Abstract
Helicobacter pylori neutrophil-activating protein (NAP) is a toll-like receptor 2 (TLR2) agonist and potent immunomodulator inducing Th1-type immune response. Here we present data about characterization of the humoral immune response against NAP-tagged antigens, encoded by attenuated measles virus (MV) vector platform, in MV infection susceptible type I interferon receptor knockout and human CD46 transgenic (Ifnarko-CD46Ge) mice. Immunogenicity of MV expressing a full-length human immunoglobulin lambda light chain (MV-lambda) was compared to that of MV expressing lambda-NAP chimeric protein (MV-lambda-NAP). MV-lambda-NAP immunized Ifnarko-CD46Ge mice developed significantly higher (6–20-fold) anti-lambda ELISA titers as compared to the MV-lambda-immunized control animal group, indicating that covalently-linked NAP co-expression significantly enhanced lambda immunogenicity. In contrast, ELISA titers against MV antigens were not significantly different between the animals vaccinated with MV-lambda or MV-lambda-NAP. NAP-tagged antigen expression did not affect development of protective anti-measles immunity. Both MV-lambda and MV-lambda-NAP-immunized groups showed strong virus neutralization serum titers in plaque reduction microneutralization test. These results demonstrated that MV-encoded lambda-NAP is highly immunogenic as compared to the unmodified full-length lambda chain. Boost of immune response to poor immunogens using live vectors expressing NAP-tagged chimeric antigens is an attractive approach with potential application in immunoprophylaxis of infectious diseases and cancer immunotherapy.
Keywords: Helicobacter pylori, Neutrophil-activating protein, Chimeric antigens, Immunogenicity, Measles virus vaccine
1. Introduction
Induction of strong and long-lasting protective immunity is the ultimate goal of vaccine development. Immunogenicity is a key characteristic of vaccine preparations related to ability of their components to elicit immune response in vaccinees. Immunogenicity could be influenced by multiple factors however, including the antigen nature (protein or polysaccharide), form (purified antigen or crude preparations) and route of administration. Many pathogens evolve to evade or confuse the immune system by expression of superantigens or masking the protective epitopes. For example, immunodominant epitopes of the human immunodeficiency virus envelope protein are located in the hypervariable regions [1, 2]. Common protective hemagglutinin epitopes of influenza A viruses can remain unrecognized by immune system, because the humoral response is directed against serotype specific immunodominant epitopes [3–5]. These findings indicate the need for advances in vaccine development based on synthetic antigens or engineering of chimeric molecules carrying the epitopes with desired specificity. Purified antigens are usually weak immunogens, which require formulation with adjuvants in order to stimulate efficiently the immune response [6, 7]. Because of self-tolerance, tumor associated antigens (TAA) can escape host immune surveillance. Successful anti-cancer immunization could be achieved by co-administration of TAA with strong immunoadjuvants or co-expression with Th1 cytokines by viral or bacterial vector systems [8–11]. Genetically engineered antigens can be covalently linked to T-cell epitopes that can boost immunity to poorly immunogenic antigens [12]. Anti-idiotypic vaccination against lymphomas follows a similar approach using a chemical conjugation of unique surface lymphoma immunoglobulin determinants to keyhole limpet hemocyanin as carrier protein [13].
Bacterial cells and bacterial cell wall components are potent immunostimulators and are used as components of formulated vaccines or as live vectors. Helicobacter pylori neutrophil-activating protein (NAP) is a key virulence factor and one of the protective antigens against Helicobacter infection [14]. NAP is a small dodecameric structure forming protein composed of 144 amino acid residues [15]. It is a homolog of Enterobacteriaceae bacterioferritins with iron-binding and DNA protective function. NAP acts as a toll-like receptor (TLR) 2 ligand and potent Th1 response immunomodulator by induction of interleukin-12 (IL-12) and IL-23 expression [16]. NAP can reverse Th2 polarization of the immune response and reduced IgE serum level and eosinophilia in models of allergic diseases [17, 18]. Local treatment with purified NAP induced T-cell infiltration, reduced vascularization and inhibited bladder cancer growth in mice [19]. It has been established that immunostimulatory properties of NAP are mediated by the C-terminus of molecule and do not require dodecamer formation [20].
The potent immunostimulatory effect and the short length of the protein make NAP an attractive transgene insert capable to boost immunogenicity of virus vector vaccines. Recently, we generate recombinant measles virus (MV) expressing secretory NAP forms based on the Edmonston vaccine strain platform [21] and developed immunoassays for detection of the NAP transgene [22]. Immunization of measles infection permissive interferon receptor type I knockout and human CD46 transgenic (Ifnarko-CD46Ge) mice with these vectors triggered strong antibody and cell-mediated anti-NAP immunity. Biological activity of MV-encoded NAP was confirmed both in vitro and in vivo. Treatment with MV strains expressing secretory NAP induced local inflammatory cytokine release and significantly improved survival in mouse models of metastatic breast cancer [23].
Here, using human lambda immunoglobulin as an antigen model we demonstrate that MV-encoded NAP-tagged chimeric antigen can induce significantly stronger immune response than the control strain expressing lambda chain alone following single immunization of MV susceptible Ifnarko-CD46Ge transgenic mice.
2. Materials and methods
2.1. Cell lines, MV strains and MV propagation
African green monkey Vero cells (ATCC) were maintained in DMEM culture medium supplemented with 10% fetal bovine serum (FBS) (Invitrogen). Generation and characterization of MV-lambda and MV-lambda-NAP has been described recently [21, 24]. MV-lambda expresses a full-length human immunoglobulin lambda light chain transgene introduced upstream of nucleoprotein (N protein) gene in the genome of MV Edmonston vaccine strain. In MV-lambda-NAP a major part of the variable lambda-immunoglobulin domain was substituted by NAP of H. pylori strain 26695 (Fig. 1). The lambda-NAP transgene expressed the constant lambda domain and the immunoglobulin leader sequence that allowed extracellular secretion of the chimeric protein. Both viruses were grown and titrated on Vero cells as previously described [21, 24]. Virus titer was determined by both plaque-forming units (PFU/ml) and tissue culture infectious doses 50% (TCID50) [21]. Purified viral stock from MV strain expressing sodium iodide symporter (MV-NIS) [25] was used as antigen in antigen-mediated ELISA. MV-NIS growth and purification procedure were performed as previously described [26].
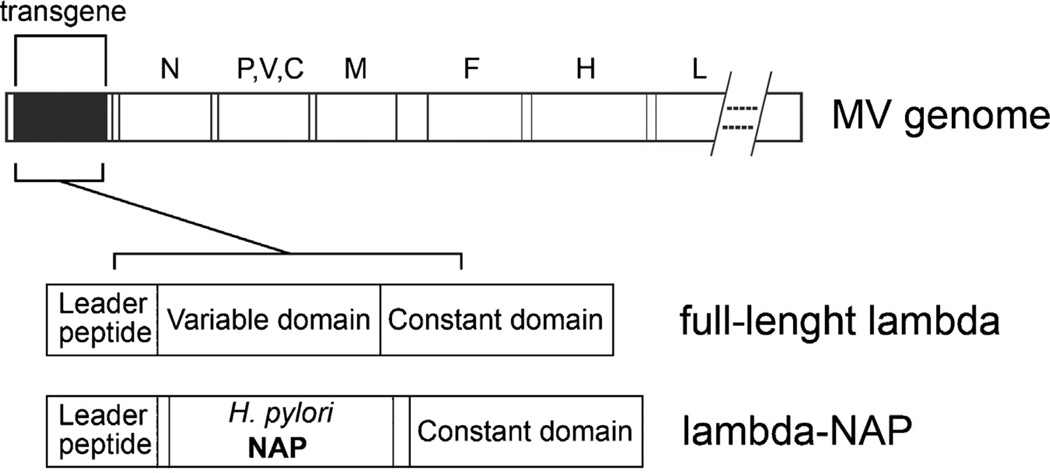
Fig. 1.
Schematic representation of the recombinant MV strains used in the study. The lambda or lambda-NAP were cloned as an additional transcription unit upstream of N protein gene in MV Edmonston vaccine strain genome. MV-lambda expressed a full-length human immunoglobulin lambda light chain. MV-lambda-NAP encoded a chimeric construct with H. pylori NAP replacing a major portion of the variable lambda domain. The lambda-NAP insert expressed the lambda immunoglobulin leader peptide and the constant lambda chain domain.
2.2. Animal experiments
Since rodents are naturally resistant to measles (innate immunity and absence of viral receptors), Ifnarko-CD46 transgenic mice [27] are suitable small animal model for studying pathogenesis and immune response mechanisms against MV infection [28–30]. Mice were maintained in the animal facilities of Mayo Clinic, Rochester MN. The study was reviewed and approved by Mayo Foundation Institutional Animal Care and Use Committee.
2.3. Immunization and immune response in MV susceptible mice
Female 6–8-week old Ifnarko-CD46Ge mice (9–10 mice per group) were immunized with 2 × 105 PFU of MV-lambda or MV-lambda-NAP by an intraperitoneal (i.p.) route. Mice were bled before MV injection and on day 18 and 32 of the study. Serum samples were heat inactivated at 56 °C for 30 min and tested for response against human lambda immunoglobulin antigen, MV neutralization titer and total anti-MV ELISA titer. The experiment was repeated twice with Ifnarko-CD46Ge mice of different age: 10–12-week and 16-week-old (n = 9–10). Sera were collected four weeks and six months post-immunization.
2.4. Antigen-mediated enzyme-linked immunosorbent assay (ELISA)
For human lambda chain specific antibody response tests, ELISA 96-well plates (Nunc) were coated overnight with 0.3 µg/well of human IgG lambda (Bethyl Laboratories) in carbonate-bicarbonate buffer (CBB), pH 9.6. Plates were blocked with 1% bovine serum albumin (BSA) for 1 h. Serum dilutions (2-fold or 4-fold) were added and incubated for 1 h at room temperature. Then plates were washed in phosphate-buffered saline (PBS) with 0.05% Tween 20 (PBS/T) and incubated with the secondary anti-mouse polyvalent immunoglobulin (G, A, M) horseradish peroxidase (HRP) conjugated antibody (Sigma) or secondary HRP-conjugated (human protein pre-absorbed) goat anti-mouse IgG antibody (Santa Cruz Biotechnology). Secondary antibodies were diluted 1:2000–4000 in 1% BSA in PBS/T. After 1-h incubation plates were washed 5 times in PBS/T and reaction was developed using a TMB substrate (Bethyl Laboratories).
The titer of the different IgG isotypes against human lambda chain was determined using isotype specific anti-mouse IgG1, IgG2a, IgG2b and IgG3 HRP-conjugated secondary antibodies (Santa Cruz Biotechnology).
To determine the serum titer against whole MV antigen ELISA plates were coated with 2 × 104 TCID50 per well of heat-inactivated (60 °C/1 h) MV-NIS resuspended in CBB. After overnight incubation plates were blocked in 1% BSA and assay followed the steps described above.
Serum endpoint titers were determined as the highest dilution with readings (absorbance >0.100 or >4 × SD) above that of the control samples. Sera collected on day 0 before immunization or from age-matched non-immunized animals were used as controls.
2.5. Serum antibody avidity test
Avidity of serum antibodies against human lambda immunoglobulin was determined in antigen-mediated ELISA using 6 M urea in PBS as dissociating agent [31, 32]. ELISA plates were coated with 0.3 µg/well of human IgG lambda in CBB. Serum samples collected 6 months after immunization of the mice were diluted and incubated with the antigen as described for antigen-mediated ELISA. Then plates were washed once with PBS/T and incubated for 5 min with 6 M urea in PBS or PBS alone (for control wells). HRP-conjugated anti-mouse IgG (Santa Cruz Biotechnology) was used as a secondary antibody and reaction development followed the steps described above.
2.6. Capture ELISA for measurement of lambda chain transgene expression
Vero cell monolayers were inoculated either with MV-lambda-NAP or MV-lambda at multiplicity of infection (MOI) of 1.0 in Opti-MEM medium (Invitrogen). After 4-h incubation inoculum was replaced with fresh DMEM supplemented with 2% FBS. Supernatants were collected at 24, 48, and 72 h and frozen at −20 °C. Lambda or chimeric lambda-NAP was quantified by human lambda immunoglobulin chain-specific ELISA (Bethyl Laboratories).
2.7. Virus neutralization (VN) test
MV plaque reduction neutralization antibody titer 50% (PNT50) of mice immunized with MV-lambda-NAP or MV-lambda control strain was measured by plaque-reduction microneutralization assay as previously described [33].
2.8. Statistical analysis
Results were analyzed using GraphPad Prism software (Graph-Pad Software, San Diego CA).
3. Results
3.1. Expression of lambda chain transgene by MV-lambda-NAP and MV-lambda infected cells
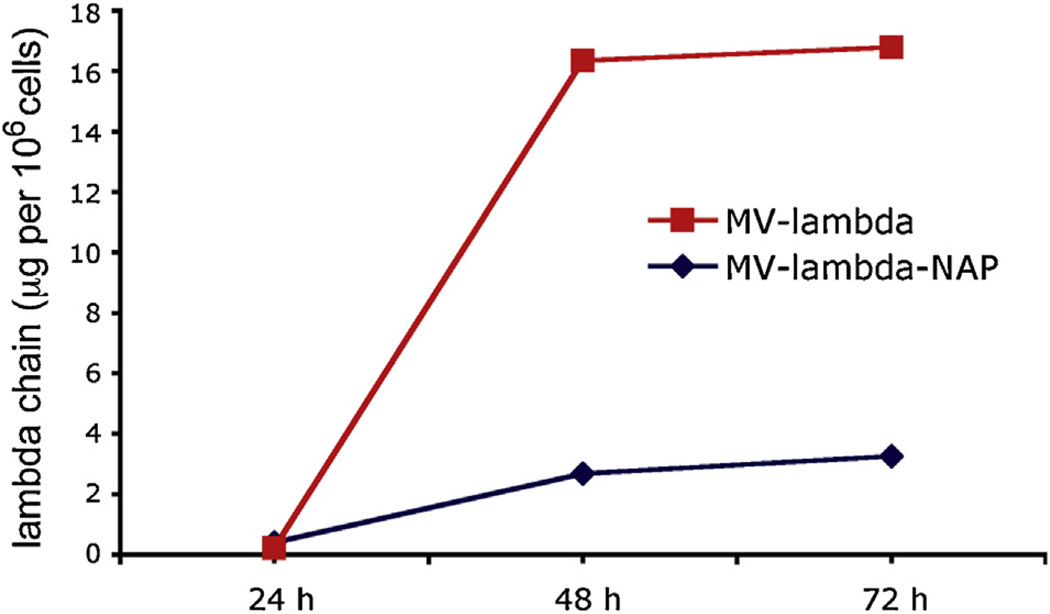
Supernatants from infected Vero cells were collected at 24-h, 48-h and 72-h time points. Since lambda-specific determinants are located in the constant lambda domain, both lambda and lambda-NAP transgene expression could be measured by lambda chain-specific ELISA (a schematic representation of the MV constructs used in the study is shown in Fig. 1). Transgene protein accumulation in the supernatants reached the maximum accumulation at 72 h post-infection with MV strains (Fig. 2). Concentration was calculated as lambda protein expression per 106 infected Vero cells. Data analysis showed that native lambda chain expression was >5-fold higher than chimeric lambda-NAP produced by MV-lambda-NAP-infected cells: 16.78 vs. 3.26 µg/106 cells.
Fig. 2.
Expression of lambda chain or lambda-NAP transgene by MV-lambda or MV-lambda-NAP infected cells. Vero cells were inoculated at MOI = 1.0 with ether MV-lambda or MV-lambda-NAP. Concentration of human lambda antigen in the supernatants was measured at 24, 48 and 72 h and transgene expression per 106 infected cells was calculated.
3.2. Immunization with MV-lambda-NAP induced strong anti-lambda humoral immune response
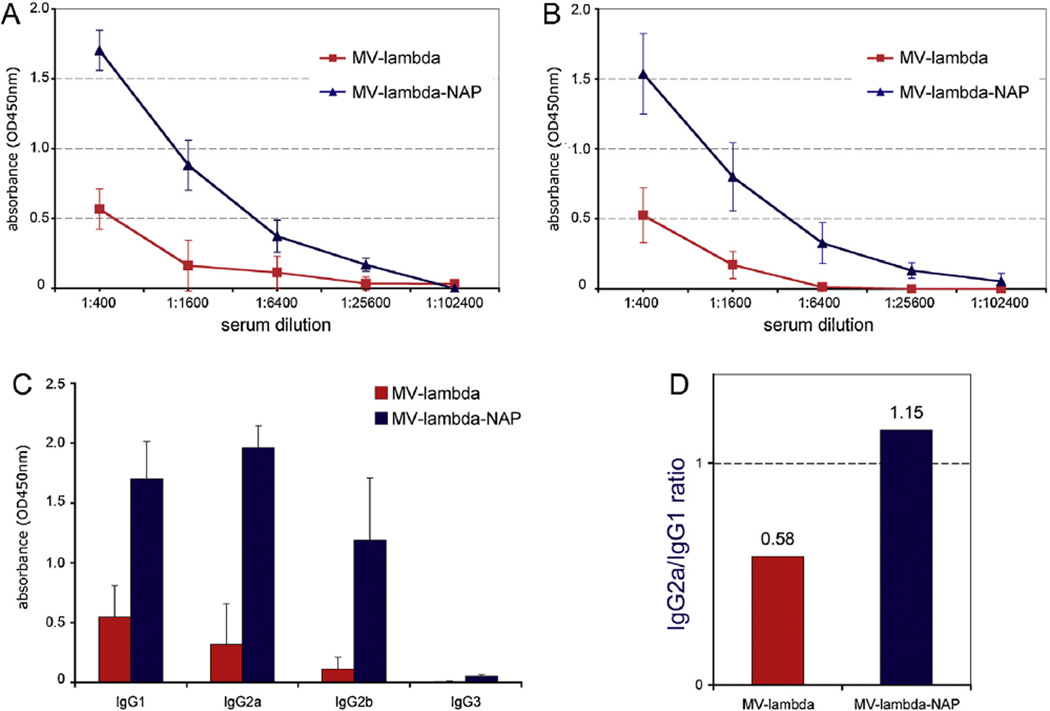
Serum samples of 6–8-week-old Ifnarko-CD46Ge mice (9–10 per group) were collected on day 18 and 32 post-immunization (single i.p. injection of 2 × 105 PFU) with MV-lambda-NAP or MV-lambda. Polyvalent anti-mouse IgG, IgA and IgM secondary antibody allowed detection of the different immunoglobulin classes in antigen-mediated ELISA. Although a live replicating vector used for immunization, anti-lambda response in MV-lambda vaccinated group was weak (up to 1:6400), indicating that human lambda chain is a weak immunogen for the mice. In contrast, the day 18 anti-lambda immunoglobulin specific serum titers were 1:25,600 in 9 of 10 MV-lambda-NAP immunized mice (1:12,800 in one mouse), significantly higher than the MV-lambda injected group (titers 1:400–1:6400 (n = 9)) (Fig. 3A). On day 32 the total antibody titers (lambda-specific IgG, IgA, IgM) were 1:6400–1:102,400 for MV-lambda-NAP immunized group vs. 1:200–1:6400 for the MV-lambda-treated mice (Fig. 3B and Table 1). The higher titer observed in some animals on day 18 compared to day 32 could be attributed to the presence of higher specific IgM levels at this earlier time point in development of primary antibody response. IgG1, IgG2a and IgG2b were the pre-dominant immunoglobulin isotypes of the antibodies against lambda antigen on day 32 (Fig. 3C). Significantly higher IgG2a/IgG1 ratio for MV-lambda-NAP immunized group could indicate Th1 switch of the immune response to NAP-tagged antigen (Fig. 3D).
Fig. 3.
Serum antibody reactivity against human lambda immunoglobulin at day 18 (A) or day 32 (B) following immunization of 6–8-week-old Ifnarko-CD46Ge mice with MV-lambda or MV-lambda-NAP. The results are from representative antigen-mediated ELISA using 4-fold dilutions of sera from MV-lambda or MV-lambda-NAP immunized animals. IgG isotype lambda-specific reactivity of the serum samples from the same animal groups (n = 9–10) diluted 1:1000 (C). The IgG2a/IgG1 ratio for MV-lambda and MV-lambda-NAP-immunized groups (D). Pre-immune sera collected before immunization were used as controls.
Table 1.
Serum titers of the immunized Ifnarko-CD46Ge mice against human lambda immunoglobulin chain in antigen-mediated ELISA. The titers were measured on day 32 (for the 6–8-old mice) or 4 weeks post immunization (for the other two experiments).
| Experiment | Immunization | Anti-lambda titer | Difference (in fold) |
|---|---|---|---|
| 6–8-Week old mice (day 32) | MV-lambda-NAP | 1:6400–1:102,400a (1:36,480)b | 19.78c (p = 0.013) |
| MV-lambda | 1:200–1:6400 (1:1844) | ||
| 10–12-Week old mice (4 weeks) | MV-lambda-NAP | 1:1600–1:25,600 (10,720) | 9.08 (p = 0.010) |
| MV-lambda | 1:100–1:1600 (1:1180) | ||
| 16-Week old mice (4 weeks) | MV-lambda-NAP | 1:6400–1:25,600 (1:16,000) | 6.21 (p < 0.001) |
| MV-lambda | 1:800–1:6400 (1:2578) |
ELISA titer range.
Mean antibody titer.
Difference in titers MV-lambda-NAP vs. MV-lambda immunized groups in fold with the p value.
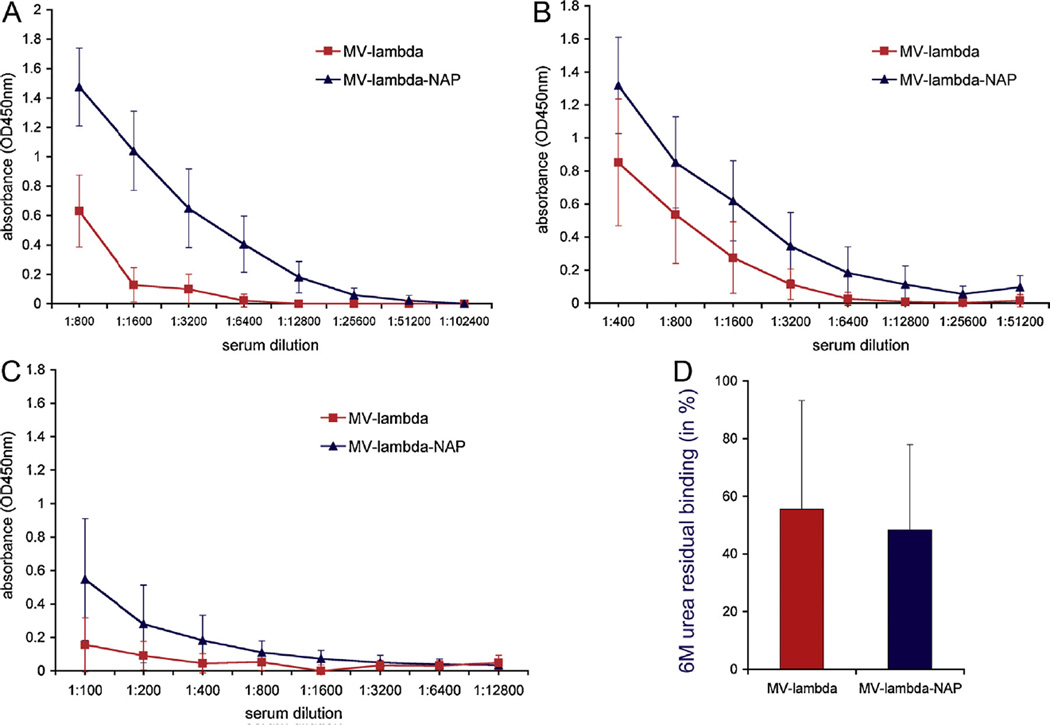
Immunization experiments were repeated twice with mice of different age – 16-week-old and 10–12-week-old. The average serum titers of MV-lambda-NAP immune mice 4 weeks post-vaccination were more than 6-fold higher as compared those of MV-lambda-injected control mice (Fig. 4A, B and Table 1). In contrast to the immunization experiment with younger (6–8-week-old) animals, there was no significant difference in lambda-specific IgG2a/IgG1 ratio between the groups (not shown). Six months post-immunization anti-lambda humoral response was detected in all 10 mice in MV-lambda-NAP group. In contrast, low anti-lambda titers were detected in 7 of 10 animals in MV-lambda control group (Fig. 4C). Avidity test confirm the strong antigen-binding of serum anti-lambda antibodies in the presence of 6M urea as dissociating agent (Fig. 4D). There was no significant difference of antibody avidity between the two groups.
Fig. 4.
Antibody titers against lambda chain 4 weeks post-immunization of 16-week-old (A) or 10–12-week-old (B) Ifnarko-CD46Ge mice. Single i.p. injection of 2–2.5 × 105 TCID50 of MV-lambda or MV-lambda-NAP were used for immunization. All mice (representative ELISA for the experiment with 10–12-week-old mice) injected with MVlambda-NAP had a detectable antibody response to lambda immunoglobulin at 6 months post-vaccination (C). At 6 months the antibody affinity against lambda antigen was similar for both animal groups (D). Serum samples from the 10 mice vaccinated with MV-lambda-NAP were diluted 1:100–400. The positive in ELISA sera from the MV-lambda group (7 of 10 mice) were tested in dilution 1:100.
These data demonstrated that covalent linkage to NAP enhanced immunogenicity of other protein antigens and MV-expressed chimeric lambda-NAP induced stronger response than the native lambda chain encoded by MV-lambda control strain.
3.3. Both MV-lambda-NAP and MV-lambda induced strong protective immunity against measles
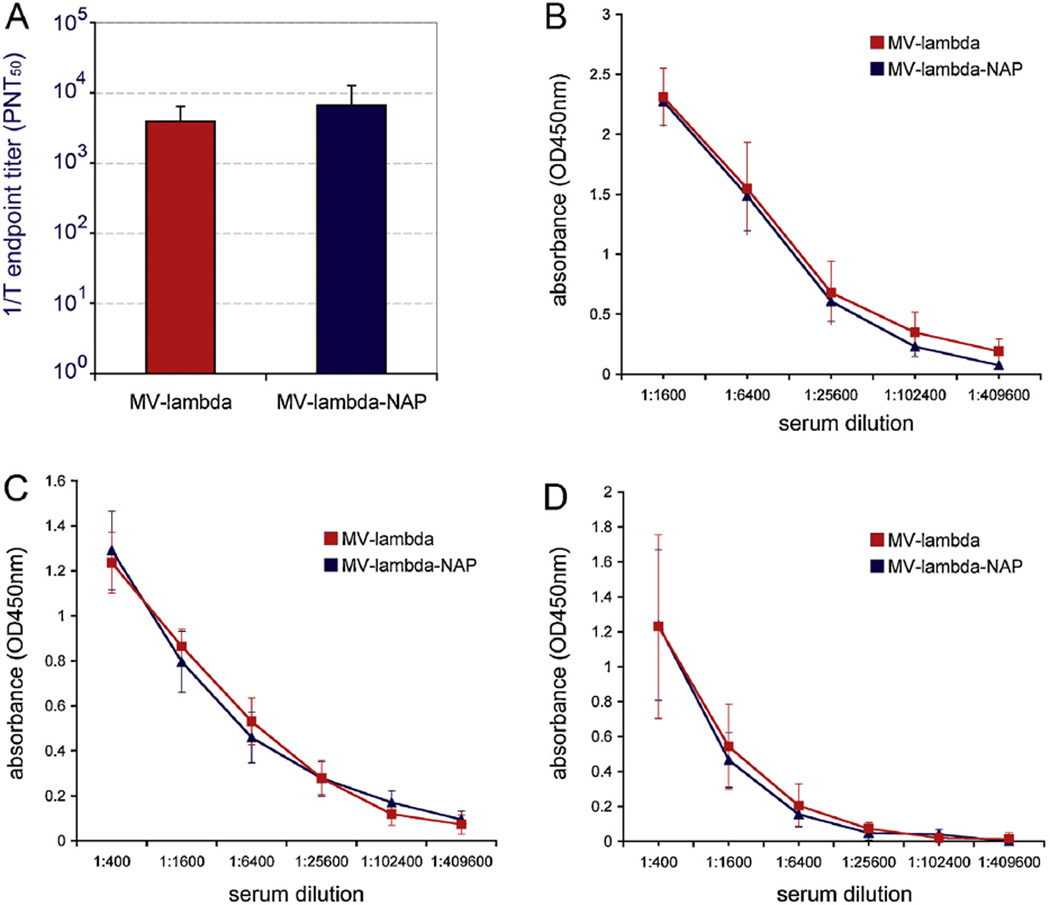
MV microneutralization test results confirmed that both recombinant MV strains stimulated high titers of virus-neutralizing antibodies in Ifnarko-CD46Ge mice at day 32 after vaccination. The mean VN titer (in PNT50) for MV-lambda-immunized mice was 1:3883 (median titer – 1:3922; titer range between the individual animals – 1:722–1:8604) and 1:6648 (median titer – 1:4652; range – 1:1246–1:14,378) for MV-lambda-NAP group. Although the average titer in MV-lambda-NAP-injected mice was higher, there was no statistically significant difference (p = 0.214) between the groups (Fig. 5A). Serum antibody response in all animals was highly protective – above the titer level of 1:120 PNT50 considered protective against measles infection in humans [34, 35].
Fig. 5.
There was no significant difference in VN titers (A) and total anti-MV ELISA titers (B) at day 32 between MV-lambda and MV-lambda-NAP-immunized 6–8-week-old Ifnarko-CD46Ge mice. Serum antibodies from 10 to 12-week-old mice (10 per group) showed strong reactivity against MV antigens in ELISA 4-weeks (C) or 6 months (D) post-immunization with MV-lambda or MV-lambda-NAP.
Ifnarko-CD46Ge mice developed strong antibody response against the total MV antigens (plates coated with whole MV particles) as shown in antigen-mediated ELISA (Fig. 5B and C). The post-vaccination titers in all animals were 1:102,400 or higher (up to 1:409,600) at day 28–32 with no differences between the groups. Serum samples collected at 6 months maintained a strong ELISA reactivity indicating that the vaccination with both strains promoted long-lasting anti-measles immunity (Fig. 5D). In contrast to anti-lambda response, there was no IgG2a biased response against MV antigens in 6–8-week old mice immunized with MV-lambda-NAP strain (not shown). These data indicated that replication competent MV vector encoding chimeric lambda-NAP selectively boosted the anti-lambda response and that NAP expression did not impact negatively the development of protective immunity against MV.
4. Discussion
Whole bacterial cells and bacterial cell wall components, including lipopolysaccharide of Gram-negative bacteria, muramyldipeptide, flagellins etc., are potent immunoadjuvants stimulating antigen-presenting cells through TLR signaling and inflammatory cytokine release [36]. Combined with purified antigens they are capable to boost vaccine immunogenicity and development of strong adaptive immunity. NAP immunomodulatory activity has been explored since NAP was identified as a major inflammation-triggering factor in the course of H. pylori infection [16].
Purified NAP has been used as a Th1 response-triggering component of experimental vaccines and immunomodulatory preparations. We chose to test an alternative approach: to express covalently-linked NAP-tagged antigens by a live vaccine vector platform. Live viral vectors represent efficient stimulators of immune response. Immune response however, is mainly directed against immunodominant epitopes of the vector and control of viral vector infection. Attenuated MV Edmonston strains proved to be an excellent vaccine platform for expression of foreign antigens of other pathogens [21, 37–40]. MV is an enveloped virus with negative-strand RNA genome that belongs to Paramyxoviridae family. Measles is the most contagious known human infectious disease. Immunoprophylaxis with the live attenuated MV vaccine has an excellent safety record and reduced significantly measles morbidity and mortality worldwide [41]. Cloning and rescue of MV from cDNA allowed engineering of recombinant MV derivative strains encoding foreign genes. Insertion of foreign genes in the MV genome is genetically stable and expression of additional proteins does not impact negatively viral replication [24, 25, 42]. Recently, we demonstrated that NAP cloned upstream of the first gene in MV genome is expressed at high level in biologically active form without interference with the development of protective anti-measles immunity [21].
Here, we used a human lambda immunoglobulin chain as a weak protein immunogen prototype and compared its immunogenicity to a modified NAP-tagged lambda using vectored MV vaccine strain for delivery. Ifnarko-CD46Ge mice are considered a relevant small animal model to study MV pathogenesis [27]. Knockout of interferon response and expression of one of the MV vaccine strain human receptors – CD46 made mouse cells permissive for infection and MV replication. Since macrophages are the primary target of MV infection in Ifnarko-CD46Ge mice [29, 30], i.p. injection was chosen as the route of vaccine administration. Both strains used in the study (MV-lambda-NAP and MV-lambda) encoded the lambda chain leader peptide and infected cells expressed secretory lambda-NAP or native lambda protein [21]. Immunization with MV-lambda strain encoding a native lambda immunoglobulin sequence did not induce lambda-specific antibody titers in ELISA higher than 1:6400 in any of the individual animals (see Table 1). In contrast, MV-lambda-NAP-immunized mice developed a 6–20-fold higher mean titer against lambda antigen. In addition, MV-lambda-NAP induced a long-term anti-lambda response as compared to the MV-lambda strain. Since MV-lambda-infected cells expressed significantly higher lambda antigen levels, the stronger immune response in MV-lambda-NAP immune group could not be attributed to a larger amount of antigen production. ELISA and VN test against MV confirmed that there is no difference between the two strains in the development of anti-measles immunity. VN serum titers were significantly higher than the protective for human levels of >1:120 PNT50 [33] in both animal groups. MV-specific antibody titers in ELISA were high and long lasting, indicating that the NAP-tagged antigen expression did not affect negatively anti-MV immunity. MV neutralizing antibodies represent fraction of the total antibody response directed against protective epitopes on MV H and F surface glycoproteins [43]. These results demonstrated that NAP is a potent immunostimulator when it is expressed covalently attached to the foreign protein and that MV expressing NAP-tagged antigens is an excellent vector platform for induction of primary immune response to weak immunogens. If it is necessary to boost secondary response it could be achieved by injection of purified formulated NAP-tagged antigens or delivered by different NAP-tagged antigen-encoding vectors. MV-expressed lambda and lambda-NAP were detected in biological fluids following infection of human xenograft tumors in mice [24, 30]. Secretory antigens released by MV infected cells could reach lymphoid tissue and activate B-cells and other immune cells at distant than vector infection focus sites. We hypothesized that NAP as a TLR2 agonist could boost immunity to NAP-tagged antigens by direct stimulation of antigen-presenting cells and inflammatory cytokine production. It is possible that simultaneous cross-linking of both TLR2 and B-cell receptor by chimeric protein can have a direct activation effect on B cells and subsequent antibody production. We observed a Th1 type biased IgG2a production in the immunization experiment with the younger (6–8-week-old) Ifnarko-CD46Ge mice but not in the older animal groups. However, further studies are required in order to determine the precise mechanisms of the enhanced immunogenicity of vector-encoded NAP-tagged antigens.
In conclusion, NAP-tagged antigens expressed by replication competent MV vector could boost strong immunity by their engineered intrinsic immunoadjuvant activity. These data are a proof of concept that covalent attachment of NAP could enhance immunogenicity of poor immunogens as a novel approach in vaccine development and immunotherapy.
Acknowledgements
We wish to thank our colleagues from the Mayo Clinic Viral Vector Production Laboratory for MV-NIS preparation and Toxicology and Pharmacology Laboratory in the Mayo Clinic Comprehensive Cancer Center Gene and Virus Therapy Shared Resource, Mayo Clinic, Rochester MN for providing the Ifnarko-CD46Ge mice.
This work was supported by the NIH grants CA 136547 and CA 154348, Atwater Grant, and Paul Leibson Memorial Fund.
References
- 1.Boudet F, Girard M, Theze J, Zouali M. Antibodies of HIV-1 positive subjects and experimentally immunized primates and rodents bind to sequence divergent regions of the third variable domain (V3) of gp120. International Immunology. 1992;4:283–294. doi: 10.1093/intimm/4.2.283. [DOI] [PubMed] [Google Scholar]
- 2.Skinner MA, Langlois AJ, McDanal CB, McDougal JS, Bolognesi DP, Matthews TJ. Neutralizing antibodies to an immunodominant envelope sequence do not prevent gp120 binding to CD4. Journal of Virology. 1988;62:4195–4200. doi: 10.1128/jvi.62.11.4195-4200.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okuno Y, Isegawa Y, Sasao F, Ueda S. A common neutralizing epitope conserved between the hemagglutinins of influenza A virus H1 and H2 strains. Journal of Virology. 1993;67:2552–2558. doi: 10.1128/jvi.67.5.2552-2558.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bui HH, Peters B, Assarsson E, Mbawuike I, Sette A. Ab and T cell epitopes of influenza A virus, knowledge and opportunities. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:246–251. doi: 10.1073/pnas.0609330104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krause JC, Tsibane T, Tumpey TM, Huffman CJ, Basler CF, Crowe JE., Jr A broadly neutralizing human monoclonal antibody that recognizes a conserved, novel epitope on the globular head of the influenza H1N1 virus hemagglutinin. Journal of Virology. 2011;85:10905–10908. doi: 10.1128/JVI.00700-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barr TA, Carlring J, Heath AW. Co-stimulatory agonists as immunological adjuvants. Vaccine. 2006;24:3399–3407. doi: 10.1016/j.vaccine.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 7.Soler E, Houdebine LM. Preparation of recombinant vaccines. Biotechnology Annual Review. 2007;13:65–94. doi: 10.1016/S1387-2656(07)13004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grange JM, Bottasso O, Stanford CA, Stanford JL. The use of mycobacterial adjuvant-based agents for immunotherapy of cancer. Vaccine. 2008;26:4984–4990. doi: 10.1016/j.vaccine.2008.06.092. [DOI] [PubMed] [Google Scholar]
- 9.Shahabi V, Maciag PC, Rivera S, Wallecha A. Live, attenuated strains of Listeria and Salmonella as vaccine vectors in cancer treatment. Bioengineered Bugs. 2010;1:235–243. doi: 10.4161/bbug.1.4.11243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh M, Billeter MA. A recombinant measles virus expressing biologically active human interleukin-12. Journal of General Virology. 1999;80:101–106. doi: 10.1099/0022-1317-80-1-101. [DOI] [PubMed] [Google Scholar]
- 11.Osada T, Berglund P, Morse MA, Hubby B, Lewis W, Niedzwiecki D, et al. Kim Lyerly H. Co-delivery of antigen and IL-12 by Venezuelan equine encephalitis virus replicon particles enhances antigen-specific immune responses and antitumor effects. Cancer Immunology, Immunotherapy. 2012;61:1941–1951. doi: 10.1007/s00262-012-1248-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chengalvala MV, Bhat RA, Bhat BM, Vernon SK, Lubeck MD. Enhanced immunogenicity of hepatitis B surface antigen by insertion of a helper T cell epitope from tetanus toxoid. Vaccine. 1999;17:1035–1041. doi: 10.1016/s0264-410x(98)00318-1. [DOI] [PubMed] [Google Scholar]
- 13.Timmerman JM, Czerwinski DK, Davis TA, Hsu FJ, Benike C, Hao ZM, et al. Idiotype-pulsed dendritic cell vaccination for B-cell lymphoma: clinical and immune responses in 35 patients. Blood. 2002;99:1517–1526. doi: 10.1182/blood.v99.5.1517. [DOI] [PubMed] [Google Scholar]
- 14.Satin B, Del Giudice G, Della Bianca V, Dusi S, Laudanna C, Tonello F, et al. The neutrophil-activating protein (HP-NAP) of Helicobacter pylori is a protective antigen and a major virulence factor. Journal of Experimental Medicine. 2000;191:1467–1476. doi: 10.1084/jem.191.9.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tonello F, Dundon WG, Satin B, Molinari M, Tognon G, Grandi G, et al. The Helicobacter pylori neutrophil-activating protein is an iron-binding protein with dodecameric structure. Molecular Microbiology. 1999;34:238–246. doi: 10.1046/j.1365-2958.1999.01584.x. [DOI] [PubMed] [Google Scholar]
- 16.Amedei A, Cappon A, Codolo G, Cabrelle A, Polenghi A, Benagiano M, et al. The neutrophil-activating protein of Helicobacter pylori promotes Th1 immune responses. Journal of Clinical Investigation. 2006;116:1092–1101. doi: 10.1172/JCI27177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Codolo G, Mazzi P, Amedei A, Del Prete G, Berton G, D’Elios MM, et al. The neutrophil-activating protein of Helicobacter pylori down-modulates Th2 inflammation in ovalbumin-induced allergic asthma. Cellular Microbiology. 2008;10:2355–2363. doi: 10.1111/j.1462-5822.2008.01217.x. [DOI] [PubMed] [Google Scholar]
- 18.Del Prete G, Chiumiento L, Amedei A, Piazza M, D’Elios MM, Codolo G, et al. Immunosuppression of TH2 responses in Trichinella spiralis infection by Helicobacter pylori neutrophil-activating protein. Journal of Allergy and Clinical Immunology. 2008;122:908–913. doi: 10.1016/j.jaci.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 19.Codolo G, Fassan M, Munari F, Volpe A, Bassi P, Rugge M, et al. HP-NAP inhibits the growth of bladder cancer in mice by activating a cytotoxic Th1 response. Cancer Immunology, Immunotherapy. 2012;61:31–40. doi: 10.1007/s00262-011-1087-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kottakis F, Papadopoulos G, Pappa EV, Cordopatis P, Pentas S, Choli-Papadopoulou T. Helicobacter pylori neutrophil-activating protein activates neutrophils by its C-terminal region even without dodecamer formation, which is a prerequisite for DNA protection – novel approaches against Helicobacter pylori inflammation. FEBS Journal. 2008;275:302–317. doi: 10.1111/j.1742-4658.2007.06201.x. [DOI] [PubMed] [Google Scholar]
- 21.Iankov ID, Haralambieva IH, Galanis E. Immunogenicity of attenuated measles virus engineered to express Helicobacter pylori neutrophil-activating protein. Vaccine. 2011;29:1710–1720. doi: 10.1016/j.vaccine.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iankov ID, Penheiter AR, Carlson SK, Galanis E. Development of monoclonal antibody-based immunoassays for detection of Helicobacter pylori neutrophilactivating protein. Journal of Immunological Methods. 2012;384:1–9. doi: 10.1016/j.jim.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iankov ID, Allen C, Federspiel MJ, Myers RM, Peng KW, Ingle JN, et al. Expression of immunomodulatory neutrophil-activating protein of Helicobacter pylori enhances the antitumor activity of oncolytic measles virus. Molecular Therapy. 2012;20:1139–1147. doi: 10.1038/mt.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iankov ID, Hillestad ML, Dietz AB, Russell SJ, Galanis E. Converting tumorspecific markers into reporters of oncolytic virus infection. Molecular Therapy. 2009;17:1395–1403. doi: 10.1038/mt.2009.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dingli D, Peng KW, Harvey ME, Greipp PR, O’Connor MK, Cattaneo R, et al. Image-guided radiovirotherapy for multiple myeloma using a recombinant measles virus expressing the thyroidal sodium iodide symporter. Blood. 2004;103(5):1641–1646. doi: 10.1182/blood-2003-07-2233. [DOI] [PubMed] [Google Scholar]
- 26.Langfield KK, Walker HJ, Gregory LC, Federspiel MJ. Manufacture of measles viruses. Methods in Molecular Biology. 2011;737:345–366. doi: 10.1007/978-1-61779-095-9_14. [DOI] [PubMed] [Google Scholar]
- 27.Mrkic B, Pavlovic J, Rülicke T, Volpe P, Buchholz CJ, Hourcade D, et al. Measles virus spread and pathogenesis in genetically modified mice. Journal of Virology. 1998;72:7420–7427. doi: 10.1128/jvi.72.9.7420-7427.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mrkic B, Odermatt B, Klein MA, Billeter MA, Pavlovic J, Cattaneo R. Lymphatic dissemination and comparative pathology of recombinant measles viruses in genetically modified mice. Journal of Virology. 2000;74:1364–1372. doi: 10.1128/jvi.74.3.1364-1372.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roscic-Mrkic B, Schwendener RA, Odermatt B, Zuniga A, Pavlovic J, Billeter MA, et al. Roles of macrophages in measles virus infection of genetically modified mice. Journal of Virology. 2001;75:3343–3351. doi: 10.1128/JVI.75.7.3343-3351.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iankov ID, Pandey M, Harvey M, Griesmann GE, Federspiel MJ, Russell SJ. Immunoglobulin G antibody-mediated enhancement of measles virus infection can bypass the protective antiviral immune response. Journal of Virology. 2006;80:8530–8540. doi: 10.1128/JVI.00593-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song MK, Vindurampulle CJ, Capozzo AV, Ulmer J, Polo JM, Pasetti MF, et al. Characterization of immune responses induced by intramuscular vaccination with DNA vaccines encoding measles virus hemagglutinin and/or fusion proteins. Journal of Virology. 2005;79:9854–9861. doi: 10.1128/JVI.79.15.9854-9861.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iankov ID, Penheiter AR, Griesmann GE, Carlson SK, Federspiel MJ, Galanis E. Neutralization capacity of measles virus H protein specific IgG determines the balance between antibody-enhanced infectivity and protection in microglial cells. Virus Research. 2013;172:15–23. doi: 10.1016/j.virusres.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haralambieva IH, Ovsyannikova IG, Vierkant RA, Poland GA. Development of a novel efficient fluorescence-based plaque reduction microneutralization assay for measles virus immunity. Clinical and Vaccine Immunology. 2008;15:1054–1059. doi: 10.1128/CVI.00008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen RT, Markowitz LE, Albrecht P, Stewart JA, Mofenson LM, Preblud SR, et al. Measles antibody: reevaluation of protective titers. Journal of Infectious Diseases. 1990;162(5):1036–1042. doi: 10.1093/infdis/162.5.1036. [DOI] [PubMed] [Google Scholar]
- 35.Ward BJ, Aouchiche S, Martel N, Bertley FM, Bautista-Lopez N, Serhir B, et al. Measurement of measles virus-specific neutralizing antibodies: evaluation of the syncytium inhibition assay in comparison with the plaque reduction neutralization test. Diagnostic Microbiology and Infectious Disease. 1999;33(3):147–152. doi: 10.1016/s0732-8893(98)00069-8. [DOI] [PubMed] [Google Scholar]
- 36.Warshakoon HJ, Hood JD, Kimbrell MR, Malladi S, Wu WY, Shukla NM, et al. Potential adjuvantic properties of innate immune stimuli. Human Vaccines. 2009;5:381–394. doi: 10.4161/hv.5.6.8175. [DOI] [PubMed] [Google Scholar]
- 37.Zuniga A, Wang Z, Liniger M, Hangartner L, Caballero M, Pavlovic J, et al. Attenuated measles virus as a vaccine vector. Vaccine. 2007;25:2974–2983. doi: 10.1016/j.vaccine.2007.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brandler S, Marianneau P, Loth P, Lacôte S, Combredet C, Frenkiel MP, et al. Measles vaccine expressing the secreted form of West Nile virus envelope glycoprotein induces protective immunity in squirrel monkeys, a new model of West Nile virus infection. Journal of Infectious Diseases. 2012;206:212–219. doi: 10.1093/infdis/jis328. [DOI] [PubMed] [Google Scholar]
- 39.Stebbings R, Février M, Li B, Lorin C, Koutsoukos M, Mee E, et al. Immunogenicity of a recombinant measles-HIV-1 clade B candidate vaccine. PLoS ONE. 2012;7:e50397. doi: 10.1371/journal.pone.0050397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.del Valle JR, Devaux P, Hodge G, Wegner NJ, McChesney MB, Cattaneo R. A vectored measles virus induces hepatitis B surface antigen antibodies while protecting macaques against measles virus challenge. Journal of Virology. 2007;81:10597–1605. doi: 10.1128/JVI.00923-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Centers for Disease Control and Prevention (CDC) Global control and regional elimination of measles, 2000–2011. MMWR Morbidity and Mortality Weekly Report. 2013;62:27–31. [PMC free article] [PubMed] [Google Scholar]
- 42.Duprex WP, McQuaid S, Hangartner L, Billeter MA, Rima BK. Observation of measles virus cell-to-cell spread in astrocytoma cells by using a green fluorescent protein-expressing recombinant virus. Journal of Virology. 1999;73:9568–9575. doi: 10.1128/jvi.73.11.9568-9575.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bouche FB, Ertl OT, Muller CP. Neutralizing B cell response in measles. Viral Immunology. 2002;15:451–471. doi: 10.1089/088282402760312331. [DOI] [PubMed] [Google Scholar]