression after chemotherapy treatment for acute lymphoblastic leukemia, with 33% of the patients having a concomitant decrease in free T4.
Pathophysiology
Clinical hypothyroidism is a pervasive deficit in thyroid hormone actions, leading to the alteration of calorigenesis and oxygen consumption throughout the body and organ-specific effects. Deficiency of T3 actions at the genomic level cause hormonal, biochemical, ion-transport, and mechanical changes in target tissues. T4 is the principal hormone produced by the thyroid gland and in circulation. Monodeiodination of its outer ring in both the cytoplasm and nucleus of target tissues converts it to T3 by the function of three tissue-specific deiodinases [72]. The actions of T3 are mediated by its binding to one of three receptor isoforms (TRα1, TRβ1, and TRβ2), which in turn form dimers with another T3 receptor or with other nuclear receptors. Subsequent DNA binding with specific orientations occurs at the 5′ regulatory regions of thyroid hormone-responsive genes to either activate or repress transcription [73]. Based on this genetic description, some clinical manifestations are understood at the molecular level, including short stature as a result of failure to stimulate the growth hormone gene in pituitary somatotrophs, decreased low-density lipoprotein (LDL) cholesterol clearance because of a deficit in the expression of the hepatic LDL receptor gene regulated by sterol regulatory element binding transcription factor 2 and impaired diastolic and systolic ventricular function resulting from decreased myocardial sarcoplasmic reticulum ATPase and α-myosin heavy chain expression. T3 also regulates cellular uptake of glucose and amino acids, augments cardiomyocyte calcium-ATPase activity, and alters adenosine triphosphate generation by the mitochondria [74]. As novel therapies continue to be identified for the treatment of neuroendocrine malignancies, hypothyroidism may also result because of the interaction of thyroid hormone with G protein-coupled membrane receptors and the mitogen activated protein kinase pathways.
Diagnosis
Signs and Symptoms
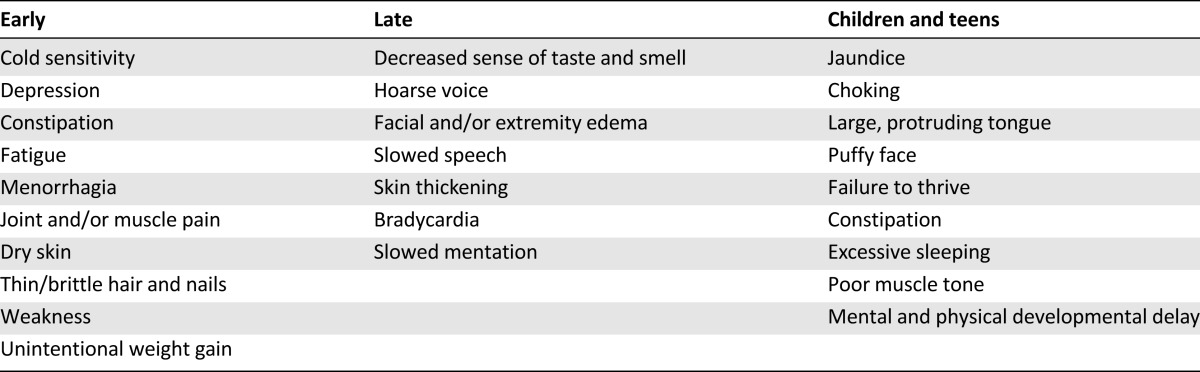
Common clinical features associated with hypothyroidism are tiredness and fatigue, weight gain, dry skin, cold intolerance, constipation, muscle weakness, facial edema, hoarse voice, and poor memory (Table 4). These symptoms can be present in other diseases, including malignancy, and can be side effects of cancer therapies. The Colorado study showed a range of 2.9%–24.5% sensitivity for individual symptoms, with the likelihood of the presence of the disease being proportional to the number of symptoms but including the inability to exclude the diagnosis in the absence of symptoms [19, 75]. These symptoms are not disease specific and are common in the euthyroid population as well as the cancer population. Symptoms that are new or occur in combination are more likely to indicate hypothyroidism. In children and adolescents, additional presentations include growth failure with delayed bone maturation, slipped capital femoral epiphysis, delayed eruption of permanent teeth, anemia, muscle pseudohypertrophy, pituitary enlargement, galactorrhea, and delayed or precocious puberty [76–81].
Table 4.
Commonly presenting symptoms of hypothyroidism
Atypical Clinical Presentation
In rare instances, patients can present with hypothermia, congestive heart failure, pericardial and pleural effusions, ileus or intestinal pseudo-obstruction, or coagulopathy [82–89]. The association of hypothyroidism with neurocognitive deficits should not be taken lightly. Memory deficits are common, and hypothyroidism must be included in the differential diagnosis when evaluating elderly patients with dementia [90]. The most common psychiatric disorder associated with hypothyroidism is depression. Other neurologic manifestations, more commonly noticed in patients with severe hypothyroidism or myxedema, include psychosis, ataxia, seizures, and coma [91–94].
Laboratory Diagnosis
The first-line diagnostic test for hypothyroidism is serum TSH, also known as TSH measurement [95, 96]. Elevated levels identify patients with primary hypothyroidism, regardless of the cause or severity. Normal levels in disease-free individuals range from 0.4 mIU/L to 4.0 mIU/L, in a logarithmic distribution, with a mean of 1.5 mIU/L [10]. In recent years, controversies surrounding the TSH reference range have surfaced. In the general population, TSH is not normally distributed because >95% of healthy individuals have a TSH level of <2.5 mIU/L, suggesting that the upper limit of the reference range is skewed by occult thyroid dysfunction [97]. In pregnancy, trimester-specific reference ranges are now used to assess thyroid function, and when not available, the upper limit of normal in the first trimester is 2.5 mIU/L and 3 mIU/L in later trimesters [98, 99]. Age and ethnicity are known to influence TSH distribution, with blacks having a lower TSH level on average. Consequently, the use of age- and race-standardized TSH reference ranges has been suggested [100]. Even with the suggestion of lowering the upper limit of the reference range from 4.5 mIU/L to 2.5 mIU/L, the variation within an individual is narrower than it is in the general population, supporting the concept of individualized reference ranges [101–104].
Treatment
Levothyroxine Sodium
There is consensus that levothyroxine sodium (also known as L-thyroxine) is beneficial in the treatment of clinical (overt) hypothyroidism, despite the lack of a systematic review or randomized control trial comparing levothyroxine and placebo. In comparison to its combination with liothyronine, there is moderate-quality evidence that levothyroxine is as effective at reducing body pain, fatigue, anxiety, cognitive function, and depression and improves quality of life [105]. Hyperthyroidism can also occur as a result of overdosing. Pertinent side effects can occur if TSH is suppressed and include a reduction in bone mass in postmenopausal women and the development of atrial fibrillation. There is no evidence of an increased fracture rate. The optimum daily dose is related to body weight (approximately 1.8 µg/kg in adults) and age, with children and older adults requiring lower doses (0.5 µg/kg) [106, 107]. Supplementation requirements are lower for those patients with autoimmune disease because they have some remaining residual functioning thyroid tissue. Patients with gastrointestinal disorders or previous small bowel bypass surgery and those taking certain medications and on certain diets including calcium carbonate, cholestyramine, sucralfate, dietary soy, and fiber have higher requirements as a result of decreased absorption. Patients with autoimmune thyroiditis and absorption difficulty can have serum TSH, free T4, and free T3 levels monitored on a regular basis (every 6–8 weeks) to guide therapy and minimize symptoms.
Liothyronine
Liothyronine (T3) is the active form of thyroid hormone in the peripheral tissues. Because of its short half-life, the usual dose is three times a day to achieve a target TSH of 0.5–1.5 mIU/L. In a small randomized, double-blind, crossover trial, Celi et al. noted improved lipid profiles and reduced body weight after 6 weeks of therapy but no difference in cardiovascular function or quality of life scores compared with patients prescribed levothyroxine [108]. Despite having a serum TSH within the reference range, many patients on levothyroxine do not achieve a ratio of physiologic free T3 to free T4. In addition, rodent studies have shown that all tissues do not achieve adequate levels of T3 with T4 replacement, whereas a combination of levothyroxine and tri-iodothryonine does [109–111]. Taking into consideration that the current formulation of tri-iodothryonine does not result in a normal physiologic profile and the associated fluctuations in free T3 levels, treatment with T3 alone or in combination may be effective only in a subgroup of patients.
Desiccated Pig Thyroid Extract
Desiccated pig thyroid extract (Armour Thyroid; Forest Laboratories, Inc., New York, NY, http://www.armourthyroid.com/) contains both T4 and tri-iodothryonine in a supraphysiologic ratio of 4 to 1. This eliminates the need to take multiple daily doses of medication. No evidence exists to show its efficacy over levothyroxine.
Monitoring Thyroid Function During Treatment
Serum TSH is measured 6–8 weeks after initiation of or a change in levothyroxine dose. Once a stable dose is achieved, annual monitoring is recommended. Supplementation is adequate when serum TSH is in the lower half of the normal range, <2.5 mIU/L but no lower than 0.1 mIU/L, to avoid adverse skeletal health [1, 112].
Post-Thyroidectomy Management for Follicular Thyroid Cancer
TSH suppression after thyroidectomy for follicular and papillary thyroid cancers (differentiated thyroid cancer) is crucial in minimizing and monitoring recurrence. This is especially pertinent in patients with lymphadenopathy or metastatic disease. Patients are either administered 0.9 mg recombinant human TSH for 2 days or exposed to 2 weeks of thyroid hormone withdrawal prior to radioactive iodine therapy and are subsequently maintained on thyroid hormone supplementation to suppress TSH levels. The optimal degree of suppression continues to be debated; however, it is has been shown that maintaining undetectable thyroglobulin levels (<1.0 µg/L) with T4 decreases recurrence [113–115]. Inadequate T4 supplementation and TSH suppression can place the patient in subclinical or overt hypothyroidism, with dire consequences including cardiac disease and recurrence of the malignancy. In the pediatric population, adequate thyroid supplementation is pertinent because overt hypothyroidism can delay or retard both physical and mental development.
Controversies in Diagnosis and Management
Thyroid hormone has convenient pharmacokinetic properties, a high degree of effectiveness, and a small risk of adverse reactions. The majority of general and specialty practitioners routinely prescribe levothyroxine and only evaluate serum TSH levels. This results in 20% of patients receiving an inadequate dose and 20% given an excessive amount of medication [1]. Most physicians initiate treatment with a dose at the lower end of the anticipated requirement of 1.6 µg/kg per day. Differences in management include initiation at the full dose versus titration up from a low dose, the addition of T3 in the presence of persistent symptoms, monitoring free T4 and free T3 as well as TSH, and treatment of patients with subclinical hypothyroidism. Even adequately treated hypothyroid patients have constitutional and neuropsychological symptoms and a decreased sense of well-being compared with euthyroid individuals.
It is well known that the magnitude of replacement or suppressive doses of levothyroxine is based on body weight and is affected by gender, weight, cause of hypothyroidism, other medications, comorbidities, diet, and etiology (malignancy vs. autoimmune disease). Estimates based on body weight range from 1.6 µg/kg to 2.56 µg/kg [116–118]; however, patients may not fall within this range, especially those with persistent symptoms and other comorbidities. Illnesses including malignancies and those requiring critical care intervention not only can mask the symptoms, and hence the diagnosis, of hypothyroidism but also can affect thyroid hormone metabolism. Recent studies suggest body mass index (BMI) as the optimal tool, as opposed to weight, in determining the appropriate dose in patients after thyroidectomy. In this study, the standard weight-based replacement regimen failed to return the majority of patients to a euthyroid state by overdosing patients with a BMI <25 kg/m2 and underdosing patients with a BMI >30 kg/m2 [119]. Initiating therapy at the estimated full dose is effective in many patients; however, in patients at extremes of age, with more lean body mass, or with significant comorbidities, it may prove more efficacious to start at a lower dose and titrate up to the targeted dose while evaluating serum TSH and free thryoxine and T3 levels. Using T3 as the primary therapy may be warranted in the small subset of patients in whom levothyroxine has not been proven to alleviate symptoms and who can be compliant with multiple daily doses. Further studies with larger sample sizes and longer follow-up are necessary before its adoption in routine clinical practice. Combining T3 with levothyroxine is more common than single therapy, with some success noted in reported well-being; however, a meta-analysis of randomized controlled trials (RCTs) concluded no difference in effectiveness with combination therapy [120, 121]. Despite multiple RCTs showing the lack of benefit of combining the two medications, T3 is formulated to mimic normal physiologic profiles, hence the reported better outcomes by some patients.
Supplementing patients diagnosed with subclinical hypothyroidism has not been universally adopted. Despite testing and treatment being relatively inexpensive, safe, and effective, the clinical consequences are not considered important and reversible in a significant proportion of affected patients for experts to justify widespread screening and treatment [122]. Both overt and subclinical hypothyroidism are associated with chronic diseases that are common in the general population, including cardiovascular disease, peripheral vascular disease, and obesity; however, treating patients with thyroid function abnormalities, even those with subclinical hypothyroidism, can potentially minimize the morbidity and mortality associated with these chronic diseases. T4 supplementation can prevent the progression to overt hypothyroidism, specifically in the elderly and in patients with autoimmune thyroiditis. Reduction of future cardiovascular disease is crucial, considering that even patients with mild disease have higher mean serum totals and LDL cholesterol concentrations, and subtle, reversible changes in myocardial function have been noted on echocardiography [123–125]. Even though the higher risk of clinical cardiovascular disease has been noted, the cardiovascular benefit of thyroid hormone treatment has yet to be rigorously tested in an RCT. Last, several small controlled, double-blinded trials have shown improvements in patients’ symptoms and neuropsychological performance indices when compared with placebo; however, they have not been confirmed, and there is a lack of large prospective randomized trials [126–129]. Subclinical hypothyroidism is common in the general population. There is a higher number of patients with raised TSH and thyroid antibodies who progress to overt hypothyroidism [129]; however, patients who present with symptoms and elevated TSH without antibodies may warrant testing. One study demonstrated 3% of these patients progressing to overt hypothyroidism [130]. No RCTs exist showing that treatment with levothyroxine decreases the incidence of cardiovascular events or mortality. There is expert support for treating patients with serum TSH of ≥10 mIU/L as well as women who are pregnant or intend to become pregnant and for a short-term trial for symptomatic patients with serum TSH <10 mIU/L [112].
Conclusion
Hypothyroidism is a common disease in the general population and is usually easily managed. Crucial populations in which both subclinical and overt disease can be masked include patients with malignancies, patients treated for malignancy, older patients, and critically ill patients. In patients exhibiting numerous symptoms associated with hypothyroidism, evaluation of the serum TSH level is warranted. In managing patients, if symptoms persist regardless of a serum TSH level in the reference range, therapy should be individualized, with evaluation of the free T4 and T3 levels and dosing based on ideal body weight. Considering the morbidity and mortality associated with inadequate or lack of treatment, including cardiovascular morbidity and mortality, congenital birth defects, and myxedema, evaluation and treatment of this common disease is crucial, especially in cancer patients and cancer survivors.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Acknowledgment
This research was supported by National Institutes of Health/National Cancer Institute Supplemental Grant R01CA12115-S1 (Y.C., H.C.).
Author Contributions
Conception/Design: Yvette Carter, Herbert Chen, Rebecca S. Sippel
Provision of study material or patients: Yvette Carter, Herbert Chen, Rebecca S. Sippel
Collection and/or assembly of data: Yvette Carter, Herbert Chen, Rebecca S. Sippel
Data analysis and interpretation: Yvette Carter, Herbert Chen, Rebecca S. Sippel
Manuscript writing: Yvette Carter, Herbert Chen, Rebecca S. Sippel
Final approval of manuscript: Yvette Carter, Herbert Chen, Rebecca S. Sippel
Disclosures
The authors indicated no financial relationships.
Section Editors: Stan Sidhu: None.
Reviewer ”A“: None
References
- 1.Roberts CG, Ladenson PW. Hypothyroidism. Lancet. 2004;363:793–803. doi: 10.1016/S0140-6736(04)15696-1. [DOI] [PubMed] [Google Scholar]
- 2. Hashimoto H. Zur Kenntnis der lymphomatosen Veranderung der Schilddruse (struma lymphomatosa). Archiv fur Klinische Chirurgie 1912; 91:219–248. [Google Scholar]
- 3.Campbell PN, Doniach D, Hudson RV, et al. Auto-antibodies in Hashimoto’s disease (lymphadenoid goitre) Lancet. 1956;271:820–821. doi: 10.1016/s0140-6736(56)92249-8. [DOI] [PubMed] [Google Scholar]
- 4.Kendall EC. Reminiscences on the isolation of thyroxine. Mayo Clin Proc. 1964;39:548–552. [PubMed] [Google Scholar]
- 5.Harington CR, Barger G. Chemistry of thyroxine: Constitution and synthesis of thyroxine. Biochem J. 1927;21:169–183. doi: 10.1042/bj0210169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gross J, Pitt-Rivers R. The identification of 3:5:3′-L-triiodothyronine in human plasma. Lancet. 1952;1:439–441. doi: 10.1016/s0140-6736(52)91952-1. [DOI] [PubMed] [Google Scholar]
- 7.Braverman LE, Ingbar SH, Sterling K. Conversion of thyroxine (T4) to triiodothyronine (T3) in athyreotic human subjects. J Clin Invest. 1970;49:855–864. doi: 10.1172/JCI106304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mayberry WE, Gharib H, Bilstad JM, et al. Radioimmunoassay for human thyrotrophin. Clinical value in patients with normal and abnormal thyroid function. Ann Intern Med. 1971;74:471–480. doi: 10.7326/0003-4819-74-4-471. [DOI] [PubMed] [Google Scholar]
- 9.Tunbridge WM, Evered DC, Hall R, et al. The spectrum of thyroid disease in a community: The Whickham survey. Clin Endocrinol (Oxf) 1977;7:481–493. doi: 10.1111/j.1365-2265.1977.tb01340.x. [DOI] [PubMed] [Google Scholar]
- 10.Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–499. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 11.Chopra IJ, Solomon DH, Chopra U, et al. Abnormalities in thyroid function in relatives of patients with Graves’ disease and Hashimoto’s thyroiditis: Lack of correlation with inheritance of HLA-B8. J Clin Endocrinol Metab. 1977;45:45–54. doi: 10.1210/jcem-45-1-45. [DOI] [PubMed] [Google Scholar]
- 12.Tamai H, Ohsako N, Takeno K, et al. Changes in thyroid function in euthyroid subjects with a family history of Graves’ disease: a follow-up study of 69 patients. J Clin Endocrinol Metab. 1980;51:1123–1127. doi: 10.1210/jcem-51-5-1123. [DOI] [PubMed] [Google Scholar]
- 13.Muller AF, Drexhage HA, Berghout A. Postpartum thyroiditis and autoimmune thyroiditis in women of childbearing age: Recent insights and consequences for antenatal and postnatal care. Endocr Rev. 2001;22:605–630. doi: 10.1210/edrv.22.5.0441. [DOI] [PubMed] [Google Scholar]
- 14.Ogilvy-Stuart AL, Shalet SM, Gattamaneni HR. Thyroid function after treatment of brain tumors in children. J Pediatr. 1991;119:733–737. doi: 10.1016/s0022-3476(05)80288-4. [DOI] [PubMed] [Google Scholar]
- 15.Schmiegelow M, Feldt-Rasmussen U, Rasmussen AK, et al. A population-based study of thyroid function after radiotherapy and chemotherapy for a childhood brain tumor. J Clin Endocrinol Metab. 2003;88:136–140. doi: 10.1210/jc.2002-020380. [DOI] [PubMed] [Google Scholar]
- 16.Sklar C, Whitton J, Mertens A, et al. Abnormalities of the thyroid in survivors of Hodgkin’s disease: Data from the Childhood Cancer Survivor Study. J Clin Endocrinol Metab. 2000;85:3227–3232. doi: 10.1210/jcem.85.9.6808. [DOI] [PubMed] [Google Scholar]
- 17.Desai J, Yassa L, Marqusee E, et al. Hypothyroidism after sunitinib treatment for patients with gastrointestinal stromal tumors. Ann Intern Med. 2006;145:660–664. doi: 10.7326/0003-4819-145-9-200611070-00008. [DOI] [PubMed] [Google Scholar]
- 18.Mannavola D, Coco P, Vannucchi G, et al. A novel tyrosine-kinase selective inhibitor, sunitinib, induces transient hypothyroidism by blocking iodine uptake. J Clin Endocrinol Metab. 2007;92:3531–3534. doi: 10.1210/jc.2007-0586. [DOI] [PubMed] [Google Scholar]
- 19.Canaris GJ, Manowitz NR, Mayor G, et al. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160:526–534. doi: 10.1001/archinte.160.4.526. [DOI] [PubMed] [Google Scholar]
- 20.Dayan CM, Daniels GH. Chronic autoimmune thyroiditis. N Engl J Med. 1996;335:99–107. doi: 10.1056/NEJM199607113350206. [DOI] [PubMed] [Google Scholar]
- 21.McLachlan SM, Rapoport B. Why measure thyroglobulin autoantibodies rather than thyroid peroxidase autoantibodies? Thyroid. 2004;14:510–520. doi: 10.1089/1050725041517057. [DOI] [PubMed] [Google Scholar]
- 22.Phillips D, McLachlan S, Stephenson A, et al. Autosomal dominant transmission of autoantibodies to thyroglobulin and thyroid peroxidase. J Clin Endocrinol Metab. 1990;70:742–746. doi: 10.1210/jcem-70-3-742. [DOI] [PubMed] [Google Scholar]
- 23.Weiss RE, Refetoff S. Resistance to thyroid hormone. Rev Endocr Metab Disord. 2000;1:97–108. doi: 10.1023/a:1010072605757. [DOI] [PubMed] [Google Scholar]
- 24.Katz SM, Vickery AL., Jr The fibrous variant of Hashimoto’s thyroiditis. Hum Pathol. 1974;5:161–170. doi: 10.1016/s0046-8177(74)80063-8. [DOI] [PubMed] [Google Scholar]
- 25.Zimmerman RS, Brennan MD, McConahey WM, et al. Hashimoto’s thyroiditis. An uncommon cause of painful thyroid unresponsive to corticosteroid therapy. Ann Intern Med. 1986;104:355–357. doi: 10.7326/0003-4819-104-3-355. [DOI] [PubMed] [Google Scholar]
- 26.Neufeld M, Maclaren NK, Blizzard RM. Two types of autoimmune Addison’s disease associated with different polyglandular autoimmune (PGA) syndromes. Medicine (Baltimore) 1981;60:355–362. doi: 10.1097/00005792-198109000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Limonard EJ, Bisschop PH, Fliers E, et al. Thyroid function after subtotal thyroidectomy in patients with Graves’ hyperthyroidism. ScientificWorldJournal. 2012;2012:548796. doi: 10.1100/2012/548796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lilley JS, Lomenick JP. Delayed diagnosis of hypothyroidism following excision of a thyroglossal duct cyst. J Pediatr. 2013;162:427–428. doi: 10.1016/j.jpeds.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Becker DV, Hurley JR. Complications of radioiodine treatment of hyperthyroidism. Semin Nucl Med. 1971;1:442–460. doi: 10.1016/s0001-2998(71)81039-5. [DOI] [PubMed] [Google Scholar]
- 30.Goldsmith JR, Grossman CM, Morton WE, et al. Juvenile hypothyroidism among two populations exposed to radioiodine. Environ Health Perspect. 1999;107:303–308. doi: 10.1289/ehp.99107303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Order SE, Sleeper AM, Stillwagon GB, et al. Current status of radioimmunoglobulins in the treatment of human malignancy. Oncology (Williston Park) 1989;3:115–120. [PubMed] [Google Scholar]
- 32.Edwards CQ, Kelly TM, Ellwein G, et al. Thyroid disease in hemochromatosis. Increased incidence in homozygous men. Arch Intern Med. 1983;143:1890–1893. [PubMed] [Google Scholar]
- 33.Bogazzi F, Tomisti L, Bartalena L, et al. Amiodarone and the thyroid: A 2012 update. J Endocrinol Invest. 2012;35:340–348. doi: 10.3275/8298. [DOI] [PubMed] [Google Scholar]
- 34.Goldberg RC, Chaikoff IL, Chaikoff IL, et al. Histopathological changes induced in the normal thyroid and other tissues of the rat by internal radiation with various doses of radioactive iodine. Endocrinology. 1950;46:72–90. doi: 10.1210/endo-46-1-72. [DOI] [PubMed] [Google Scholar]
- 35.Nishiyama K, Tanaka E, Tarui Y, et al. A prospective analysis of subacute thyroid dysfunction after neck irradiation. Int J Radiat Oncol Biol Phys. 1996;34:439–444. doi: 10.1016/0360-3016(95)02079-9. [DOI] [PubMed] [Google Scholar]
- 36.Grande C. Hypothyroidism following radiotherapy for head and neck cancer: Multivariate analysis of risk factors. Radiother Oncol. 1992;25:31–36. doi: 10.1016/0167-8140(92)90192-w. [DOI] [PubMed] [Google Scholar]
- 37.Hancock SL, Cox RS, McDougall IR. Thyroid diseases after treatment of Hodgkin’s disease. N Engl J Med. 1991;325:599–605. doi: 10.1056/NEJM199108293250902. [DOI] [PubMed] [Google Scholar]
- 38.Bonato C, Severino RF, Elnecave RH. Reduced thyroid volume and hypothyroidism in survivors of childhood cancer treated with radiotherapy. J Pediatr Endocrinol Metab. 2008;21:943–949. doi: 10.1515/jpem.2008.21.10.943. [DOI] [PubMed] [Google Scholar]
- 39.Boomsma MJ, Bijl HP, Langendijk JA. Radiation-induced hypothyroidism in head and neck cancer patients: A systematic review. Radiother Oncol. 2011;99:1–5. doi: 10.1016/j.radonc.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Garcia-Serra A, Amdur RJ, Morris CG, et al. Thyroid function should be monitored following radiotherapy to the low neck. Am J Clin Oncol. 2005;28:255–258. doi: 10.1097/01.coc.0000145985.64640.ac. [DOI] [PubMed] [Google Scholar]
- 41.Norris AA, Amdur RJ, Morris CG, et al. Hypothyroidism when the thyroid is included only in the low neck field during head and neck radiotherapy. Am J Clin Oncol. 2006;29:442–445. doi: 10.1097/01.coc.0000217831.23820.85. [DOI] [PubMed] [Google Scholar]
- 42.Mercado G, Adelstein DJ, Saxton JP, et al. Hypothyroidism: A frequent event after radiotherapy and after radiotherapy with chemotherapy for patients with head and neck carcinoma. Cancer. 2001;92:2892–2897. doi: 10.1002/1097-0142(20011201)92:11<2892::aid-cncr10134>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 43.Chow LM, Nathan PC, Hodgson DC, et al. Survival and late effects in children with Hodgkin’s lymphoma treated with MOPP/ABV and low-dose, extended-field irradiation. J Clin Oncol. 2006;24:5735–5741. doi: 10.1200/JCO.2006.05.6879. [DOI] [PubMed] [Google Scholar]
- 44.Metzger ML, Hudson MM, Somes GW, et al. White race as a risk factor for hypothyroidism after treatment for pediatric Hodgkin’s lymphoma. J Clin Oncol. 2006;24:1516–1521. doi: 10.1200/JCO.2005.05.0195. [DOI] [PubMed] [Google Scholar]
- 45.Mahipal A, Tijani L, Chan K, et al. A pilot study of sunitinib malate in patients with metastatic uveal melanoma. Melanoma Res. 2012;22:440–446. doi: 10.1097/CMR.0b013e328358b373. [DOI] [PubMed] [Google Scholar]
- 46.Rutkowski P, Bylina E, Klimczak A, et al. The outcome and predictive factors of sunitinib therapy in advanced gastrointestinal stromal tumors (GIST) after imatinib failure—one institution study. BMC Cancer. 2012;12:107–114. doi: 10.1186/1471-2407-12-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Del Fabbro E, Dev R, Cabanillas ME, et al. Extreme hypothyroidism associated with sunitinib treatment for metastatic renal cancer. J Chemother. 2012;24:221–225. doi: 10.1179/1973947812Y.0000000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feldt S, Schüssel K, Quinzler R, et al. Incidence of thyroid hormone therapy in patients treated with sunitinib or sorafenib: A cohort study. Eur J Cancer. 2012;48:974–981. doi: 10.1016/j.ejca.2012.01.036. [DOI] [PubMed] [Google Scholar]
- 49.Faivre S, Demetri G, Sargent W, et al. Molecular basis for sunitinib efficacy and future clinical development. Nat Rev Drug Discov. 2007;6:734–745. doi: 10.1038/nrd2380. [DOI] [PubMed] [Google Scholar]
- 50.Cella D, Michaelson MD, Bushmakin AG, et al. Health-related quality of life in patients with metastatic renal cell carcinoma treated with sunitinib vs interferon-alpha in a phase III trial: Final results and geographical analysis. Br J Cancer. 2010;102:658–664. doi: 10.1038/sj.bjc.6605552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wolter P, Stefan C, Decallonne B, et al. The clinical implications of sunitinib-induced hypothyroidism: A prospective evaluation. Br J Cancer. 2008;99:448–454. doi: 10.1038/sj.bjc.6604497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Groot JWB, Zonnenberg BA, Plukker JTM, et al. Imatinib induces hypothyroidism in patients receiving levothyroxine. Clin Pharmacol Ther. 2005;78:433–438. doi: 10.1016/j.clpt.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 53.Goss GD, Arnold A, Shepherd FA, et al. Randomized, double-blind trial of carboplatin and paclitaxel with either daily oral cediranib or placebo in advanced non-small-cell lung cancer: NCIC clinical trials group BR24 study. J Clin Oncol. 2010;28:49–55. doi: 10.1200/JCO.2009.22.9427. [DOI] [PubMed] [Google Scholar]
- 54.Kim TD, Schwarz M, Nogai H, et al. Thyroid dysfunction caused by second-generation tyrosine kinase inhibitors in Philadelphia chromosome-positive chronic myeloid leukemia. Thyroid. 2010;20:1209–1214. doi: 10.1089/thy.2010.0251. [DOI] [PubMed] [Google Scholar]
- 55.Phan GQ, Attia P, Steinberg SM, et al. Factors associated with response to high-dose interleukin-2 in patients with metastatic melanoma. J Clin Oncol. 2001;19:3477–3482. doi: 10.1200/JCO.2001.19.15.3477. [DOI] [PubMed] [Google Scholar]
- 56.Franzke A, Peest D, Probst-Kepper M, et al. Autoimmunity resulting from cytokine treatment predicts long-term survival in patients with metastatic renal cell cancer. J Clin Oncol. 1999;17:529–533. doi: 10.1200/JCO.1999.17.2.529. [DOI] [PubMed] [Google Scholar]
- 57.Weijl NI, Van der Harst D, Brand A, et al. Hypothyroidism during immunotherapy with interleukin-2 is associated with antithyroid antibodies and response to treatment. J Clin Oncol. 1993;11:1376–1383. doi: 10.1200/JCO.1993.11.7.1376. [DOI] [PubMed] [Google Scholar]
- 58.Atkins MB, Mier JW, Parkinson DR, et al. Hypothyroidism after treatment with interleukin-2 and lymphokine-activated killer cells. N Engl J Med. 1988;318:1557–1563. doi: 10.1056/NEJM198806163182401. [DOI] [PubMed] [Google Scholar]
- 59. doi: 10.1016/j.ajem.2008.07.012. Chen SY, Kao PC, Lin ZZ et al. Sunitinib-induced myxedema coma. Am J Emerg Med 2009;27:370.e1-370.e3. [DOI] [PubMed] [Google Scholar]
- 60.Collinson FJ, Vasudev NS, Berkin L, et al. Sunitinib-induced severe hypothyroidism with cardiac compromise. Med Oncol. 2011;28(Suppl 1):S699–S701. doi: 10.1007/s12032-010-9757-z. [DOI] [PubMed] [Google Scholar]
- 61.Di Lorenzo G, Autorino R, Bruni G, et al. Cardiovascular toxicity following sunitinib therapy in metastatic renal cell carcinoma: A multicenter analysis. Ann Oncol. 2009;20:1535–1542. doi: 10.1093/annonc/mdp025. [DOI] [PubMed] [Google Scholar]
- 62.Golden WM, Weber KB, Hernandez TL, et al. Single-dose rexinoid rapidly and specifically suppresses serum thyrotropin in normal subjects. J Clin Endocrinol Metab. 2007;92:124–130. doi: 10.1210/jc.2006-0696. [DOI] [PubMed] [Google Scholar]
- 63.Gopal AK, Rajendran JG, Gooley TA, et al. High-dose [131I]tositumomab (anti-CD20) radioimmunotherapy and autologous hematopoietic stem-cell transplantation for adults > or = 60 years old with relapsed or refractory B-cell lymphoma. J Clin Oncol. 2007;25:1396–1402. doi: 10.1200/JCO.2006.09.1215. [DOI] [PubMed] [Google Scholar]
- 64.Press OW, Unger JM, Braziel RM, et al. Phase II trial of CHOP chemotherapy followed by tositumomab/iodine I-131 tositumomab for previously untreated follicular non-Hodgkin’s lymphoma: Five-year follow-up of Southwest Oncology Group Protocol S9911. J Clin Oncol. 2006;24:4143–4149. doi: 10.1200/JCO.2006.05.8198. [DOI] [PubMed] [Google Scholar]
- 65.Kaminski MS, Tuck M, Estes J, et al. 131I-tositumomab therapy as initial treatment for follicular lymphoma. N Engl J Med. 2005;352:441–449. doi: 10.1056/NEJMoa041511. [DOI] [PubMed] [Google Scholar]
- 66.Rose SR. Cranial irradiation and central hypothyroidism. Trends Endocrinol Metab. 2001;12:97–104. doi: 10.1016/s1043-2760(00)00359-3. [DOI] [PubMed] [Google Scholar]
- 67.Segal-Lieberman G, Karasik A, Shimon I. Hypopituitarism following closed head injury. Pituitary. 2000;3:181–184. doi: 10.1023/a:1011407910913. [DOI] [PubMed] [Google Scholar]
- 68.Bell NH. Endocrine complications of sarcoidosis. Endocrinol Metab Clin North Am. 1991;20:645–654. [PubMed] [Google Scholar]
- 69.McNeil LW, McKee LC, Jr, Lorber D, et al. The endocrine manifestations of hemochromatosis. Am J Med Sci. 1983;285:7–13. doi: 10.1097/00000441-198305000-00002. [DOI] [PubMed] [Google Scholar]
- 70.Sherman SI, Gopal J, Haugen BR, et al. Central hypothyroidism associated with retinoid X receptor-selective ligands. N Engl J Med. 1999;340:1075–1079. doi: 10.1056/NEJM199904083401404. [DOI] [PubMed] [Google Scholar]
- 71.Baronio F, Battisti L, Radetti G. Central hypothyroidism following chemotherapy for acute lymphoblastic leukemia. J Pediatr Endocrinol Metab. 2011;24:903–906. doi: 10.1515/jpem.2011.407. [DOI] [PubMed] [Google Scholar]
- 72.Bianco AC, Salvatore D, Gereben B, et al. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev. 2002;23:38–89. doi: 10.1210/edrv.23.1.0455. [DOI] [PubMed] [Google Scholar]
- 73.Koenig RJ. Thyroid hormone receptor coactivators and corepressors. Thyroid. 1998;8:703–713. doi: 10.1089/thy.1998.8.703. [DOI] [PubMed] [Google Scholar]
- 74.Davis PJ, Davis FB. Nongenomic actions of thyroid hormone. Thyroid. 1996;6:497–504. doi: 10.1089/thy.1996.6.497. [DOI] [PubMed] [Google Scholar]
- 75.Canaris GJ, Steiner JF, Ridgway EC. Do traditional symptoms of hypothyroidism correlate with biochemical disease? J Gen Intern Med. 1997;12:544–550. doi: 10.1046/j.1525-1497.1997.07109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Foley TP. Acquired hypothyroidism in infants, children, and adolescents. In: Braverman LE, Utiger RD, editors. The Thyroid: A Fundamental and Clinical Text. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2000. pp. 983–988. [Google Scholar]
- 77.Hirano T, Stamelos S, Harris V, et al. Association of primary hypothyroidism and slipped capital femoral epiphysis. J Pediatr. 1978;93:262–264. doi: 10.1016/s0022-3476(78)80514-9. [DOI] [PubMed] [Google Scholar]
- 78.Najjar SS. Muscular hypertrophy in hypothyroid children: The Kocher-Debré-Semelaigne syndrome. A review of 23 cases. J Pediatr. 1974;85:236–239. doi: 10.1016/s0022-3476(74)80403-8. [DOI] [PubMed] [Google Scholar]
- 79.Vagenakis AG, Dole K, Braverman LE. Pituitary enlargement, pituitary failure, and primary hypothyroidism. Ann Intern Med. 1976;85:195–198. doi: 10.7326/0003-4819-85-2-195. [DOI] [PubMed] [Google Scholar]
- 80.Chattopadhyay A, Kumar V, Marulaiah M. Polycystic ovaries, precocious puberty and acquired hypothyroidism: The Van Wyk and Grumbach syndrome. J Pediatr Surg. 2003;38:1390–1392. doi: 10.1016/s0022-3468(03)00403-2. [DOI] [PubMed] [Google Scholar]
- 81.Barnes ND, Hayles AB, Ryan RJ. Sexual maturation in juvenile hypothyroidism. Mayo Clin Proc. 1973;48:849–856. [PubMed] [Google Scholar]
- 82.Reuler JB. Hypothermia: pathophysiology, clinical settings, and management. Ann Intern Med. 1978;89:519–527. doi: 10.7326/0003-4819-89-4-519. [DOI] [PubMed] [Google Scholar]
- 83.Patrassi G. [So-called secondary or thyroid-resistant myxedema] Munch Med Wochenschr. 1950;92:950–955. [PubMed] [Google Scholar]
- 84.Ladenson PW, Sherman SI, Baughman KL, et al. Reversible alterations in myocardial gene expression in a young man with dilated cardiomyopathy and hypothyroidism. Proc Natl Acad Sci USA. 1992;89:5251–5255. doi: 10.1073/pnas.89.12.5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Quin JD, McDonald A, Russell R, et al. Hypothyroidism presenting with cardiac tamponade. Scott Med J. 1994;39:82. doi: 10.1177/003693309403900309. [DOI] [PubMed] [Google Scholar]
- 86.Lin CT, Liu CJ, Lin TK, et al. Myxedema associated with cardiac tamponade. Jpn Heart J. 2003;44:447–450. doi: 10.1536/jhj.44.447. [DOI] [PubMed] [Google Scholar]
- 87.Gottehrer A, Roa J, Stanford GG, et al. Hypothyroidism and pleural effusions. Chest. 1990;98:1130–1132. doi: 10.1378/chest.98.5.1130. [DOI] [PubMed] [Google Scholar]
- 88.Boruchow IB, Miller LD, Fitts WT., Jr Paralytic ileus in myxedema. Arch Surg. 1966;92:960–963. doi: 10.1001/archsurg.1966.01320240148033. [DOI] [PubMed] [Google Scholar]
- 89.Nickel SN, Frame B. Neurologic manifestations of myxedema. Neurology. 1958;8:511–517. doi: 10.1212/wnl.8.7.511. [DOI] [PubMed] [Google Scholar]
- 90.Asher R. Myxoedematous madness. BMJ. 1949;2:555–562. doi: 10.1136/bmj.2.4627.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Logothetis J. Psychotic behavior as the initial indicator of adult myxedema. J Nerv Ment Dis. 1963;136:561. [Google Scholar]
- 92.Price TR, Netsky MG. Myxedema and ataxia. Cerebellar alterations and “neural myxedema bodies.”. Neurology. 1966;16:957–962. doi: 10.1212/wnl.16.10.957. [DOI] [PubMed] [Google Scholar]
- 93.Woods KL, Holmes GK. Myxoedema coma presenting in status epilepticus. Postgrad Med J. 1977;53:46–48. doi: 10.1136/pgmj.53.615.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nicoloff JT, LoPresti JS. Myxedema coma. A form of decompensated hypothyroidism. Endocrinol Metab Clin North Am. 1993;22:279–290. [PubMed] [Google Scholar]
- 95.Ross DS. Serum thyroid-stimulating hormone measurement for assessment of thyroid function and disease. Endocrinol Metab Clin North Am. 2001;30:245–264, vii. doi: 10.1016/s0889-8529(05)70186-9. [DOI] [PubMed] [Google Scholar]
- 96.Spencer CA, Takeuchi M, Kazarosyan M. Current status and performance goals for serum thyrotropin (TSH) assays. Clin Chem. 1996;42:140–145. [PubMed] [Google Scholar]
- 97.Spencer CA, Hollowell JG, Kazarosyan M, et al. National Health and Nutrition Examination Survey III thyroid-stimulating hormone (TSH)-thyroperoxidase antibody relationships demonstrate that TSH upper reference limits may be skewed by occult thyroid dysfunction. J Clin Endocrinol Metab. 2007;92:4236–4240. doi: 10.1210/jc.2007-0287. [DOI] [PubMed] [Google Scholar]
- 98.Abalovich M, Amino N, Barbour LA, et al. Management of thyroid dysfunction during pregnancy and postpartum: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2007;92(Suppl):S1–S47. doi: 10.1210/jc.2007-0141. [DOI] [PubMed] [Google Scholar]
- 99.Stagnaro-Green A, Abalovich M, Alexander E, et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2011;21:1081–1125. doi: 10.1089/thy.2011.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Surks MI, Boucai L. Age- and race-based serum thyrotropin reference limits. J Clin Endocrinol Metab. 2010;95:496–502. doi: 10.1210/jc.2009-1845. [DOI] [PubMed] [Google Scholar]
- 101.Wartofsky L, Dickey RA. The evidence for a narrower thyrotropin reference range is compelling. J Clin Endocrinol Metab. 2005;90:5483–5488. doi: 10.1210/jc.2005-0455. [DOI] [PubMed] [Google Scholar]
- 102.Surks MI, Goswami G, Daniels GH. The thyrotropin reference range should remain unchanged. J Clin Endocrinol Metab. 2005;90:5489–5496. doi: 10.1210/jc.2005-0170. [DOI] [PubMed] [Google Scholar]
- 103.Brabant G, Beck-Peccoz P, Jarzab B, et al. Is there a need to redefine the upper normal limit of TSH? Eur J Endocrinol. 2006;154:633–637. doi: 10.1530/eje.1.02136. [DOI] [PubMed] [Google Scholar]
- 104.Andersen S, Pedersen KM, Bruun NH, et al. Narrow individual variations in serum T(4) and T(3) in normal subjects: A clue to the understanding of subclinical thyroid disease. J Clin Endocrinol Metab. 2002;87:1068–1072. doi: 10.1210/jcem.87.3.8165. [DOI] [PubMed] [Google Scholar]
- 105. Nygaard B. Hypothyroidism (primary). Clin Evid (Online). Available at http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3275323/. Published July 19, 2010. [Google Scholar]
- 106.Fish LH, Schwartz HL, Cavanaugh J, et al. Replacement dose, metabolism, and bioavailability of levothyroxine in the treatment of hypothyroidism. Role of triiodothyronine in pituitary feedback in humans. N Engl J Med. 1987;316:764–770. doi: 10.1056/NEJM198703263161302. [DOI] [PubMed] [Google Scholar]
- 107.Sawin CT, Geller A, Hershman JM, et al. The aging thyroid. The use of thyroid hormone in older persons. JAMA. 1989;261:2653–2655. doi: 10.1001/jama.261.18.2653. [DOI] [PubMed] [Google Scholar]
- 108.Celi FS, Zemskova M, Linderman JD, et al. Metabolic effects of liothyronine therapy in hypothyroidism: a randomized, double-blind, crossover trial of liothyronine versus levothyroxine. J Clin Endocrinol Metab. 2011;96:3466–3474. doi: 10.1210/jc.2011-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Saravanan P, Chau WF, Roberts N, et al. Psychological well-being in patients on ‘adequate’ doses of l-thyroxine: Results of a large, controlled community-based questionnaire study. Clin Endocrinol (Oxf) 2002;57:577–585. doi: 10.1046/j.1365-2265.2002.01654.x. [DOI] [PubMed] [Google Scholar]
- 110.Escobar-Morreale HF, Obregón MJ, Escobar del Rey F, et al. Replacement therapy for hypothyroidism with thyroxine alone does not ensure euthyroidism in all tissues, as studied in thyroidectomized rats. J Clin Invest. 1995;96:2828–2838. doi: 10.1172/JCI118353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Escobar-Morreale HF, del Rey FE, Obregón MJ, et al. Only the combined treatment with thyroxine and triiodothyronine ensures euthyroidism in all tissues of the thyroidectomized rat. Endocrinology. 1996;137:2490–2502. doi: 10.1210/endo.137.6.8641203. [DOI] [PubMed] [Google Scholar]
- 112.Vaidya B, Pearce SH. Management of hypothyroidism in adults. BMJ. 2008;337:a801. doi: 10.1136/bmj.a801. [DOI] [PubMed] [Google Scholar]
- 113.Rivkees SA, Mazzaferri EL, Verburg FA, et al. The treatment of differentiated thyroid cancer in children: Emphasis on surgical approach and radioactive iodine therapy. Endocr Rev. 2011;32:798–826. doi: 10.1210/er.2011-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cooper DS, Specker B, Ho M, et al. Thyrotropin suppression and disease progression in patients with differentiated thyroid cancer: Results from the National Thyroid Cancer Treatment Cooperative Registry. Thyroid. 1998;8:737–744. doi: 10.1089/thy.1998.8.737. [DOI] [PubMed] [Google Scholar]
- 115.Burmeister LA, Goumaz MO, Mariash CN, et al. Levothyroxine dose requirements for thyrotropin suppression in the treatment of differentiated thyroid cancer. J Clin Endocrinol Metab. 1992;75:344–350. doi: 10.1210/jcem.75.2.1639933. [DOI] [PubMed] [Google Scholar]
- 116.Gordon MB, Gordon MS. Variations in adequate levothyroxine replacement therapy in patients with different causes of hypothyroidism. Endocr Pract. 1999;5:233–238. doi: 10.4158/EP.5.5.233. [DOI] [PubMed] [Google Scholar]
- 117.Santini F, Pinchera A, Marsili A, et al. Lean body mass is a major determinant of levothyroxine dosage in the treatment of thyroid diseases. J Clin Endocrinol Metab. 2005;90:124–127. doi: 10.1210/jc.2004-1306. [DOI] [PubMed] [Google Scholar]
- 118.Slawik M, Klawitter B, Meiser E, et al. Thyroid hormone replacement for central hypothyroidism: A randomized controlled trial comparing two doses of thyroxine (T4) with a combination of T4 and triiodothyronine. J Clin Endocrinol Metab. 2007;92:4115–4122. doi: 10.1210/jc.2007-0297. [DOI] [PubMed] [Google Scholar]
- 119.Ojomo KA, Schneider DF, Reiher AE, et al. Using body mass index to predict optimal thyroid dosing after thyroidectomy. J Am Coll Surg. 2013;216:454–460. doi: 10.1016/j.jamcollsurg.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bunevicius R, Kazanavicius G, Zalinkevicius R, et al. Effects of thyroxine as compared with thyroxine plus triiodothyronine in patients with hypothyroidism. N Engl J Med. 1999;340:424–429. doi: 10.1056/NEJM199902113400603. [DOI] [PubMed] [Google Scholar]
- 121.Grozinsky-Glasberg S, Fraser A, Nahshoni E, et al. Thyroxine-triiodothyronine combination therapy versus thyroxine monotherapy for clinical hypothyroidism: Meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2006;91:2592–2599. doi: 10.1210/jc.2006-0448. [DOI] [PubMed] [Google Scholar]
- 122.Chu JW, Crapo LM. The treatment of subclinical hypothyroidism is seldom necessary. J Clin Endocrinol Metab. 2001;86:4591–4599. doi: 10.1210/jcem.86.10.7961. [DOI] [PubMed] [Google Scholar]
- 123.Danese MD, Ladenson PW, Meinert CL, et al. Clinical review 115: Effect of thyroxine therapy on serum lipoproteins in patients with mild thyroid failure: A quantitative review of the literature. J Clin Endocrinol Metab. 2000;85:2993–3001. doi: 10.1210/jcem.85.9.6841. [DOI] [PubMed] [Google Scholar]
- 124.Biondi B, Palmieri EA, Lombardi G, et al. Effects of subclinical thyroid dysfunction on the heart. Ann Intern Med. 2002;137:904–914. doi: 10.7326/0003-4819-137-11-200212030-00011. [DOI] [PubMed] [Google Scholar]
- 125.Brenta G, Mutti LA, Schnitman M, et al. Assessment of left ventricular diastolic function by radionuclide ventriculography at rest and exercise in subclinical hypothyroidism, and its response to L-thyroxine therapy. Am J Cardiol. 2003;91:1327–1330. doi: 10.1016/s0002-9149(03)00322-9. [DOI] [PubMed] [Google Scholar]
- 126.Cooper DS, Halpern R, Wood LC, et al. L-Thyroxine therapy in subclinical hypothyroidism. A double-blind, placebo-controlled trial. Ann Intern Med. 1984;101:18–24. doi: 10.7326/0003-4819-101-1-18. [DOI] [PubMed] [Google Scholar]
- 127.Nyström E, Caidahl K, Fager G, et al. A double-blind cross-over 12-month study of L-thyroxine treatment of women with ‘subclinical’ hypothyroidism. Clin Endocrinol (Oxf) 1988;29:63–75. doi: 10.1111/j.1365-2265.1988.tb00250.x. [DOI] [PubMed] [Google Scholar]
- 128.Jaeschke R, Guyatt G, Gerstein H, et al. Does treatment with L-thyroxine influence health status in middle-aged and older adults with subclinical hypothyroidism? J Gen Intern Med. 1996;11:744–749. doi: 10.1007/BF02598988. [DOI] [PubMed] [Google Scholar]
- 129.Monzani F, Del Guerra P, Caraccio N, et al. Subclinical hypothyroidism: Neurobehavioral features and beneficial effect of L-thyroxine treatment. Clin Investig. 1993;71:367–371. doi: 10.1007/BF00186625. [DOI] [PubMed] [Google Scholar]
- 130.Vanderpump MP, Tunbridge WM, French JM, et al. The incidence of thyroid disorders in the community: A twenty-year follow-up of the Whickham Survey. Clin Endocrinol (Oxf) 1995;43:55–68. doi: 10.1111/j.1365-2265.1995.tb01894.x. [DOI] [PubMed] [Google Scholar]


