Abstract
Rapid prototyping (RP), also known as additive manufacturing (AM), has been well received and adopted in the biomedical field. The capacity of this family of techniques to fabricate customized 3D structures with complex geometries and excellent reproducibility has revolutionized implantology and regenerative medicine. In particular, nozzle-based systems allow the fabrication of high-resolution polylactic acid (PLA) structures that are of interest in regenerative medicine. These 3D structures find interesting applications in the regenerative medicine field where promising applications including biodegradable templates for tissue regeneration purposes, 3D in vitro platforms for studying cell response to different scaffolds conditions and for drug screening are considered among others. Scaffolds functionality depends not only on the fabrication technique, but also on the material used to build the 3D structure, the geometry and inner architecture of the structure, and the final surface properties. All being crucial parameters affecting scaffolds success. This Commentary emphasizes the importance of these parameters in scaffolds’ fabrication and also draws the attention toward the versatility of these PLA scaffolds as a potential tool in regenerative medicine and other medical fields.
Keywords: scaffolds, polylactic acid (PLA), tissue engineering, rapid prototyping, biodegradable, drug screening, 3D in vitro culture system, regenerative medicine, composite material, calcium phosphate glass
Introduction
Additive manufacturing techniques have been welcome in the biomaterials field. This family of techniques also known as Rapid Prototyping (RP) has become part of the set of techniques currently used in the development of new implants and 3D scaffolds for tissue engineering.1–4 Owing to their capacity to build custom-made 3D structures, RP techniques have arisen special interest within the regenerative medicine community. In addition to revolutionize implantology and regenerative therapies by introducing new possibilities to reconstruct and regenerate tissues in a patient-specific manner, RP also provides a tremendous tool to fabricate scaffolds on demand to obtain in vitro platforms for studying the effect of various parameters such as scaffolds architecture, pore size, geometry, topography, wettability, and mechanical properties among others, on cells behavior including inflammatory response.
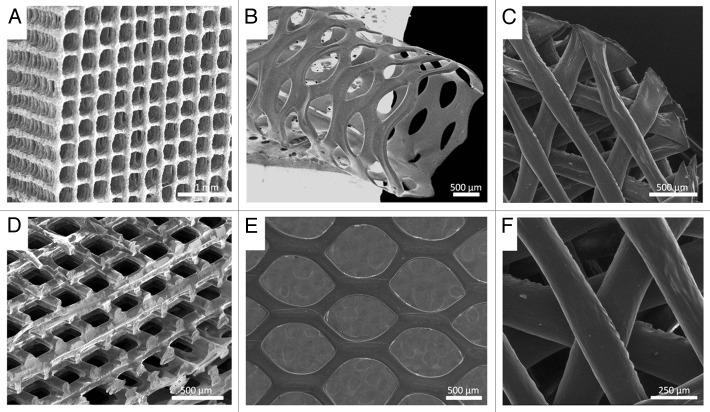
Within the additive manufacturing techniques family, nozzle-deposition-based ones have shown great versatility. The approach consisting in a controlled dispensing system integrated with pumping technology and a CAD/CAM system allows the precise and reproducible fabrication of 3D structures with well-defined predetermined geometries. In particular, the use of this technique to fabricate “high-resolution” polylactic acid (PLA) 3D structures has been recently reported by us.5 In that work we describe the fabrication of PLA based scaffolds with different geometries and provide valuable information on the importance of the different fabrication parameters on both bulk and surface properties, and their impact on cell adhesion. In brief, both PLA and PLA/glass scaffolds were 3D-printed using the nozzle-deposition-based system (Tissue Engineering 3-Dn-300, Sciperio/nScrypt Inc., available in the Rapid Prototyping service of the Biomedical Networking Center, CIBER-BBN, and IBEC www.ibecbarcelona.eu/biomaterials). Homogeneous polymer and polymer/glass solutions in chloroform (5% w/v) were prepared and printed at 3mm/s and a pressure between 40–80 psi, through a G27 (200 µm) nozzle. Structures with two different architectures were fabricated: a) orthogonal structures (Fig. 1a) with distance between struts axes (~500µm) and struts diameter around ~70 µm, and b) displaced double-layer structures (Fig. 1d) with a distance between struts axes of ~250 µm and two layers dispensed in each direction.5 Structural, mechanical and surface properties of the developed scaffolds were studied as well as in vitro cell adhesion. Results shown in that work illustrate and highlight the significance of factors such as: (1) the material used to fabricate the 3D structure, (2) the geometry and architecture of the final structure, and (3) surface properties of the scaffolds.
Figure 1. SEM images of biodegradable 3D structures with various materials, geometries and architectures, (A) PLA/CaP glass composite orthogonal structure; (B) PLA tubular hexagonal mesh; (C and F) Chitosan orthogonal-diagonal structure; (D) PLA orthogonal-displaced structure, (E) PLA hexagonal mesh.
Importance of Materials in 3D Printing
Choosing the right material is crucial to achieve functional 3D structures. Materials’ intrinsic properties may affect both surface and bulk properties of the final structure. Moreover, materials’ properties have a direct effect on the attainment of certain predetermined geometries. All these aspects affect the mechanical properties and the overall performance of the 3D scaffold.
In the case of PLA, processing with the already mentioned 3D printing tool allows obtaining highly precise structures with better resolution than the ones obtained with other currently used methods.6,7 This improvement in resolution is due to a particular interplay between a set of temperature/plastiziser/printing parameters and the post-processing shrinkage of the struts due to solvent evaporation that must be carefully tuned in order to obtain such structures.5 However, if a completely different material such as a hydrogel or a PLA/glass composite material is used, results are completely different. This is the case of chitosan scaffolds, chitosan is a natural hydrogel that requires in situ cross-linking to keep the scaffold structural integrity. Moreover, chitosan swells when in contact with aqueous media. Thus, structures with significantly larger struts diameter than the pre-defined ones are obtained.
In the case of composite materials such as PLA/glass particles, though the overall structural results are the same as with PLA, the increase of viscosity due to the addition of glass particles in the printing solution implies some changes in the printing parameters and the morphology of the final structures. Furthermore, from the morphological point of view, incorporation of glass particles in the polymer matrix has a significant effect on surface topography. In addition, the presence of glass particles in the scaffold affects the mechanical behavior of the structure and its degradation rate.8,9 In fact, when 50% of glass particles (< 40 µm) are blended in the polymer solution, the elastic modulus of the structures increases substantially (PLA = 28.38 ± 3.99 MPa, PLA/glass = 44.19 ± 2.67 MPa; n = 3).5 Therefore, it is clear that materials’ properties have to be carefully considered since each material requires different and specific processing conditions, and each material leads to different structures.
Design and Architecture of 3D Scaffolds
Design and inner architecture of the 3D structure strongly depends on its final application; in this sense, a wide variety of geometries can be developed as the ones depicted in Figure 1. An important parameter affecting cell response is the scaffold geometry including pores size, shape, and struts size and orientation among others. Scaffolds architecture not only affects their mechanical performance but also affects their permeability, nutrients diffusion and cell response.10 Indeed, it has been reported that mesenchymal stem cells (MSCs) differentiation and proliferation of pre-osteoblastic cells is highly affected by the geometry of individual pores within the scaffold.11,12
Also, it has been published that the separation between struts as well as the morphology of pores and the angle formed between struts affect macrophages reponse.13 In fact, we have recently confirmed that the variation of scaffold geometry from an orthogonal configuration (squared pores) to a diagonal configuration (triangular pores) (see Fig. 1) affects both macrophages morphology and cytokine expression (data not shown). Furthermore, orthogonal scaffolds promoted the presence of rounded multinucleated giant cells, whereas diagonal ones lead to elongated macrophages.
Moreover, the distribution of struts and their thickness may also affect significantly on the mechanical properties of the final scaffolds. As a matter of fact, it has been shown that by modifying scaffolds geometry from an orthogonal design to a displaced or shifted one (Fig. 1A and D), there is a substantial variation on the compressive modulus of both scaffolds (Eorthogonal = 93.32 ± 2.18; Edisplaced = 28.38 ± 3.99MPa; n = 3) as described by us.5 Thus, depending on the mechanical requirements of the final application, the right material/design combination has to be chosen in order to get the most adequated structures.
Additionally to struts’ orientation and conformation, struts’ thickness plays an important role. Thinner rods contribute to increasing the specific area of the scaffolds considerably and therefore the contact area between material and cells increases. However, diminishing struts diameter implies that the number of rods required to build a specific volume increases and therefore longer fabrication times are required. Nonetheless, higher scaffolds resolution is a valuable asset as pointed by Hollister et al.14 who stated that one of the technical constraints of currently used solid free form techniques in order to fulfil scaffolds translation to clinic is their limitation in terms of feature size resolution to the hundreds of micron scale. Albeit this is the case in most of the work published in this area, the work reported by us reveals that high resolution structures with features below the hundreds of microns are possible with nozzle-based printing technology.5
Enhancing Cell Response by Controlling Surface Properties
Overall, the success of a biomaterial strongly depends on its interaction with the biological environment. There are applications where a direct and tight contact between the tissue and the material is required while there are other applications where a rather antifouling behavior is needed. Hence, it is clear that biomaterials surfaces are crucial to enhance and control the biological response of scaffolds and implantable devices. Both surface chemistry and surface topography are the most important features affecting biomaterials biological response.
Modification of surface chemistry is the most direct way to influence protein adsorption and therefore cell behavior. By tailoring functional groups available at the material surface it is possible to modify its surface properties, and consequently its wettability, surface electrical charges, and free energy are also changed. As a result, the affinity of some proteins and cellular response for a particular substrate is altered. Nowadays, different techniques aimed to modify scaffolds’ surface have been developed,15–18 among them surface functionalization with proteins or peptide sequences as well as the incorporation of an inorganic bioactive phase that triggers specific cell events have been successfully achieved.
Surface functionalization with bioactive molecules
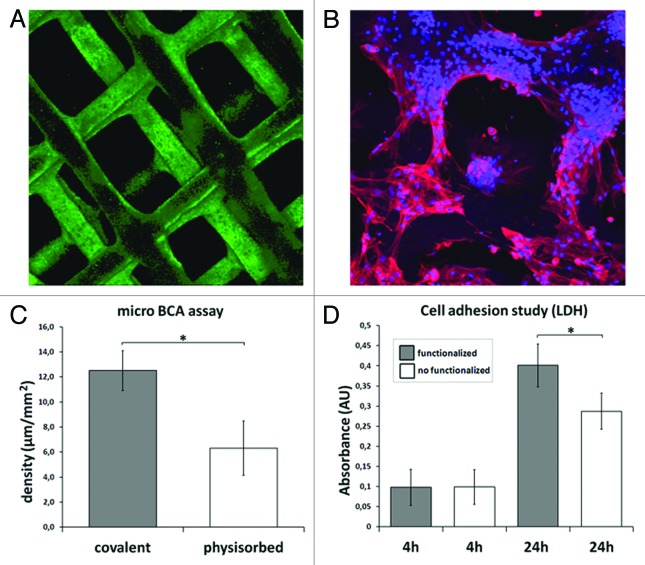
Coupling functional groups, or specific biomolecules such as functional peptides to the surface by means of chemical treatments is one the most currently studied methods for improving biomaterials bioactivity. In this context, surface functionalization of the already mentioned PLA 3D printed scaffolds with collagen has been investigated. Both, physisorbed and covalently bonded collagen surface coatings have been achieved. In the case of physical absorption, the scaffolds were directly immersed in a solution containing collagen, whereas to achieve covalent bonding the surface was previously treated with NaOH + EDC/NHS in order to activate the accessible functional groups and subsequently immersed in the collagen solution (100 µg/ml in PBS, 24 h at room temperature). Both types of samples were evaluated and characterized by CBQCA and Micro BCA assays to evaluate the amount and distribution of the biomolecule on the surface of the scaffolds. The experiment was performed in triplicate. As observed in Figure 2, fully and homogeneously collagen covered scaffolds were obtained (Fig. 2A). In addition, a higher protein density was quantified on the covalently functionalized scaffolds (Fig. 2C). Also, a cell viability assay was performed to measure cell adhesion on both non-functionalized and functionalized (covalently attached collagen) scaffolds. Mesenchymal stem cells (3 × 105 cells/scaffold) were seeded in the scaffolds, polystyrene microplate wells were used as control. Cell adhesion was studied at 4 and 24 h using a LDH assay kit. Results shown in Figure 2D are expressed as the average absorbance levels of three samples (Fig. 2D). An ANOVA analysis was performed to establish possible statistical significant differences (p ≤ 0.05) in the absorbance values. Cell studies showed a positive cell response in the functionalized scaffolds. In fact, after 72 h of culture, mesenchymal stem cells were very well spread and completely covering the scaffold surface (Fig. 2B).
Figure 2. Surface functionalization of PLA 3D scaffolds: (A) Fully and homogeneously collagen covered scaffold; (B) rMSCs cultured on the functionalized scaffolds after 72 h; (C) Quantification of the amount of collagen on the scaffolds surface. Covalently functionalized scaffolds showed a significantly higher protein density than physisorbed ones; (D) LDH assay of adhered rMSCs after 4 and 24h of culture on both covalently- and non-functionalized PLA scaffolds. Functionalized scaffolds showed a higher number of viable cells after 24h. The values marked with asterisk (*) showed statistical significant differences (p ≤ 0.05).
Improving surface bioactivity by adding inorganic particles
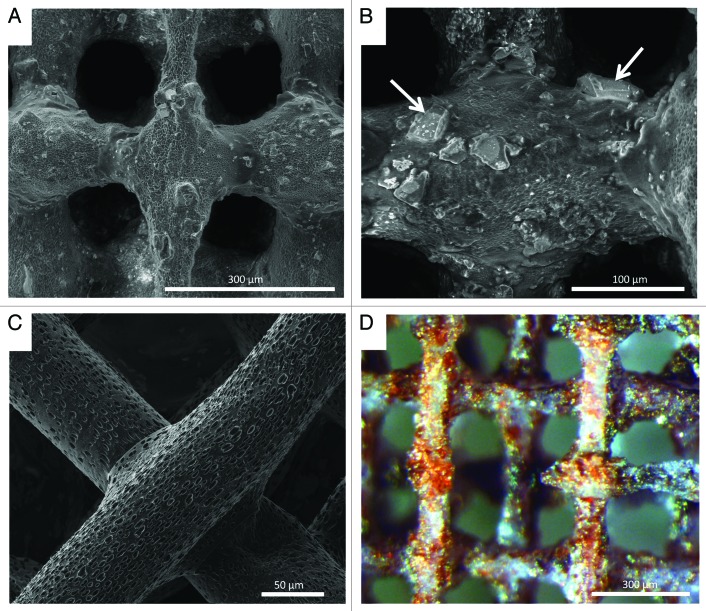
As previously mentioned, other method for modifying surface chemistry is by adding bioactive inorganic particles to the PLA matrix. In this line, addition of a soluble, bioactive CaP glass, known as G5,19,20 in particles shape to reinforce and improve bioactivity of PLA scaffolds has been explored.9 It is known that G5 glass is highly hydrophilic (contact angle = 29.8°).21 Thus, addition of G5 particles contributes to decrease PLA contact angle. Previous studies have demonstrated a preferential attachment and spreading of cells on G5 glass particles than in the polymer matrix.10,21 This soluble glass degrades along time while releasing different ions to the surrounding media.19 It has been demonstrated that the presence of G5 glass particles not only increases cell attachment and spreading but also triggers angiogenesis and bone formation owing the Ca2+ release and stiffness of the glass.22,23 Indeed, several studies have demonstrated the relevant role of ions on tissue regeneration.24,25 Given that the glass particles incorporated into the polymer scaffold are partially exposed in the surface, as demonstrated by an alizarin red assay (where only the calcified inorganic phase is stained in red color, Fig. 3D), their presence contribute both to modify surface chemistry and surface topography.
Figure 3. SEM images of (A and B) PLA/CaP glass composite scaffolds showing glass distribution and glass/polymer interface, white arrows indicate glass particles; (C) Struts of a PLA scaffold showing the micro and nanoporosity left after solvent evaporation; (D) PLA/CaP glass scaffold after Alizarin red staining. Red colored areas denote the CaP inorganic phase indicating the glass particles exposed on the scaffold surface.
Addition of G5 particles into the polymer matrix introduced an interesting topography to the scaffold surface as observed in Figure 3A and B. In addition, the surface of the polymer struts showed micro and nanopores left by the evaporation of the solvent (see Fig. 3C). Thus, the final structures presented a combination of porosities and other topography features ranging from the macroscale due to the pores initially designed to the micro and nanoscale due to solvent evaporation and the presence of glass particles. According to the interferometry results, the addition of glass particles significantly increased the average roughness (Sa) of the surface in comparison to PLA (PLA = 117.72 ± 60.50 nm, PLA/glass = 1003.89 ± 228.45 nm; n = 9).5 It is known that both surface micro and nanoporosity play important roles in protein adhesion and therefore on cell response.26 Surface nanoporosity not only increases the contact surface area between the material and biological entities but also generates nanoscale topographical cues affecting cell behavior. In fact, enhancement of cell interaction due to nanotopography has been reported.27,28 Cells do not interact directly with biomaterials, but with an absorbed protein layer that provides anchoring sequences to cells. Protein adsorption is highly dependent on surface properties; in particular, it is believed that since the dimensions of surface nanofeatures are closer to proteins, their interaction is stronger. Thus, controlled nanoporosity might play an important role in biological interactions.
We reported on the results of rMSCs response to PLA and PLA/CaP glass scaffolds with similar architecture at short periods of time. Cell viability (WST assay, n = 3) and morphology (confocal microscopy observation upon nuclei and cytoskeleton staining) were evaluated after 4 and 24 h of adhesion in contact with the materials. Results revealed that although both materials displayed similar cell viability results, noteworthy differences were observed in cells’ morphology when comparing both types of scaffolds. After 4 h, immunofluorescence images showed well spread cells with extended cytoskeleton on the scaffolds with CaP glass particles and relatively rounded cells on the PLA scaffolds. Thus, the obtained results suggested clear early cell morphological differences due to topographical and chemical changes.5
Conclusions
Data reported by us is a glimpse that opens new possibilities to produce novel PLA scaffolds with finely tuned architectures at a higher resolution than currently used methods. As described in the previous sections, the success of 3D scaffolds depends on the combination of the appropriate materials with the right design and the right fabrication technique and fabrication conditions that lead to the attainment of tailored 3D structures adapted to specific needs. Current fabrication tools allow obtaining tridimensional structures with complex architectures and surface properties on demand. In this sense, the possibility to build customized scaffolds combining various bulk and surface properties is of main interest not only in the tissue/organs engineering field, where the aim is to obtain temporal templates with properties adapted to the tissue to be regenerated; but also, in drug screening and in certain malignancies therapeutics such as in cancer.29 Three-dimensional scaffolds provide an environment able to recapitulate in vivo conditions in a more resembling way than traditional 2D in vitro cell culture systems. In particular, in the case of cancer, it has been reported that tumor phenotype is governed by the 3D tumor microenvironment. Thus, 3D scaffolds seem to be a good option to mimic tumor architecture and the in vivo scenario.
In the same direction, it could be expected that in a near future advances in the fabrication techniques and development of 3D structures will provide scaffolds that allow a better replica of the in vivo milieu. Having templates that better mimic the in vivo microenvironment could lead to both, better scaffolds for tissue regeneration and more accelerated drug screening systems with a significant reduction on animal testing.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank the Spanish MINECO for funding MN through the Ramón y Cajal program and for funding TS through the “Personal Técnico de Apoyo” subprogram.
Footnotes
Previously published online: www.landesbioscience.com/journals/organogenesis/article/26048
References
- 1.Hollister SJ. Porous scaffold design for tissue engineering. Nat Mater. 2005;4:518–24. doi: 10.1038/nmat1421. [DOI] [PubMed] [Google Scholar]
- 2.Hutmacher DW, Schantz JT, Lam CXF, Tan KC, Lim TC. State of the art and future directions of scaffold-based bone engineering from a biomaterials perspective. J Tissue Eng Regen Med. 2007;1:245–60. doi: 10.1002/term.24. [DOI] [PubMed] [Google Scholar]
- 3.Moroni L, Elisseeff J. Biomaterials engineered for integration. Mater Today. 2008;11:44–51. doi: 10.1016/S1369-7021(08)70089-0. [DOI] [Google Scholar]
- 4.Yeong WY, Chua CK, Leong KF, Chandrasekaran M. Rapid prototyping in tissue engineering: challenges and potential. Trends Biotechnol. 2004;22:643–52. doi: 10.1016/j.tibtech.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Serra T, Planell JA, Navarro M. High-resolution PLA-based composite scaffolds via 3-D printing technology. Acta Biomater. 2013;9:5521–30. doi: 10.1016/j.actbio.2012.10.041. [DOI] [PubMed] [Google Scholar]
- 6.Melchels FP, Feijen J, Grijpma DW. A poly(D,L-lactide) resin for the preparation of tissue engineering scaffolds by stereolithography. Biomaterials. 2009;30:3801–9. doi: 10.1016/j.biomaterials.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 7.Xiong Z, Yan Y, Wang S, Zhang R, Zhang C. Fabrication of porous scaffolds for bone tissue engineering via low-temperature deposition. Scr Mater. 2002;46:771–6. doi: 10.1016/S1359-6462(02)00071-4. [DOI] [Google Scholar]
- 8.Navarro M, Ginebra MP, Planell JA, Barrias CC, Barbosa MA. In vitro degradation behavior of a novel bioresorbable composite material based on PLA and a soluble CaP glass. Acta Biomater. 2005;1:411–9. doi: 10.1016/j.actbio.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Navarro M, Aparicio C, Charles-Harris M, Ginebra MP, Engel E, Planell JA. Development of a biodegrdable composite scaffold for bone tissue engineering: physicochemical, topographical, mechanical, degradation, and biological properties. Adv Polym Sci. 2006;200:209–31. doi: 10.1007/12_068. [DOI] [Google Scholar]
- 10.Charles-Harris M, Koch MA, Navarro M, Lacroix D, Engel E, Planell JAA. A PLA/calcium phosphate degradable composite material for bone tissue engineering: an in vitro study. J Mater Sci Mater Med. 2008;19:1503–13. doi: 10.1007/s10856-008-3390-9. [DOI] [PubMed] [Google Scholar]
- 11.Bidan CM, Kommareddy KP, Rumpler M, Kollmannsberger P, Fratzl P, Dunlop JW. Geometry as a factor for tissue growth: towards shape optimization of tissue engineering scaffolds. Adv Healthc Mater. 2013;2:186–94. doi: 10.1002/adhm.201200159. [DOI] [PubMed] [Google Scholar]
- 12.Kilian KA, Bugarija B, Lahn BT, Mrksich M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc Natl Acad Sci U S A. 2010;107:4872–7. doi: 10.1073/pnas.0903269107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saino E, Focarete ML, Gualandi C, Emanuele E, Cornaglia AI, Imbriani M, Visai L. Effect of electrospun fiber diameter and alignment on macrophage activation and secretion of proinflammatory cytokines and chemokines. Biomacromolecules. 2011;12:1900–11. doi: 10.1021/bm200248h. [DOI] [PubMed] [Google Scholar]
- 14.Hollister SJ, Murphy WL. Scaffold translation: barriers between concept and clinic. Tissue Eng Part B Rev. 2011;17:459–74. doi: 10.1089/ten.teb.2011.0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshida M, Langer R, Lendlein A, Lahann J. From advanced biomedical coatings to multi-functionalized biomaterials. Pol Rev. 2006;46:347–75. [Google Scholar]
- 16.Kim JE, Lee EJ, Kim HE, Koh YH, Jang JH. The impact of immobilization of BMP-2 on PDO membrane for bone regeneration. J Biomed Mater Res A. 2012;100:1488–93. doi: 10.1002/jbm.a.34089. [DOI] [PubMed] [Google Scholar]
- 17.Miyagi Y, Chiu LL, Cimini M, Weisel RD, Radisic M, Li RK. Biodegradable collagen patch with covalently immobilized VEGF for myocardial repair. Biomaterials. 2011;32:1280–90. doi: 10.1016/j.biomaterials.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Chung HJ, Park TG. Surface engineered and drug releasing pre-fabricated scaffolds for tissue engineering. Adv Drug Deliv Rev. 2007;59:249–62. doi: 10.1016/j.addr.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 19.Navarro M, Ginebra MP, Clement J, Martinez S, Avila G, Planell JA. Physico-chemical degradation of resorbable phosphate glasses stabilized with TiO2. J Am Ceram Soc. 2003;86:1345–52. doi: 10.1111/j.1151-2916.2003.tb03474.x. [DOI] [Google Scholar]
- 20.Navarro M, Ginebra MP, Planell JA. Cellular response to calcium phosphate glasses with controlled solubility. J Biomed Mater Res A. 2003;67:1009–15. doi: 10.1002/jbm.a.20014. [DOI] [PubMed] [Google Scholar]
- 21.Navarro M, Engel E, Planell JA, Amaral I, Barbosa M, Ginebra MP. Surface characterization and cell response of a PLA/CaP glass biodegradable composite material. J Biomed Mater Res A. 2008;85:477–86. doi: 10.1002/jbm.a.31546. [DOI] [PubMed] [Google Scholar]
- 22.Aguirre A, González A, Navarro M, Castaño O, Planell JA, Engel E. Control of microenvironmental cues with a smart biomaterial composite promotes endothelial progenitor cell angiogenesis. Eur Cell Mater. 2012;24:90–106, discussion 106. doi: 10.22203/ecm.v024a07. [DOI] [PubMed] [Google Scholar]
- 23.Vila OF, Bagó JR, Navarro M, Alieva M, Aguilar E, Engel E, Planell JA, Rubio N, Blanco J. Calcium phosphate glass improves angiogenesis capacity of poly(lactic acid) scaffolds and stimulates differentiation of adipose tissue-derived mesenchymal stromal cells to the endothelial lineage. J Biomed Mater Res A. 2013;101:932–41. doi: 10.1002/jbm.a.34391. [DOI] [PubMed] [Google Scholar]
- 24.Mouriño V, Cattalini JP, Boccaccini AR. Metallic ions as therapeutic agents in tissue engineering scaffolds: an overview of their biological applications and strategies for new developments. J R Soc Interface. 2012;9:401–19. doi: 10.1098/rsif.2011.0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin RA, Yne S, Hanna JV, Lee PD, Newport RJ, Smith ME, Jones JR. Characterizing the hierarchical structures of bioactive sol-gel silicate glass and hybrid scaffolds for bone regeneration. . Philos Transct A Math Phys Eng Sci. 2012;370:1422–43. doi: 10.1098/rsta.2011.0308. [DOI] [PubMed] [Google Scholar]
- 26.Biggs MJP, Richards RG, McFarlane S, Wilkinson CDW, Oreffo ROC, Dalby MJ. Adhesion formation of primary human osteoblasts and the functional response of mesenchymal stem cells to 330nm deep microgrooves. J R Soc Interface. 2008;5:1231–42. doi: 10.1098/rsif.2008.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dalby MJ, Gadegaard N, Tare R, Andar A, Riehle MO, Herzyk P, Wilkinson CDW, Oreffo ROC. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat Mater. 2007;6:997–1003. doi: 10.1038/nmat2013. [DOI] [PubMed] [Google Scholar]
- 28.McMurray RJ, Gadegaard N, Tsimbouri PM, Burgess KV, McNamara LE, Tare R, Murawski K, Kingham E, Oreffo ROC, Dalby MJ. Nanoscale surfaces for the long-term maintenance of mesenchymal stem cell phenotype and multipotency. Nat Mater. 2011;10:637–44. doi: 10.1038/nmat3058. [DOI] [PubMed] [Google Scholar]
- 29.Fong EL, Lamhamedi-Cherradi SE, Burdett E, Ramamoorthy V, Lazar AJ, Kasper FK, Farach-Carson MC, Vishwamitra D, Demicco EG, Menegaz BA, et al. Modeling Ewing sarcoma tumors in vitro with 3D scaffolds. Proc Natl Acad Sci U S A. 2013;110:6500–5. doi: 10.1073/pnas.1221403110. [DOI] [PMC free article] [PubMed] [Google Scholar]