Abstract
Abstract Both voluntary and involuntary movements activate sensors in the muscles, skin, tendon and joints. As limb movement can result from a mixture of spinal reflexes and voluntary motor commands, the cortical centres underlying conscious proprioception might either aggregate or separate the sensory inputs generated by voluntary movements from those generated by involuntary movements such as spinal reflexes. We addressed whether healthy volunteers could perceive the contribution of a spinal reflex during movements that combined both reflexive and voluntary contributions. Volunteers reported the reflexive contribution in leg movements that were partly driven by the knee-jerk reflex induced by a patellar tendon tap and partly by voluntary motor control. In one condition, participants were instructed to kick back in response to a tendon tap. The results were compared to reflexes in a resting baseline condition without voluntary movement. In a further condition, participants were instructed to kick forwards after a tap. Volunteers reported the perceived reflex contribution by repositioning the leg to the perceived maximum displacement to which the reflex moved the leg after each tendon tap. In the resting baseline condition, the reflex was accurately perceived. We found a near-unity slope of linear regressions of perceived on actual reflexive displacement. Both the slope value and the quality of regression fit in individual volunteers were significantly reduced when volunteers were instructed to generate voluntary backward kicks as soon as they detected the tap. In the kick forward condition, kinematic analysis showed continuity of reflex and voluntary movements, but the reflex contribution could be estimated from electromyography (EMG) recording on each trial. Again, participants’ judgements of reflexes showed a poor relation to reflex EMG, in contrast to the baseline condition. In sum, we show that reflexes can be accurately perceived from afferent information. However, the presence of voluntary movement significantly impairs reflex perception. We suggest that perceptual separation between voluntary and reflex movement is poor at best. Our results imply that the brain has no clear marker for perceptually separating voluntary and involuntary movement. Attribution of body movement to voluntary or involuntary motor commands is surprisingly poor when both are present.
Key points.
Voluntary motor commands and spinal reflexes both produce body movements that activate sensors located in the muscles, joints, tendon and skin.
It is unknown whether perceptions generated by the sensory inputs from voluntary movements can be distinguished from perception of inputs due to spinal reflexes. Surprisingly, the perception of reflexes remains largely unaddressed.
Knee-jerk reflexes were perceived accurately on the basis of proprioceptive inputs alone, but perception was poorer when volunteers were instructed to voluntarily kick backwards or forwards as rapidly as possible in response to the tendon tap.
This demonstrates that sensory inputs from a spinal reflex lead to movement perception. However, there is no clear perceptual landmark separating reflexes from voluntary movements, even when the two movements involve antagonistic muscles.
These findings help us to understand how sensory feedback from body movement leads to movement perception and awareness of action.
Introduction
Our body can be moved voluntarily or involuntarily. All movements result in a barrage of sensory inputs from the muscles, joints, tendon and skin. Efferent cortical motor commands that drive voluntary movement may also contribute directly to the perception of movement. For example, a comparator process may cancel sensory reafference against predictions based on motor commands (Blakemore & Frith, 2003). However, not all movements require cortical commands. For instance, the well-known spinal reflex arc can be driven with sensory inputs alone (Sherrington, 1907). Anecdotal experience shows that we clearly perceive such spinal reflexes, but (perhaps surprisingly) there appear to have been few systematic scientific investigations of reflex perception. In particular, it remains unclear how perception of reflexes may differ from perception of voluntary movements.
On the other hand, several studies have compared the perception of voluntary and passive movements. Some studies report more accurate perception of voluntary actions than of passive movements (Browne & Lee, 1954; Paillard & Brouchon, 1968; Gritsenko et al. 2007). Other studies emphasise the suppression of afferent input during voluntary action (Papakostopoulos et al. 1975; Chapman et al. 1987), which might suggest less accurate movement perception during voluntary action. Thus, perception of movement may be either enhanced or impaired during voluntary action, depending on conditions. The precision of both execution and perception of human movement are nevertheless remarkable; arm displacements as small as 2 mm can be reliably perceived (Hall & McCloskey, 1983).
Most actions involve an overlapping mix of voluntary/cortical and involuntary movements. Whether the cortex can separate the proprioceptive inputs from voluntary movements and from reflexes is not known. This ability seems important for ‘credit assignment’. To regulate voluntary motor commands or reflex gains for future actions, the brain must compute whether any error in the current movement should be attributed to an inappropriate voluntary motor command or an inappropriate reflex (Wolpert et al. 2011). The remarkable ability to improve voluntary motor commands through motor learning implies that the brain is able to solve this credit assignment problem. Thus, the brain appears able to correctly separate afferent inputs into those caused by voluntary and by involuntary movement. Few computational studies have investigated how this is done. Here we have investigated whether people can perceptually distinguish between limb movement caused by voluntary and by reflex motor commands. Our aim was to identify whether conscious perception of movement signals could form the basis of credit assignment in sensorimotor control.
Both cortical and spinal movements produce multiple afferent signals that are potentially informative about the action itself. First, muscle contractions directly activate the Golgi tendon organs. Whether this input contributes to conscious perception is contentious (Matthews, 1982). In addition, muscle stretch causes signals from muscle spindles. Joint receptors and cutaneous sensors add further inputs. Each of these inputs can result in a sense of movement when activated experimentally in the absence of motion (Burgess et al. 1982; Proske & Gandevia, 2009). Finally, the efference copies of the motor command may also produce perception, in conjunction with a forward predictive model (Wolpert & Flanagan, 2001). However, the level of perceptual detail provided by the efference remains unclear (Gandevia & Rothwell, 1987; Proske & Gandevia, 2009). The dynamic perception of movement provides some of the strongest evidence in favour of an efferent contribution. For instance, awareness of voluntary movement can occur before the body actually moves (Dassonville, 1995). When spinal and cortical movements occur in close relation to one another, computational models suggest that the cortical component is attenuated by a sensorimotor cancellation process (Blakemore & Frith, 2003), so that perception is dominated by proprioceptive inputs of reflex origin. However, neurophysiological studies have suggested that voluntary motor commands lead to alteration of all afferent signals, due to both spinal attenuation (Seki & Fetz, 2012) and increased noise (Wise et al. 1998). Degraded proprioceptive input during movement would presumably affect the separation of afferent signals into those linked to voluntary movement and those linked to reflexes.
This study focuses on the well-known knee-jerk stretch reflex. Striking the patellar tendon results in the activation of quadriceps muscle, driving a reflex arc that contracts the same muscle. In a sitting position with the leg hanging vertically this reflex causes a forward swing of the lower leg. We asked volunteers to report the amplitude of this excursion. By comparing the reported and real amplitudes we demonstrate that reflexes can be accurately perceived in themselves. We also tested two further conditions, involving different mixtures of voluntary and reflex movement. In one condition, we instructed volunteers to kick voluntarily against the reflex, in response to the tendon tap. In a separate condition, we instructed participants to kick forward along with the reflex, in response to the tendon tap. These instructions produced movements that were part spinal and part cortical. In the kick back condition, volunteers reported the maximal forward displacement that the leg reached, before the backward movement. In the kick forward condition, reflex and voluntary movement have overlapping effects. We investigated how well participants could perceive the separate contribution of the reflex to the leg movement. We therefore asked them to judge the limb position at which their voluntary motor command began to drive the leg forward. Both judgements revealed a poor relationship between the actual and perceived movement. The pattern of data suggests that proprioceptive inputs from the spinal movements cannot be perceptually separated from the inputs generated by cortical movements.
Methods
Ethical approval
The University College London Research Ethics Committee approved the experiments (project ID, 3025/001) and our conduct also conformed to the Declaration of Helsinki.
Experimental volunteers
Male and female volunteers between the age of 18 and 35 years (median 21) participated on the basis of written informed consent. A total of 29 volunteers were considered, eight of whom were rejected before conducting perceptual tests. Four of the eight had subcutaneous fat that interfered with the electromyography (EMG) recordings. In the remaining four we could not induce a knee-jerk reflex.
Tendon tap induced knee-jerk reflex and passive movements
A Queen Square hammer or an MLA 93 Tendon Hammer (ADInstruments GmbH, Spechbach, Germany) was used to tap a 13 mm diameter target on the left knee. While using the former instrument, the knee and the hammer were covered with rubber pads, and a force-sensitive resistor (12.7 mm, SparkFun Electronics, USA; https://www.sparkfun.com/) was placed on the kneepad. The latter hammer was used on the bare knee and its piezo-electric sensor generated voltages proportional to the striking force. All the trials considered in this study were generated from taps that displayed 20–35% of the maximum tap force possible by the experimenter (A.G.). At least 90% of the trials of each volunteer fell within this range. The tap force was registered using a custom written LabVIEW program reading from the data acquired using NI USB-6008 connected to the sensor (National Instruments, Austin, TX, USA). A microphone placed close to the tap additionally monitored the audio signals from the impact. The volunteers were seated upright with arms crossed above their waist. The legs hung vertically and were made invisible to the volunteer using a baffle. The volunteers fixated on a 0.1 cm diameter filled circle on the screen in front of them.
For passive movements volunteers were seated on the table with the leg hanging vertically. Just prior to each movement the leg was held back for 1–4 s until the volunteer was completely relaxed. The left foot was released from a voltage-controlled electromagnet (GT-60; Isliker Magnate, Andelfingen, Switzerland), to drop and swing forwards passively. The height of the drop was adjusted by using a pulley to vary the initial leg position.
Delivery of instructions
In the baseline condition the volunteers were instructed to ‘relax’ and to remain relaxed throughout the knee-jerk caused by the tendon tap. At rest the knee joint angle was 85–92°, and remained within this range throughout the experimental session. After each reflex, we asked participants to report their perception of the amplitude of the reflex movement. To do this, they moved their leg to the perceived location of the maximum forward excursion it had reached due to the reflex. They indicated verbally when they had done this, and held that posture for 2 s. To ensure that this method did not bias participants’ perceptual reports, we explicitly informed them that after a tendon tap a movement may or may not occur. Verbal response and leg position were recorded by a video camera with a side view. In all 21 volunteers the reflex conditions were tested in blocks of 10 trials. Each condition was tested in a separate block, and block order was pseudorandomised. Participants were reminded to ‘relax’, ‘kick back’ or ‘kick forward’ and asked if they were ready, prior to each trial.
For the voluntary movement conditions, volunteers were instructed to quickly kick as far back or forward as possible as soon as they felt the tendon tap. Because the kick forward condition involved strong overlap between reflex and voluntary movement, we investigated two possible perceptual markers for separating the contribution of each. Thus, one group (n= 10) was instructed to report ‘the position of the leg at which they began to voluntarily move it’, while a second group was instructed to report ‘how far forward the reflex moved their leg’ (n= 11).
We also tested perception of passive movements, to investigate the contribution of afferent signals to movement perception in the absence of motor commands. Volunteers were seated at rest and asked to ‘relax’ as the foot was pulled backwards and upwards, and then released by switching off an electromagnet. Again they reported how far forward the leg moved. In a further condition, volunteers attempted to kick back as soon as they felt the foot drop.
Kinematics and EMG
In 10 volunteers three 5 mm diameter LEDs were placed on the ankle, knee joint and thigh. These were used as optical markers for the video record to document the angular displacements around the knee joint. The video was recorded at 60 frames per second. In 11 volunteers a goniometer (MLTS700 Joint Angle Sensor; ADInstruments GmbH) was strapped around the knee joint. The voltage change from this sensor was recorded at 2000 Hz. For EMG, data were acquired using two pairs of surface electrodes placed on the quadriceps and hamstring. The electrode placements were according to the guidelines described by Zipp ()1982. Briefly, the quadriceps electrodes were placed along a diagonal line from the inner kneecap to the outer hip, and inter-electrode distance was maintained at one-fifth of the line. This configuration mainly lay over the vastus medialis but not without cross talk from the other quadriceps muscles. Similarly, the surface electrodes were configured over the hamstring at one-fifth of a diagonal line between the outer kneecap and the gluteus maximus belly. This mainly lay over the long head of the biceps femoris but again not without cross talk from the semitendinosus muscle. A ground electrode was placed on the lower leg. For both configurations the distal (to the body) electrode was placed at 7 cm from the kneecap. EMG data from the quadriceps and hamstrings were acquired by using a pair of isolated amplifiers (1902, Cambridge Electronic Design, Cambridge, UK), sampled at 2000 Hz and passed through a 50 Hz notch filter online. The EMG recordings and the camera were synchronized using TTL pulses and an LED driven by the same pulses as visible to the camera.
Data analysis and statistics
As mentioned above we measured the angular displacements around the knee joint by using two different methods. For the optical markers the images were processed using an Image J plug-in (SpotTracker 2D, EPFL, Lausanne, Switzerland). The traced coordinates were processed using Matlab scripts to determine the change in position from rest (the mean position over 0.25 s measured before the tendon tap). The detection threshold was set at +3 times resting standard deviation (SD). For the goniometer measurements the data were converted into angular displacement in degrees by using Matlab scripts. Due to the higher sensitivity of the goniometer the detection threshold was set at +5 times resting SD. For passive movements the angular displacements were calculated relative to the resting vertical position prior to the lift and drop.
The EMG data were band pass filtered between 10 and 950 Hz. Taken together with the data acquisition settings, the filter has the potential to introduce aliasing artefacts. Still, such data acquisition settings were chosen as they are suitable for monitoring the data online in an unshielded room and the filter settings are established in the detection of fast muscular contractions (Buch et al. 2010; Neubert et al. 2010).
A threshold of +3 times resting SD was used to detect the onset and offset of the reflex in the quadriceps muscle. The area under the signal from onset to offset was quantified. Similarly, the onset of voluntary contraction was detected in the hamstrings in the kick back condition. The voluntary movement onset in quadriceps (in the kick forwards condition) was defined as the point at which the activity crossed the 3 SD threshold after the reflex offset. The reflexive data from the baseline condition provided templates during manual supervision (by an independent observer) of the analysis.
We assessed the perception of reflexes and passive movements using linear regression to relate the perceived position of the leg, as judged in each condition, to the relevant actual position. The intercept, slope and R2 values provide estimates of, respectively, perceptual bias, ability to discriminate different reflex sizes or perceptual gain, and precision or measurement noise in reflex and passive movement perception. The R2 values were compared between two groups using the non-parametric Mann–Whitney test. For pairwise comparisons of slopes and intercepts Student’s t test was used. When comparing integrated reflex EMG amplitudes in the three conditions, Kruskal–Wallis non-parametric analysis of variance (ANOVA) was followed by Wilcoxon matched pair tests. Results are presented as mean ± standard error of the mean (SEM).
Results
EMG and kinematic description of the reflex in the baseline condition
In the baseline condition subjects were instructed to remain relaxed during the knee-jerk reflex. As there are only few EMG and kinematic descriptions of this reflex in healthy humans (Hamann & Morris, 1975; Boyle et al. 1979; Lebiedowska et al. 2011), we begin with its key features. Manual taps on the patellar tendon were made using a hammer on a 13 mm diameter target. The trial-to-trial variability in reflex strength probably reflects variation in gain of spinal circuits (Schindler-Ivens & Shields, 2000), in tap amplitude and in the exact location of the tap within the 132 mm2 target. As expected, the timing of the reflex showed little variation (for data summary see Table 1). Surface EMG recordings showed that the quadriceps muscle was activated at 15.62 ± 0.62 ms (n= 11 volunteers) after the tendon tap. Physical displacement of the leg, i.e. the time taken for the goniometer signal to show a movement above 5 SD from the baseline, began 118 ± 4.9 ms from the tendon tap (Fig. 1, Table 1). It took 401.21 ± 15.76 ms to reach the maximum forward displacement.
Table 1.
Key attributes of the movements used in this study
| Attribute | Baseline | Kick back | Kick forward |
|---|---|---|---|
| Reflex EMG strength (ms mV) | 6.0 ± 1.3 | 7.5 ± 1.5 | 7.6 ± 1.9 |
| Reflex EMG onset (ms) | 15.6 ± 0.6 | 15.0 ± 0.6 | 15.4 ± 0.2 |
| Reflex forward angular displacement (deg) | 16.1 ± 2.1 | 9.4 ± 2.9 | NA |
| Reflex perceptual report (deg) | 20.4 ± 3.0 | 19.7 ± 6.2 | 26.4 ± 5.2 |
| Passive angular displacement (deg) | 18.1 ± 2.7 | 8.3 ± 3.1 | NA |
| Passive perceptual report (deg) | 17.8 ± 2.8 | 1.2 ± 2.1 | NA |
| Reflex kinematic onset (ms) | 118 ± 4.9 | 117 ± 7.1 | 116 ± 8.2 |
| Reflex kinematic offset (ms) | 401 ± 15.8 | 272 ± 18.3 | NA |
| Voluntary EMG onset (ms) | NA | 109 ± 5.2 | 125 ± 4.4 |
Each entry is the mean of means ± SEM (n= 12/13/20/21; see text for details). Latencies are from the tendon tap. NA, not applicable.
Figure 1. Perception of the knee-jerk reflex in a representative volunteer.
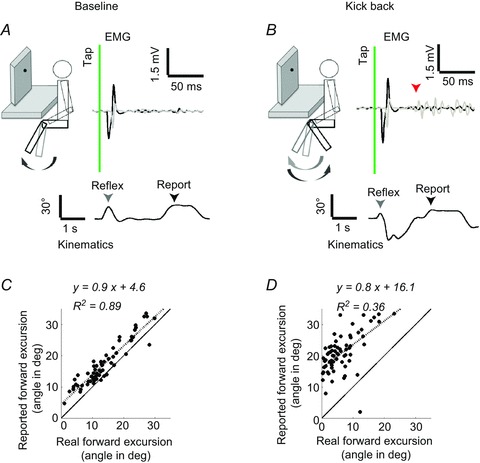
A, schematic side view of the baseline experimental set-up in which the volunteers were seated. They were tapped on the knee and the leg was invisible to the volunteer due to the cardboard baffle. The top traces show single trial EMG recordings from the hamstring (grey) and quadriceps (black). The time of the tap is shown using the vertical line. The angular displacements (lower trace) around the knee joint were documented by using joint angle sensors (in this example) or optical markers. The first arrow on the kinematic trace (grey) depicts the real reflexive movement amplitude and the second marks the reported forward excursion (black). B, when instructed to kick back, a clear activation of the hamstring muscles was seen after the reflexive contraction (arrow – voluntary EMG onset). C and D, scatter plots and linear regressions between the real forward foot movements and the reports, in the baseline (C) or kick back conditions (D). The dashed line shows the linear fit of the displayed data and continuous line depicts the hypothetical ideal observer.
Perception of the reflex in the baseline condition
In the baseline condition, volunteers reported the maximal knee extension reached during the reflexive forward swing. Plotting the perceived angular displacement (y) as a function of the actual angular displacement (x) showed that participants were clearly able to perceive the reflex movement. Moreover, participants’ perceptual accuracy could be quantified by a linear fit of perceived to actual displacement. (Fig. 1). The regression fits were generally good (mean R2= 0.7 ± 0.04, n= 21). The individual regression slopes were between 0.5 and 1.2. The mean slope was just less than unity at 0.9 ± 0.1 (t20=−3.225, P < 0.05, Student’s t test). All but three (of 21) volunteers showed a positive y-intercept (Fig. 2). The mean intercept was slightly but significantly above zero at 3.8 ± 0.9 deg (t20= 4.597, P < 0.05, Student’s t test).
Figure 2. A voluntary kick back alters reflex perception.
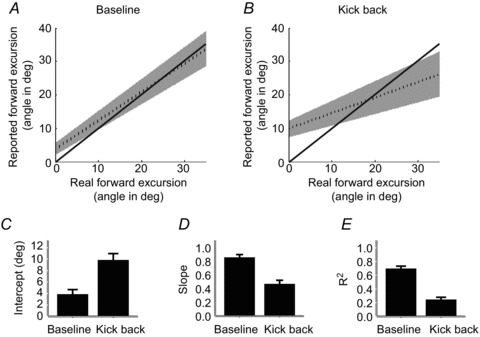
A and B, group mean linear regression line (dashed) with 95% confidence intervals in the baseline (A) and kick back (B) conditions. Solid line depicts the hypothetical ideal observer. C–E, when kicking back, data from the 21 volunteers show consistent increases in the intercept (C), and reduction in slope (D) and R2 values (E) compared to the baseline condition. The bar plots display group means ± SEM. All pairs were significantly different (P < 0.05). For significance levels, see main text.
We performed a similar analysis on the passive leg drops data (Supplementary Fig. S1). Again we used linear fits to describe the relationship between perceived and actual movement. The mean slope for passive movements was 0.8 ± 0.2 (n= 11). This value was significantly different from unity (t10=−2.46, P < 0.05). Regression fits were good (R2= 0.7 ± 0.05, n= 11). Neither the slope nor the R2 value differed between reflex and passive movements (slope reflex 0.8 ± 0.06, passive 0.8 ± 0.07, t10=−0.20, P= 0.85, Student’s t test; R2 reflex 0.6 ± 0.06, passive = 0.7 ± 0.05, P= 0.06, Mann–Whitney test). The mean intercept was not significantly above zero (0.7 ± 1.6, t10= 0.416, P= 0.7, Student’s t test) and was lower than that for reflexive movements (t10= 3.436, P < 0.05, Student’s t test).
EMG and kinematic description of the reflex in the kick back condition
We first compared the reflex itself between baseline and voluntary kick back conditions. The data are summarised in Table 1. One volunteer’s EMG data had to be eliminated due to a data acquisition error resulting in clipping of the reflex signals. The strength of the reflex was higher in the voluntary condition compared with the baseline (mean baseline integrated area of 6.0 ± 1.3 ms mV and 7.5 ± 1.5 ms mV in the voluntary condition, t19=−3.261, P < 0.05, Student’s t test). The latency of the reflex EMG onset in the voluntary condition was unchanged compared with the baseline (15.0 ± 0.6 ms, P= 0.8, Mann–Whitney test). In the voluntary kick back condition, the voluntary EMG onset occurred at 109 ± 5.2 ms (n= 20). Interestingly, the reflex had not significantly displaced the leg by the time this voluntary burst began: the mean displacement was only 0.12 ± 0.06 deg (t10= 1.97, P= 0.08). It took 272 ±18.27 ms to reach the maximum forward displacement.
The kick back did not extinguish the reflexive forward movement, but did reduce its amplitude (Table 1). The mean maximum forward excursion was 16.1 ± 2.1 deg in the baseline condition and 9.4 ± 2.9 deg in the kick back condition (t10=−4.47, P < 0.05). In the kick back condition, as in the baseline condition, the reflex amplitude varied from trial to trial. This variation produced a corresponding change in the maximum forward displacement. A linear regression of displacement against reflex EMG showed a strong relation (R2 0.5 ± 0.05, slope 1.2 ± 0.2, intercept 0.9 ± 0.6). These values were comparable to those in the baseline condition (R2 0.6 ± 0.07, slope 1.7 ± 0.3, intercept 7.0 ± 1.7).
Perception of the reflex in the kick back condition
We again used linear regressions to examine the perception of the displacement caused by the initial reflex. To investigate how the presence of voluntary kick back influenced the perception of the reflex, we consider an ideal observer model. An ideal observer would clearly yield a slope of unity, an intercept of 0 and a high R2 in this analysis. Participants might depart from the ideal observer model in a number of ways. First, perceptual noise might lead to low R2 values, without affecting slope or intercept. Second, reduced perceptual sensitivity would lead to slopes below unity and intercepts above zero: when participants do not have accurate information about the true joint angle, large differences in actual joint angle produce only small differences in perception. Rather than reporting the actual joint angle, participants report values closer to a typical or mean joint angle, overestimating small excursions and underestimating large excursions. Finally, perceptual biases would lead to changes in intercept without changes in slope (Fig. S1). Perception of the reflex movement was severely affected by the presence of the voluntary kick back movement (Fig. 1 example; Fig. 2 group data). The regression slope was significantly below unity in the kick back condition (0.47 ± 0.1, t20=−9.3, P < 0.05, Student’s t test), and significantly reduced compared to the baseline condition (t20= 6.650, P < 0.05, Student’s paired t test), although still significantly above zero (t20= 8.1, P < 0.05, Student’s t test). The intercept was also increased relative to the baseline condition (mean y-intercept 9.8 ± 1.2 deg, significantly greater than the baseline condition, t20=−4.992, P < 0.05, Student’s paired t test). Finally, the proportion of variability in perception explained by the stimulus decreased relative to the baseline condition (R2= 0.2 ± 0.04, P < 0.05, Mann–Whitney test). This pattern of results corresponds to a loss of perceptual sensitivity, and an increase in noise, relative to the ideal observer. Participants appeared to overestimate kinematic effects of small reflexes and underestimate kinematic effects of large reflexes, reporting similar values irrespective of the actual reflex amplitude.
We also addressed the perception of passive leg-drops when instructed to kick back (Fig. S1). The slope and quality of the fit showed a similar pattern to reflexes, i.e. both these measures reduced in the presence of voluntary action. Mean slope reduced to 0.6 ± 0.1 (t10= 4.829, P < 0.05, Student’s paired t test) and the R2 to 0.4 ± 0.06 (P < 0.05, Mann–Whitney test). In contrast to the rise in intercepts for reflexes, passive movements were characterized by a lower intercept value for the kick back compared to when relaxed (−6.7 ± 3.2, t10= 2.232, P < 0.05, Student’s paired t test). Therefore, the presence of a voluntary action impaired perception of a passive movement, as it did for reflexes.
EMG and kinematic description of the reflex in the kick forward condition
In a separate condition, we instructed the volunteers to kick forwards as soon as they felt a tendon tap. The tap-induced reflexive contraction of the quadriceps was followed by voluntary contraction of the same muscle (Fig. 3). The waveform of the reflex was again very stereotyped, and was followed by a burst of voluntary activity with only a brief EMG pause between the two. The reflex EMG amplitude was not significantly different from the baseline condition (mean change in EMG strength 21 ± 13.04% from baseline, t19=−1.677, P= 0.11, Student’s paired t test). As in the kick back condition, the mean latency to onset of the voluntary contraction was 125 ± 4.4 ms (n= 20, for data summary see Table 1). The mean position of the leg at this moment was 0.26 ± 0.09 deg, which was significantly different from the initial position (t10= 2.80, P < 0.05). There was no visible kinematic landmark separating the reflex from the voluntary kick – reflexive and voluntary muscular contractions resulted in a forward leg swing without any visible disruptions (Fig. 3).
Figure 3. Perception of the knee-jerk reflex in the kick forward condition in a representative volunteer.
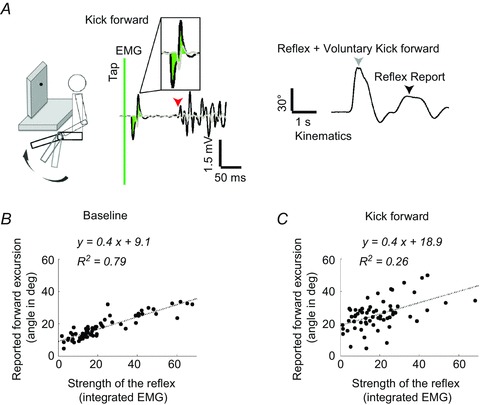
A, schematic view of the experimental set-up. Volunteers were instructed to kick forwards as soon as they felt the tendon tap. On the EMG traces, the time of tap is the green vertical bar. Note the strong reflexive and voluntary activation in quadriceps (black trace, arrow marks the voluntary EMG activity onset). The area used for analysis is shaded on the EMG trace. The grey arrow marks the end of the forward movement that consisted of both reflexive and voluntary contractions. The black arrow on the kinematic trace shows the position from which the volunteer perceived him/herself kicking forwards. B and C, strength of the reflexive contractions and the perceptual reports correlated well in the baseline condition (B) and poorly when kicking forwards (C).
Perception of the reflex in the kick forward condition
We compared the perception of reflexes in the kick forward condition to the baseline. Note that we did not attempt to compare the kick forward and kick back conditions directly, because the different movement patterns in the two cases would make interpretation difficult.
Because there was no obvious kinematic marker separating the reflex and voluntary contributions, we instructed participants to report the perceived leg position ‘at which you first began to voluntarily move’ the leg (n= 10). Another group were asked to report ‘how far forward the reflex moved your leg’ (n= 11). Both produced similar results, and the data were merged into a single group. We compared the reported position at voluntary movement onset to the strength of the reflex contraction (Fig. 3 example; Fig. 4 group data). Our interest focused on whether participants could make this judgement in the absence of any specific kinematic or proprioceptive landmark of the transition between reflex and voluntary drive.
Figure 4. A voluntary kick forward alters reflex perception.
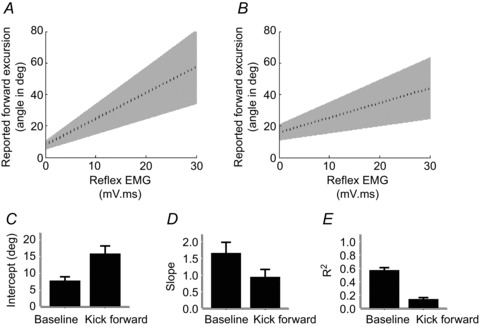
A and B, group mean linear regression line (dashed) with 95% confidence intervals in the baseline (A) and kick forward (B) conditions. C–E, similar to the kick back condition, we observed consistent increase in the intercept (C), and reduction in slope (D) and R2 values (E). For significance levels, see text.
In the kick forward condition we could not predict the perceived displacement from the actual reflex-induced displacement directly, because of the overlap between reflex and voluntary movement. We nevertheless reasoned that the reflex contribution into the movement would be related to reflex EMG amplitude. In contrast, in the voluntary condition we instructed volunteers to ‘kick forward to maximal extent’, and we expected them to produce the same strong and ballistic voluntary movement in each trial. Yet, trial-to-trial variability in reaction time and muscle contraction intensity is practically unavoidable. We assumed that any variation in voluntary command onset or amplitude must be independent of trial-to-trial variation in reflex amplitude. Therefore, we could estimate the perception of the reflex in the kick forward condition by relating reported reflex-induced leg displacement to reflex EMG amplitude. Moreover, the relationship between reflex EMG and perceptual report could be computed also for the baseline condition, and compared to the kick forward condition. This comparison is informative even if reflex EMG amplitude is only a proxy measure for the kinematic consequences of the reflex.
We used linear regressions to relate the reported reflexive movements’ excursion to reflex EMG amplitude (Fig. 3 example; Fig. 4 group data). One participant’s EMG data had to be eliminated due to a data acquisition error. At baseline, the mean slope was 1.7 ± 0.3, while the mean slope was 0.9 ± 0.2 in the kick forwards condition. Thus, in both conditions there was a highly significant relationship between reflex EMG amplitude and perceived kinematic effect (t19= 5.042, P < 0.05; t19= 4.067, P < 0.05). However, this relationship was weaker in the kick forward condition than at baseline (t19= 3.76, P < 0.05, Student’s paired t test). The mean R2 was 0.6 ± 0.04 at rest and 0.1 ± 0.03 for kick forwards (P < 0.05, Mann–Whitney test). The regression intercept was much higher than in the baseline condition (15.9 ± 2.4 deg for kick forwards and 7.8 ± 1.2 deg at rest, t19=−2.981, P < 0.05, Student’s paired t test). Thus, we found an impaired perception of the reflex contribution in the kick forward condition, relative to baseline.
Discussion
Despite the ubiquity of knee-jerk reflex testing in clinical practice, the perception of the movements generated seems scarcely to have been studied. Our findings show that the movements caused by the spinal reflex can be perceived accurately when they occur in isolation. This demonstrates the functional capacity of the proprioceptive receptors, presumably including muscle spindles, joint receptors, tendon organs and cutaneous receptors, to encode information about leg position. We further showed that reflex movements and passive movements are perceived with similar accuracy. We used a passive leg drop, which was as kinematically similar to the knee-jerk reflex as possible, to show that both reflexes and passive movement amplitude could be perceived precisely. Our analysis of regression slopes suggested that reflexes are not perceived any more accurately than passive movements. This rules out the possibility of any additional signals underlying movement perception in the reflex condition. Rather, our data are consistent with the view that the same afferent signals are used to perceive both reflex and passive movements.
The information available for perception of reflexes differs from that for active movements. Perception of voluntary movement may rely on efferent signals, and a cortical forward model (Wolpert & Flanagan, 2001). Since the reflex is spinal in origin, these cortical signals are not available. Many studies have reported better perception for voluntary movements than for passive movements (Paillard & Brouchon, 1968; Cordo et al. 2011). The cortical efferent command is thought to explain this difference (Laufer et al. 2001; Branch et al. 2008; Fuentes & Bastian, 2010). However, these cannot contribute to movements generated subcortically, such as spinal reflexes.
How is the reflex movement perceived? Several classes of proprioceptors could contribute, including the muscle spindles, cutaneous sensors and joint receptors. The tendon tap stretches the quadriceps muscle spindles to drive the reflex arc, but this brief input probably does not contain the main sensory information about movement excursion. The muscle spindles in the hamstring driven by the forward leg movement must also contribute to perception. Notably, activation of the muscle spindles around the knee joint by vibrating the patellar tendon results in an illusory leg movement (Collins et al. 2005). The cutaneous receptors around the knee joint are also remarkably sensitive to the leg movement (Edin, 2001), and stretching the skin alone can result in movement perception (Collins et al. 2005). Whether the knee joint receptors or Golgi tendon organs of the reflexively contracting quadriceps similarly produce a conscious perception is not known. The accurate perception of the passive leg drop in our experiments – which clearly does not induce muscle contractions – suggests that for movement detection the brain is not reliant on the Golgi tendon inputs, as these afferents respond during muscle contraction (Binder et al. 1977). Therefore, there are several sensors that can contribute to the perception of the reflexive movement.
The main finding of our study is a dramatic impairment in perception of the reflex contribution to movement when reflexes are immediately followed by voluntary actions. When we asked participants to kick back immediately following the tendon tap, the reflex contributed a forward leg movement, while the voluntary command caused a backward movement. Therefore, the sensory inputs from the two movements should, in principle, be from largely distinct sets of sensors. For example, the reflex would cause stretch of the hamstring muscle, while the voluntary movement would cause stretch of the quadriceps muscle. Moreover, these two muscles are stretched in a strict sequence such that the muscle spindles of the hamstring are followed by activation of the quadriceps muscle spindles. Therefore, several sources of information should be relevant to reporting the maximal displacement caused by the reflex. Nevertheless, we found that participants were surprisingly poor at reporting their reflexes: regression fits and slopes were both altered in the kick back condition compared to the baseline condition.
Two very different mechanisms, implying very different types of proprioceptive computation, might underlie this impairment. First, the presence of a voluntary motor command may alter proprioceptive afferent signals, at several levels within the CNS. The sensitivity of the muscle spindles, which may be crucial for encoding reflexive movement, may drop due to voluntary contraction. Electrophysiological experiments in cats show that muscle contraction reduces the sensitivity of the spindles to vibration or stretch (Brown et al. 1967; Wise et al. 1999). In our experiments, the hamstring muscle indeed begins to contract before the end of the reflex movement. Moreover, descending voluntary commands may attenuate afferent signals at the level of the spinal cord (Milne et al. 1988; Seki et al. 2003). This sensory suppression supposedly ‘prevents saturation of the central nervous system by the massive barrage of re-afference generated during the movement’ (Collins et al. 1998).
A second, very different account of impaired reflex perception in the kick back condition involves the computation known as ‘credit assignment’ (Wolpert et al. 2011). All forms of perception face the computational problem of attributing signals to sources. Here, the proprioceptive signals must be attributed either to the reflex movement or to the voluntary command. The forward models proposed for motor control (Wolpert & Flanagan, 2001) can also perform this credit assignment function. In our case, the comparison between predicted and actual afferent information serves to extract the portion of afferent information contributed by voluntary movement, separating this from the remaining portion contributed by the reflex (McCloskey, 1981; Blakemore & Frith, 2003). However, the poor perception of reflex movements in the kick back condition shows that this does not, in fact, occur. This computational failure could arise for any of several reasons: degraded afferent input during voluntary movement, as described above; absence of any temporal or spatial marker of the voluntary motor command; or the unavailability of such markers for conscious perception. The time taken to update the cortical forward model offers another possible explanation. The forward model is based on an estimate of the current state of the limb. When the reflex movement changes the actual state of the limb, it takes time to update the cortical model with this new state (Wolpert & Flanagan, 2001). Therefore, the model’s estimate of the afferent signals caused by the voluntary command may be outdated and incorrect. The actual afferent signal would then be subtracted from an improper state prediction, appropriate for static but not a moving limb.
We also tested a condition in which participants added a voluntary forward kick to the reflex movement. In this condition, there is no clear kinematic marker of the transition between reflex and voluntary control. Therefore, we asked participants to judge the reflex contribution to the overall movement by reporting either the position at which they began to move the leg, or how far forward the reflex had moved the leg. In addition, we used the reflex EMG amplitude as a proxy for the contribution of the reflex to the overall movement kinematics. This proxy was justified because the baseline condition showed that the perceived displacement did indeed track trial-to-trial variations in reflex EMG amplitude. We then compared the relationship between perception and reflex EMG amplitude in the kick forward and baseline conditions. We again found that the presence of a voluntary movement significantly impaired the perception of reflexes, as measured by slopes and poor regression fits.
We expected that monitoring cortical output corresponding to the voluntary motor command would allow the reflex and voluntary components to be separated by the brain even in the kick forward condition (Gandevia et al. 2006). If the brain could mark the onset of the voluntary motor command within the continuous stream of afferent signals generated by the reflex, then volunteers should have performed our task as accurately in the kick forward as in the baseline condition. This perceptual impairment suggests that brain centres involved in conscious perception of movement do not have access to an accurate marker of motor commands. Either the afferent predictions derived from the forward model may be incorrect, or the predictions may be correct but unavailable for conscious perception. Our study cannot distinguish these possibilities.
The Golgi tendon input could also, in principle, contribute to reflex perception in the kick forward condition. EMG recordings showed that reflexive muscular contraction was clearly separated in time from the voluntary burst corresponding to the forward kick. This separation would be sufficient to generate a sequence of two distinct afferent volleys from Golgi tendon organs (Jansen & Rudjord, 1964; Houk & Henneman, 1967; Gregory & Proske, 1979). However, based on our perceptual results, no cortical sensory module appears able to separate reflexive from voluntary movement using such sensory inputs.
This study cannot reveal exactly which of the above mechanisms underlie poor reflex perception in the voluntary conditions. Furthermore, our volunteers were only ever asked to report the amplitude of the forward excursion. Other attributes of reflex movements may be perceived correctly, despite the presence of superimposed voluntary movement. Moreover, voluntary commands were initiated only after the onset of reflex commands. Therefore, perceptual separation of voluntary and reflex contributions might still be possible in situations where the voluntary movement precedes the reflex.
To conclude, precise and sensitive interactions between voluntary and reflex control occur in the hand (Rothwell et al. 1982) and arm (Kimura et al. 2006). Such integration could hardly be achieved without accurate programming and credit assignment between the two components. However, our results show that these processes are relatively inaccessible to conscious perception. Specifically, perception of the knee-jerk reflex contribution to movement is impaired in the presence of co-occurring voluntary motor command. Voluntary motor commands might degrade proprioceptive afferent input. Alternatively, the brain modules responsible for credit assignment between reflex and voluntary contributions to movement may operate below the level of perceptual awareness. Conscious access to both ‘efference copy’ associated with the voluntary motor command and various sources of sensory inputs would have allowed accurate separation of the reflex and the voluntary contributions to movement – yet this was not found. Further research is necessary to examine whether the inability to make a clear perceptual distinction can be generalised to other combinations of reflexes and voluntary movements that occur close together in space and time.
Acknowledgments
We are indebted to Prof. Kevan Martin for his support and Dr Martin Schubert for his clinical insights.
Glossary
- EMG
electromyography
Additional information
Competing interests
None declared.
Author Contributions
A.G. and P.H. designed, planned, interpreted the results and prepared the manuscript. A.G. conducted all the experiments and analysed the data.
Funding
This work was supported by the Society in Science – The Branco Weiss Fellowship and a research grant from the Vontobel Stiftung awarded to A.G. P.H. was supported by a Leverhulme Trust Research Fellowship, an ESRC Professorial Fellowship, and ERC Advanced Grant, and by EU FP7 project VERE.
References
- Binder MD, Kroin JS, Moore GP, Stuart DG. The response of Golgi tendon organs to single motor unit contractions. J Physiol. 1977;271:337–349. doi: 10.1113/jphysiol.1977.sp012003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, Frith C. Self-awareness and action. Curr Opin Neurobiol. 2003;13:219–224. doi: 10.1016/s0959-4388(03)00043-6. [DOI] [PubMed] [Google Scholar]
- Boyle RS, Shakir RA, Weir AI, McInnes A. Inverted knee jerk: a neglected localising sign in spinal cord disease. J Neurol Neurosurg Psychiatry. 1979;42:1005–1007. doi: 10.1136/jnnp.42.11.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branch CH, Buxbaum LJ, Schwoebel J. Accurate reaching after active but not passive movements of the hand: evidence for forward modelling. Behav Neurol. 2008;19:117–125. doi: 10.1155/2008/972542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MC, Engberg I, Matthews PB. The relative sensitivity to vibration of muscle receptors of the cat. J Physiol. 1967;192:773–800. doi: 10.1113/jphysiol.1967.sp008330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne K, Lee J. The appreciation of passive movement of the metatarsophalangeal joint of the great toe in man. J Physiol. 1954;123:10–11P. [PubMed] [Google Scholar]
- Buch ER, Mars RB, Boorman ED, Rushworth MF. A network centered on ventral premotor cortex exerts both facilitatory and inhibitory control over primary motor cortex during action reprogramming. J Neurosci. 2010;30:1395–1401. doi: 10.1523/JNEUROSCI.4882-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess PR, Wei JY, Clark FJ, Simon J. Signaling of kinesthetic information by peripheral sensory receptors. Annu Rev Neurosci. 1982;5:171–187. doi: 10.1146/annurev.ne.05.030182.001131. [DOI] [PubMed] [Google Scholar]
- Chapman CE, Bushnell MC, Miron D, Duncan GH, Lund JP. Sensory perception during movement in man. Exp Brain Res. 1987;68:516–524. doi: 10.1007/BF00249795. [DOI] [PubMed] [Google Scholar]
- Collins DF, Cameron T, Gillard DM, Prochazka A. Muscular sense is attenuated when humans move. J Physiol. 1998;508:635–643. doi: 10.1111/j.1469-7793.1998.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DF, Refshauge KM, Todd G, Gandevia SC. Cutaneous receptors contribute to kinesthesia at the index finger, elbow, and knee. J Neurophysiol. 2005;94:1699–1706. doi: 10.1152/jn.00191.2005. [DOI] [PubMed] [Google Scholar]
- Cordo PJ, Horn JL, Kunster D, Cherry A, Bratt A, Gurfinkel V. Contributions of skin and muscle afferent input to movement sense in the human hand. J Neurophysiol. 2011;105:1879–1888. doi: 10.1152/jn.00201.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassonville P. Haptic localization and the internal representation of the hand in space. Exp Brain Res. 1995;106:434–448. doi: 10.1007/BF00231066. [DOI] [PubMed] [Google Scholar]
- Edin B. Cutaneous afferents provide information about knee joint movements in humans. J Physiol. 2001;531:289–297. doi: 10.1111/j.1469-7793.2001.0289j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes CT, Bastian AJ. Where is your arm? Variations in proprioception across space and tasks. J Neurophysiol. 2010;103:164–171. doi: 10.1152/jn.00494.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC, Rothwell JC. Knowledge of motor commands and the recruitment of human motoneurons. Brain. 1987;110:1117–1130. doi: 10.1093/brain/110.5.1117. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Smith JL, Crawford M, Proske U, Taylor JL. Motor commands contribute to human position sense. J Physiol. 2006;571:703–710. doi: 10.1113/jphysiol.2005.103093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory JE, Proske U. The responses of Golgi tendon organs to stimulation of different combinations of motor units. J Physiol. 1979;295:251–262. doi: 10.1113/jphysiol.1979.sp012966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritsenko V, Krouchev NI, Kalaska JF. Afferent input, efference copy, signal noise, and biases in perception of joint angle during active versus passive elbow movements. J Neurophysiol. 2007;98:1140–1154. doi: 10.1152/jn.00162.2007. [DOI] [PubMed] [Google Scholar]
- Hall LA, McCloskey DI. Detections of movements imposed on finger, elbow and shoulder joints. J Physiol. 1983;335:519–533. doi: 10.1113/jphysiol.1983.sp014548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann WC, Morris JG. Excitability of human gastrocnemius motor neurones at the time of the knee jerk. J Physiol. 1975;244:19P–20P. [PubMed] [Google Scholar]
- Houk J, Henneman E. Responses of Golgi tendon organs to active contractions of the soleus muscle of the cat. J Neurophysiol. 1967;30:466–481. doi: 10.1152/jn.1967.30.3.466. [DOI] [PubMed] [Google Scholar]
- Jansen JK, Rudjord T. On the silent period and Golgi tendon organs of the soleus muscle of the cat. Acta Physiol Scand. 1964;62:364–379. doi: 10.1111/j.1748-1716.1964.tb10435.x. [DOI] [PubMed] [Google Scholar]
- Kimura T, Haggard P, Gomi H. Transcranial magnetic stimulation over sensorimotor cortex disrupts anticipatory reflex gain modulation for skilled action. J Neurosci. 2006;26:9272–9281. doi: 10.1523/JNEUROSCI.3886-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufer Y, Hocherman S, Dickstein R. Accuracy of reproducing hand position when using active compared with passive movement. Physiother Res Int. 2001;6:65–75. doi: 10.1002/pri.215. [DOI] [PubMed] [Google Scholar]
- Lebiedowska MK, Sikdar S, Eranki A, Garmirian L. Knee joint angular velocities and accelerations during the patellar tendon jerk. J Neurosci Methods. 2011;198:255–259. doi: 10.1016/j.jneumeth.2011.04.018. [DOI] [PubMed] [Google Scholar]
- Matthews PB. Where does Sherrington’s ‘muscular sense’ originate? Muscles, joints, corollary discharges. Annu Rev Neurosci. 1982;5:189–218. doi: 10.1146/annurev.ne.05.030182.001201. [DOI] [PubMed] [Google Scholar]
- McCloskey DI. Corollary discharges: motor commands and perception. Compr Physiol 2011, Supplement 2: Handbook of Physiology, The Nervous System, Motor Control. 1981:1415–1447. doi: 10.1002/cphy.cp010232. [Google Scholar]
- Milne RJ, Aniss AM, Kay NE, Gandevia SC. Reduction in perceived intensity of cutaneous stimuli during movement: a quantitative study. Exp Brain Res. 1988;70:569–576. doi: 10.1007/BF00247604. [DOI] [PubMed] [Google Scholar]
- Neubert FX, Mars RB, Buch ER, Olivier E, Rushworth MF. Cortical and subcortical interactions during action reprogramming and their related white matter pathways. Proc Natl Acad Sci U S A. 2010;107:13240–13245. doi: 10.1073/pnas.1000674107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paillard J, Brouchon M. Active and passive movements in the calibration of position sense. In: Freedman SJ, editor. The Neuropsychology of Spatially Oriented Behaviour. Dorsey Press; 1968. pp. 37–55. Belmont, CA. [Google Scholar]
- Papakostopoulos D, Cooper R, Crow HJ. Inhibition of cortical evoked potentials and sensation by self-initiated movement in man. Nature. 1975;258:321–324. doi: 10.1038/258321a0. [DOI] [PubMed] [Google Scholar]
- Proske U, Gandevia SC. The kinaesthetic senses. J Physiol. 2009;587:4139–4146. doi: 10.1113/jphysiol.2009.175372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell JC, Traub MM, Marsden CD. Automatic and ‘voluntary’ responses compensating for disturbances of human thumb movements. Brain Res. 1982;248:33–41. doi: 10.1016/0006-8993(82)91144-1. [DOI] [PubMed] [Google Scholar]
- Schindler-Ivens S, Shields RK. Low frequency depression of H-reflexes in humans with acute and chronic spinal-cord injury. Exp Brain Res. 2000;133:233–241. doi: 10.1007/s002210000377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki K, Fetz EE. Gating of sensory input at spinal and cortical levels during preparation and execution of voluntary movement. J Neurosci. 2012;32:890–902. doi: 10.1523/JNEUROSCI.4958-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki K, Perlmutter SI, Fetz EE. Sensory input to primate spinal cord is presynaptically inhibited during voluntary movement. Nat Neurosci. 2003;6:1309–1316. doi: 10.1038/nn1154. [DOI] [PubMed] [Google Scholar]
- Sherrington CS. On the proprio-ceptive system, especially in its reflex aspect. Brain. 1907;29:467–482. [Google Scholar]
- Wise AK, Gregory JE, Proske U. Detection of movements of the human forearm during and after co-contractions of muscles acting at the elbow joint. J Physiol. 1998;508:325–330. doi: 10.1111/j.1469-7793.1998.325br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise AK, Gregory JE, Proske U. The responses of muscle spindles to small, slow movements in passive muscle and during fusimotor activity. Brain Res. 1999;821:87–94. doi: 10.1016/s0006-8993(99)01071-9. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Diedrichsen J, Flanagan JR. Principles of sensorimotor learning. Nat Rev Neurosci. 2011;12:739–751. doi: 10.1038/nrn3112. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Flanagan JR. Motor prediction. Curr Biol. 2001;11:R729–732. doi: 10.1016/s0960-9822(01)00432-8. [DOI] [PubMed] [Google Scholar]
- Zipp P. Recommendations for standardization of lead positions in surface electromyography. Eur J Appl Physiol. 1982;50:41–54. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


