Abstract
Recent focus on the diversity of macrophage phenotype and function signifies that these trophic cells are no longer of exclusive interest to the field of immunology. As key orchestrators of organogenesis, the contribution of macrophages to fetal development is worthy of greater attention. This review summarizes the key functions of macrophages and their primary regulator, colony-stimulating factor (CSF)-1, during development; highlighting trophic mechanisms beyond phagocytosis and outlining their roles in a range of developing organ systems. Advances in the understanding of macrophage polarization and functional heterogeneity are discussed from a developmental perspective. In addition, this review highlights the relevance of CSF-1 as a pleiotropic developmental growth factor and summarizes recent experimental evidence and clinical advancements in the area of CSF-1 and macrophage manipulation in reproduction and organogenic settings. Interrogation of embryonic macrophages also has implications beyond development, with recent attention focused on yolk sac macrophage ontogeny and their role in homeostasis and mediating tissue regeneration.
The regulatory networks that govern development involve a complex range of growth factors, signaling pathways and transcriptional regulators arising from epithelial, mesenchymal and stromal origins. A component of the organogenic milieu common to the majority of developing organs is the tissue macrophage. These hemopoietic cells are part of the mononuclear phagocyte system regulated primarily by colony-stimulating factor (CSF)-1 1, 2.
There is a resurgence in the field of CSF-1 and macrophage biology; where greater understanding of the heterogeneity of these cells is revealing contributions to tissue repair and regeneration beyond the phagocytic and inflammatory functions for which they were traditionally ascribed 3–6. The accumulation of macrophages during tissue injury is no longer viewed as simply a surrogate for disease severity, with macrophages now known to be vital in governing tissue regeneration in many settings 7–11. In particular it is the influence of CSF-1 in regulating an alternative macrophage activation state that is increasingly linked to organ repair in a range of disease models 12–17. With many similarities drawn between organogenesis and regeneration, it is pertinent to re-examine the role of CSF-1 and macrophages in organ development.
Keywords: macrophages, colony-stimulating factor-1, CSF-1, M-CSF, development, organogenesis, M2
Ontogeny of Embryonic Macrophages
Classical hemopoiesis specifies that macrophages arise from lineage-committed myeloid and subsequent monocyte precursors in the bone marrow. They are released as circulatory blood monocytes before migrating into tissues and differentiating into macrophages to replenish local populations or in response to inflammation.1 However, this traditional dogma of macrophage ontogeny is challenged during embryonic development. First observed in the mouse embryo at embryonic day (E)7.5, the presence of primordial macrophage populations before the onset of definitive hematopoiesis indicates that alternate mechanisms of macrophage derivation in embryogenesis exist.18,19 This first population represents a transient wave of maternally-derived macrophages, that provide scavenger functions in the embryo prior to the development of its own phagocytes, and rapidly declines by E8.5/9.18 The first true embryonic macrophages are evident from E8 and are derived from the primitive endoderm of the yolk sac.18,20 From E8–9, macrophages are evident throughout the yolk sac and begin to invade the embryo proper migrating into the developing head.18,20 Definitive hematopoiesis, defined as the derivation of hemopoietic cells from multipotent hemopoietic stem cells (HSCs), begins at approximately E10.5 from progenitors originating in the aorto-gonado-mesonephron (AGM) region.21,22 From E12 onwards, the liver is primary site of myeloid production. Distinct from HSC-derived myeloid cells, yolk sac-derived macrophages are Myb-independent.23
Trophic Functions of Macrophages during Development
Large numbers of macrophages are present in virtually all developing organs, with maximum numbers correlating with key periods of organogenesis.24 Moreover, mouse models deficient in tissue macrophages display a range of developmental abnormalities including skeletal and neurological deficiencies and impaired growth and fertility.25-28 Furthermore, there is no viable transgenic mutant model that is totally devoid of macrophages during organogenesis,29 highlighting the relevance of macrophages to development.
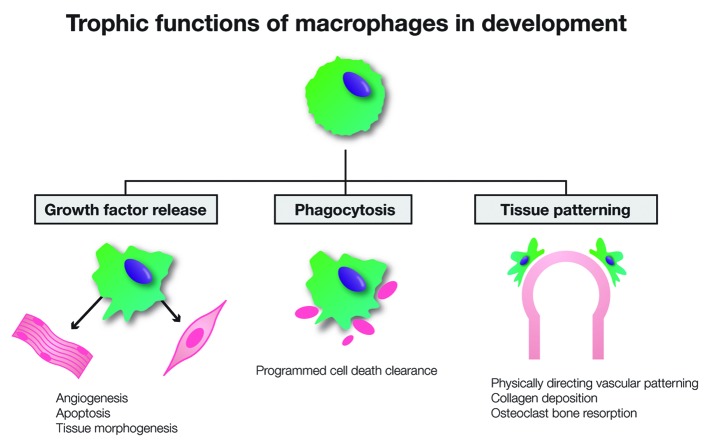
Macrophages contribute significantly to the organogenic milieu and support the morphogenic processes via a range of mechanisms (Fig. 1).3,4,19,30 Most widely recognized for their phagocytic roles, macrophage-mediated clearance of cellular debris due to programmed cell death is an essential part of the significant tissue remodelling that occurs during development.19,31 Macrophages densely populate regions of increased cell death such as the interdigital webbing during embryogenesis,20 and inactivation of the apoptotic gene psr results in perturbed development of the lungs, brain and eye due to excessive tissue and impaired cellular clearance.32 Macrophages also contribute to the induction of programmed cell death with macrophage-derived signaling directing apoptosis as part of the regulation of tissue morphogenesis.33,34 In the developing eye, interaction between angiopoietin-2 and macrophage-derived Wnt7b is essential in driving endothelial cell apoptosis associated with vascular regression and retinal remodelling.35,36 Another developmental function of macrophages is the provision of trophic support. As potent effector cells, macrophages produce a range of mediators including platelet-derived growth factors (PDGFs),37 transforming growth factors (TGFs),38 insulin-like growth factor (IGF)-139 and Wnts.40-42 Macrophage-derived factors mediate cell fate decisions, as observed in β cell differentiation in the developing pancreas43 and hepatic progenitor differentiation during liver regeneration.40 Macrophages also contribute to development through angiogenic regulation.30 In addition to the production of a range of pro- and anti-angiogenic factors,36,37,42 macrophages have been shown to assist in vascular patterning by physically directing angiogenic positioning.44
Figure 1. Key functions of macrophages in organ development. Diverse functions of macrophages contribute to the regulation of organogenesis. As potent effector cells, macrophages produce a range of growth factors that stimulate angiogenesis, induce apoptosis and regulate tissue morphogenesis. Phagocytosis and clearance of cellular debris important in programmed cell death is an essential part of the significant tissue remodelling that occurs during development. Macrophages also act to directly mediate tissue patterning through direct interactions, including physically directing vascular growth, collagen deposition in mammary bud outgrowth and the bone resorbing activity of bone-resident osteoclasts.
Classification of Macrophage Phenotype
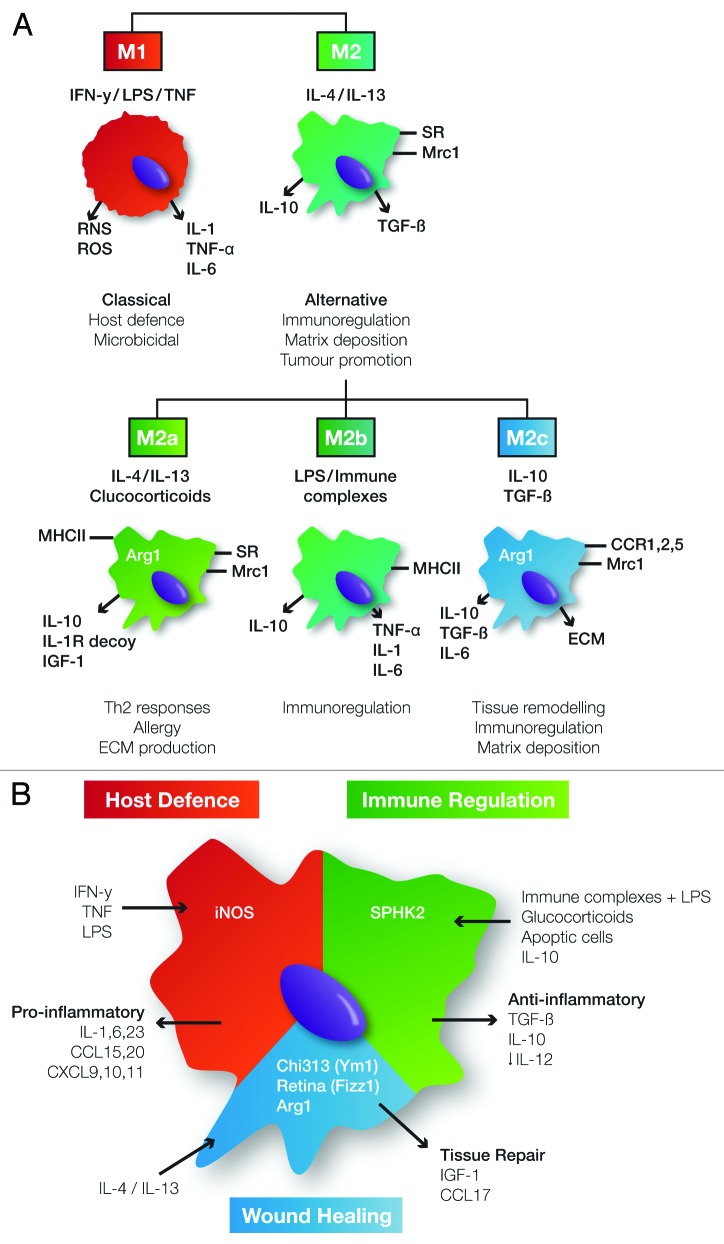
Macrophages are remarkably plastic in their ability to adapt to microenvironmental cues, evidenced by their wide-ranging locations and distinct functions.7,45,46 Such plasticity allows them to effectively respond to environmental changes or immunological challenges in order to elicit the appropriate functional response. Classification systems have been devised to categorise macrophage phenotype, physiological activation and functional activity. Emulating the T cell field, macrophages have broadly been classified into two groups; “classically activated” M1 macrophages that are associated with host defense and pro-inflammatory outcomes, and “alternatively activated” or M2 macrophages that represent a more regulatory or reparative macrophage activation state (Fig. 2).47-49
Figure 2. Macrophage heterogeneity and proposed macrophage classification systems. (A) Macrophage classification based on M1 “classical” activation and M2 “alternative” activation. M1 macrophages are activated by pro-inflammatory stimuli such as IFN-γ to induce production of pro-inflammatory factors and microbicidal reactive nitrogen (RNS) and oxygen species (ROS) to mediate host defense functions. Macrophages take on an alternative activation state in response to IL-4/IL-13 exposure and are characterized by mannose receptor (Mrc1) expression and release of anti-inflammatory factors such as IL-10 to mediate Th2-associated responses. Given the diversity within the alternative category, Martinez et al. proposed subclassification to include M2a, M2b and M2c which each have characteristic inducing factors, receptor expression and cytokine production to mediate Th2 responses, immunoregulation and tissue remodelling functions respectively.48 (B) As opposed to discrete categories, Mosser et al. proposed a more fluid macrophage classification system based on a color wheel analogy.45 They identify the three broad functional categories as host defense, wound healing and immunoregulation, induced in response to varied stimuli to provide characteristic receptor and cytokine production which mediate effector functions. However these are not discrete categories and macrophages have the ability to blend within broad subtypes to respond to varying microenvironmental cues, provide specific functional needs and generate extensive heterogeneity. IFN-γ, interferon gamma; LPS, lipopolysaccharide; TNF, tumor necrosis factor; iNOS, inducible nitric oxide synthase; MHCII, major histocompatibility complex class II; IL, interleukin; Th, T helper cell; SR, scavenger receptors; TGF-β, transforming growth factor β; Arg1, arginase 1; IGF-1, insulin-like growth factor 1; ECM, extracellular matrix; CCR, CC chemokine receptor; CXCL, CXC motif chemokine; Chi3l3, chitinase 3-like protein; Retnla, resistin-like molecule α; SPHK1, sphingosine kinase 1; Mrc1, mannose receptor 1.
M1 Macrophages
In response to inflammatory stimuli such as pathogenic invasion and necrosis, pro-inflammatory mediators interferon gamma (IFN-γ) and tumor necrosis factor (TNF) stimulate macrophages to take on a classical activation state.48,50,51 These primed macrophages secrete pro-inflammatory cytokines and chemokines such as interleukin (IL)-1, TNF-α, IL-12, IL-23 and chemokine (C-C motif) ligand (CCL)8.49,52 They also produce cytotoxic mediators including oxidative and nitrogen radicals to facilitate microbicidal and tumoricidal activities.53 This effective killing repertoire is important in host defense, however extended or inappropriate activation can lead to tissue damage as observed in chronic diseases such as rheumatoid arthritis.50,54,55
M2 Macrophages
The description of alternative activation has arisen from the improved understanding of macrophage diversity beyond mere pro-inflammatory phagocytes. Production of IL-4 and IL-13 as part of a Th2 response stimulates macrophages to acquire an M2 phenotype.47,56,57 These macrophages have decreased production of pro-inflammatory cytokines and decreased microbial killing and phagocytosis activity. Instead they produce anti-inflammatory mediators such as IL-10 and TGF-β to promote dampening of immune responses.58,59
Alternative Macrophage Activation and Development
While the M1/M2 dichotomy has arisen from studies in adult settings of disease and repair, there are many common regulatory and molecular programs during organogenesis. In this regard, regeneration and repair of organs in the adult may represent a recapitulation of developmental programs, and congruently developmental macrophages that respond to their microenvironment and instructive cues provide important pleiotropic functions. This is evidenced by the convergent phenotype of macrophages involved in development and the M2 classification of macrophages associated with tissue repair. Importantly, Rae et al. conducted comprehensive gene expression profiling of tissue macrophages during embryonic development comparing cells derived from E12.5 kidneys, brains and lungs.60 Interestingly, fetal macrophage abundance and gene expression was shown to be comparable regardless of the tissue of origin. Furthermore, these fetal macrophages expressed markers consistent with an M2 phenotype, including upregulation of mannose receptor 1 (Mrc1), macrophage scavenger receptor 2 (Msr2), complement component 1q (C1q), CD163, selenoprotein P, CCL24 and triggering receptor expressed on myeloid cells 2 (Trem 2).60 In the lung, the number of macrophages is increased and they display an M2-associated activation state, indicated by upregulation of Mrc1, Arg1 and CCL17, during tissue remodelling associated with alveolar development.61 Interestingly, but perhaps unsurprisingly, these alternatively-activated developmental macrophages are phenotypically similar to tumor-associated macrophages (TAMS).62-64 TAMS induce angiogenesis, tissue remodelling and scavenger functions which support tumor growth and metastasis; reminiscent of M2 macrophages and their key roles essential in normal organ development.62 Ojalvo et al. demonstrated significant overlap in the gene expression signatures of fetal macrophages and TAMS within breast cancer tissue.64 This included genes associated with angiogenesis and other trophic functions, supporting the recapitulation of developmental events in neoplastic growth.64
The phenotype and function of tissue macrophages that govern extracellular matrix (ECM) production and remodelling is fundamental to organogenesis. Alternative activation of macrophages is associated with ECM production and the release of trophic factors that contribute to tissue remodelling, angiogenesis and wound healing.47,65,66 Common alternative activation markers include expression of enzymes arginase 1 (Arg1), resistin-like molecule α (Retnla; Fizz1) and chitinase 3-like 3 (Chi3l3; Ym1).56,67 Other stimuli shown to promote an alternative phenotype include glucocorticoids, IL-10 and immune complexes.48,49,68,69 During embryonic development, ECM remodelling is a central part of the structural changes involved in branching morphogenesis and architectural establishment that is dependent on tissue macrophages. Matrix metalloproteinases (MMPs) contribute to matrix proteolysis and have positive roles in branch formation not only in the kidney70 but also in other branching organs including the lung71 and mammary gland.72 MMP9 is produced by the mesenchyme, and when blocked using an anti-MMP9 Ab a decrease in ureteric bud branching in metanephric organ cultures was observed.73 Furthermore in vivo, genetic deletion in Mmp9−/− mice resulted in impaired branching, increased mesenchymal apoptosis and decreased nephron formation.74
During postnatal lung development in the mouse, we have recently reported that macrophages show an upregulated expression of M2 markers (Arg1, Ccl17 and Mrc1) that correlates with the alveolarisation stage; characterized by secondary septation, alveolar wall remodelling and microvascular maturation.61 Mrc1 provides an important mechanism for cellular clearance associated with homeostasis and tissue reorganization,76 and Arg1 is associated with collagen formation and ECM production.77 This highlights the unrecognized importance of macrophages in the alveolarisation stage of lung development, and in particular the association with an M2 activation state and tissue remodelling.61
CSF-1 as a Developmental Mediator
CSF-1, also known as macrophage (M)-CSF, is a hemopoietic growth factor for the mononuclear phagocyte lineage and the primary regulator of macrophage differentiation, proliferation and survival.78-80 It elicits its effect through binding with the CSF-1 receptor (CSF-1R); a high-affinity receptor tyrosine kinase encoded by the c-fms proto-oncogene.81 CSF-1 is produced by a range of cell types and acts both locally and humorally in an autocrine and paracrine manner.78 Alternate mRNA splicing and differential proteolytic processing gives rise to three biologically-active isoforms of CSF-1: a secreted glycoprotein; a secreted proteoglycan; and a membrane spanning cell-surface glycoprotein.82 The two major cell populations regulated by CSF-1 via expression of the CSF-1R are cells of the trophoblast lineage and hemopoietic cells of the mononuclear phagocyte system,83 with distinct promoters specific to each lineage in humans.84 Expression beyond these lineages is also reported in settings including Paneth cells of the small intestine,85,86 neural stem cells87 and kidney tubular epithelium in a setting of acute injury.88
CSF-1 is essential in regulating trophic macrophages during development.4,19,28,60 CSF-1R is one of the earliest markers of these cells indicating the importance of CSF-1 in the differentiation and propagation of macrophages from the earliest stages of development.20,83 Indeed, it is the early and consistent expression of the CSF-1R that has provided an important tool for tracking developmental macrophages.20,83 The absence of CSF-1 results in a range of developmental abnormalities, including skeletal, neurological, growth and fertility defects,25-28 primarily stemming from the severe deficiency in tissue macrophages. Interestingly while these developmental defects are striking, CSF-1-deficient mice (Csf1op/op) exhibit few immunological defects. This is in contrast to granulocyte-macrophage colony-stimulating factor (GM-CSF), where Gmcsf−/− mutant mice are superficially healthy and fertile, with the exception of lung abnormalities associated with impaired surfactant clearance.89 CSF-1 has non-redundant functions in development, as administration of GM-CSF was not able to correct the growth and skeletal deficiencies in CSF-1-deficient mouse.90 Furthermore, unlike other macrophage mediators, CSF-1 levels are developmentally regulated and correlate with key periods of organogenesis.91 Interesting, CSF-1 is associated with M2 macrophage polarization.12,92 This is in contrast to macrophages responding to GM-CSF which have M1-associated phenotypic characteristics.12,52,93 During tissue injury and repair, Hamilton proposes a balanced relationship between CSF-1 and GM-CSF-mediated anti- and pro-inflammatory activity.12,15 When the ratio is tipped toward GM-CSF, a pro-inflammatory response prevails until the inflammatory stimulus diminishes and the balance shifts to a CSF-1-mediated reparative/homeostatic state.12,15
Macrophages in Organogenesis
CSF-1-responsive macrophages act as important regulators of organogenesis, and several examples of the important organ-specific roles of macrophages during development are evident. Microglia, the resident macrophages of the CNS, are a key cell type in brain development. From E11–12 in the mouse, they are found closely associated with ventricular surfaces, developing neuronal cells and the neural tube.94 Macrophages contribute to neurogenesis, synaptogenesis and apoptosis involved in brain development.34,95,96 They are also involved in remodelling arborisations of neurosecretory neurons97 and contribute to synaptic pruning.98 Another developmental function of macrophages has been observed during brain angiogenesis. Fantin et al. demonstrated that macrophages provide essential guidance of tip cell fusion in vessel anastomosis.44 Mice severely devoid of macrophages through ablation of the differentiation transcription factor PU.1 displayed fewer, less complex connections.44 Furthermore, the recruitment of angiogenic macrophages into the hindbrain was dependent on CSF-1.44 Interestingly, it is yolk sac-derived macrophages rather than monocyte-derived macrophages that contribute to the angiogenic functions. CSF-1 has a neurotrophic effect when added to embryonic neurons in culture, promoting increased neuron survival and outgrowth, and neural function is impaired in mice deficient in CSF-1.99 Furthermore, a more severe phenotype is observed in the brains of Csf1r−/− mutant mice, where a total absence of macrophages is associated with gross disruption of brain architecture as well as olfactory defects stemming from a highly perturbed olfactory bulb.100 More recently, CSF-1R expression has been reported on neural progenitor cells, and it’s activation linked to normal corticogenesis.87
Mammary gland development is one of the most elegant examples of the importance of CSF-1-responsive macrophages in branching morphogenesis. During normal development macrophages directly associate with terminal end buds, lining and directing the developing duct101 in response to local production of CSF-1.102,103 Macrophages promote collagen fibrillogenesis and terminal bud structural organization.101 A lactational disturbance in Csf1op/op mice, whereby ~90% of mothers failed to feed pups, indicates alterations in mammary gland development and function in the absence of CSF-1.104 Macrophages normally recruited to the developing ducts are absent and outgrowth and branching is reduced, resulting in atrophic, poorly branched terminal end buds,102 a phenotype corrected by transgenic restoration of local mammary epithelium-produced CSF-1.103
Macrophages in the developing murine pancreas are located principally at the duct-islet interface105 in close apposition to insulin-producing islet cells.43 These F4/80+ cells are reported to display a stellate morphology.106 Similarly in the human, macrophages cluster around developing ducts, recruited via local CSF-1 expression, and provide a supportive developmental microenvironment.107 In the Csf1op/op mouse, macrophages are scarce and insulin mass is reduced due to reduced β cell proliferation and abnormal islet morphology.106 In the eye, macrophages contribute to remodelling with macrophage-derived nerve growth factor (NGF) contributing to normal neuronal apoptosis.108 Macrophages also contribute to angiogenesis of the eye. Normally located in close contact with the retinal vasculature and bridging neighboring sprouts during anastomosis, deficiency in retinal macrophages in Csf1op/op mice is associated a less complex retinal plexus.44 The importance of CSF-1-responsive macrophages in retinal development has been elegantly demonstrated in the zebrafish, where morpholino-oligonucleotide-mediated knockdown of the CSF-1R inhibited macrophage migration and retinal colonisation, resulting in a range of developmental defects.109 Interestingly, when depleted macrophages were allowed to return, a partial rescue of the neurogenesis and retinal growth was observed.109
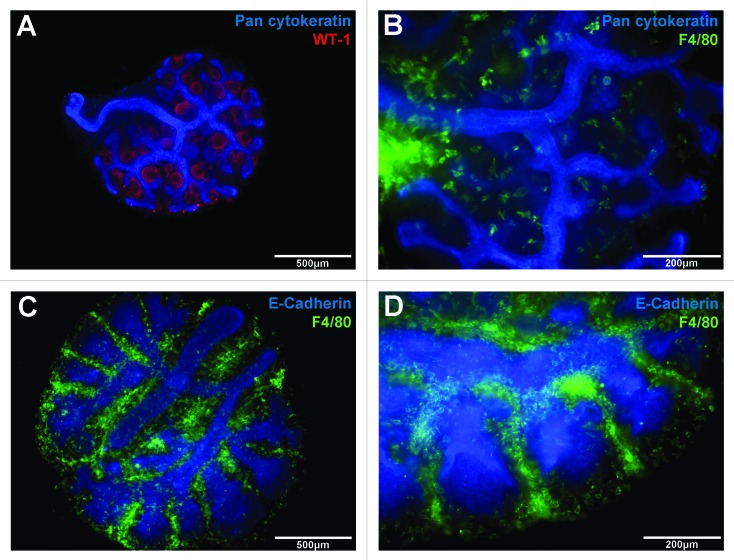
Macrophages are also abundant in the developing heart and embryonic macrophages are essential in cardiac development. In a Xenopus study,110 blockade of the primitive macrophage differentiation transcription factor spib resulted in a severe loss of macrophages and serious heart malformations. The efficiency of macrophage depletion correlated with the degree of cardiac malformation due to a lack of endocardial maturation, that could be rescued though the introduction of macrophage-containing tissue grafts.110 In the lung, macrophages are evident as early as E10 in the mouse located within the mesenchyme and surrounding the forming lung buds and elongating bronchi.111,112 We have previously observed that during branching morphogenesis, macrophages are located abundantly within developing branch points (Fig. 3).61 Macrophage number increases during postnatal life and peaks during the alveolarisation stage.61 In the absence of CSF-1, alveolar macrophage populations are severely depleted during postnatal development,113,114 and in adulthood mice develop spontaneous emphysema associated with deregulated matrix MMPs and abnormal elastin deposition.114 Furthermore, macrophages in the embryonic lung,60 and in the postnatal lung undergoing alveolarisation61 both demonstrate a gene expression profile indicative of a trophic M2 macrophage activation state.
Figure 3. Macrophages are abundant in embryonic organs during development. Embryonic organs (E12.5) cultured as explants on floating membranes provide useful assays for investigating macrophages and branching morphogenesis in kidney (A and B) and lung (C and D) development. During nephrogenesis in the kidney, indicated by pan cytokeratin-labeling of branches (A, blue) and WT-1-labeling of developing nephrons (A, red), large numbers of F4/80-labeled macrophages (B, green) are present throughout the kidney, but with particular concentration around the central branches (B). Similarly in the lung, F4/80-labeled macrophages (green) are present in large numbers during branching morphogenesis (C). In particular, macrophages are densely located within branch points of the branching epithelium (D, blue).
IL-34 – a CSF-R ligand
The recent discovery of IL-34 as the alternate ligand for the CSF-1R raises a wealth of questions regarding the differential roles of IL-34 and CSF-1 in mediating specific effects attributed to CSF-1R signaling.115 IL-34 mRNA has been located in a range of tissues, but is most abundant in the spleen.115 Although the ligands share no sequence homology and have differential receptor binding kinetics,116,117 IL-34 and CSF-1 share functional activation of the CSF-1R, with transgenic introduction of IL-34 gene expression under control of the CSF-1 promoter able to correct the phenotypic defects of CSF-1 deficient mice.117 It is the differential spatiotemporal expression patterns of the two ligands that determines their complementary and non-redundant functions.117 Embryonic mRNA expression indicates that CSF-1 may be more important in the pregnant uterus and in osteoclast regulation.117
IL-34 has particular importance in brain development, where IL-34 mRNA is expressed before CSF-1 mRNA and at higher levels in most regions of the developing brain.117 Although both ligands have complementary expression in distinct regional patterns, the constitutively high expression of IL-34 in the brain supports its key role in microglial development and homeostasis,87 and has been comprehensively examined in IL-34-deficient reporter mice.118,119 The essential role of IL-34 has been confirmed specifically in microglial and Langerhans cell development,119 and in microglial homeostasis in the adult.118 Interestingly, no overt growth or osteopetrotic phenotype, and little effect on other myeloid populations including the liver and lung, were observed in IL-34 deficient animals.118,119 Phenotypically, IL-34-mediated differentiation in vitro gives rise to macrophages with a characteristic M2 phenotype, comparable to CSF-1-responsive macrophages.120 Furthermore, in response to GM-CSF they can be polarized to take on a characteristic M1-associated phenotype.120
CSF-1 and Macrophage Manipulation in Development
The diverse functions of macrophages makes them useful candidates for a range of applications including tumor killing, tissue regeneration and promoting organogenesis. The key to their utility will be a targeted, measured activation so that specific effects can be harnessed and exploited. Manipulation of CSF-1 signaling has been used clinically as an immunological mediator in settings such as cancer and infection.121 With a growing appreciation for the importance of this pleiotropic growth factor in developmental regulation it highlights this as another area with potential clinical implications.
Reproduction
CSF-1 and macrophages have important functions in fertility and reproduction. CSF-1 is produced by the uterine epithelium during pregnancy in response to hormones such as progesterone.122 During pregnancy, uterine production of CSF-1 increases 1000-fold compared with a 2-fold increase in serum levels.123 The coincident expression of uterine CSF-1 and trophoblast CSF-1R supports the role of CSF-1 in placentation and placental growth.122,124 Furthermore, local production of CSF-1 at the maternal-fetal interface is important in immunosuppression associated with pregnancy maintenance and preventing fetal rejection.125 Defective CSF-1 production by Th2 cells is associated with pregnancy loss126 and women with reduced circulating CSF-1 have a higher rate of recurrent spontaneous abortion.127 CSF-1 also mediates reproductive function through macrophage regulation. Csf1op/op mice are severely depleted in ovarian, uterine and placental macrophages.128 Macrophages are important accessory cells in reproduction and it is the absence of CSF-1-mediated tissue macrophage regulation that is responsible for the primary fertility defects observed in Csf1op/op mice.128
Circulating CSF-1 protein levels may serve as a predictor for in vitro fertilization (IVF) outcomes.129,130 Moreover, CSF-1 has been therapeutically administered as an adjunct therapy during hormonal ovarian hyperstimulation therapy.131 Patients with documented unsuccessful responsiveness in previous hyperstimulation cycles were recruited, and CSF-1 treatment was shown to improve follicular development leading to increased numbers of mature oocytes retrieved for subsequent IVF. Concomitant CSF-1 treatment was particularly impressive in patients with low serum CSF-1 levels, and a 40% successful pregnancy rate among these 27 patients was reported.131
Growth and maturation
Trophic macrophages as essential regulatory mediators of development also provide a potential avenue for modulating growth and organogenesis. Geutskens et al. reported that the addition of CSF-1 to embryonic pancreas explants was associated with an increase in β cell differentiation and insulin production.43 Furthermore, Rae et al. demonstrated that the addition of CSF-1 to embryonic kidney explants increased branching and nephrogenesis while maintaining normal morphology.60 In these reports, enhancement of organogenesis correlated with an increase in macrophages.
The postnatal period is an important phase for CSF-1-mediated growth. In both mouse and humans, systemic CSF-1 levels normally increase in the first few days after birth.91,132 We have previously reported that the systemic supplementation of postnatal CSF-1 in mice promoted an increase in overall organ growth and body weight, that may be due in part to increased expression of IGF-1.13 This raises new information regarding the potential mechanism of trophic macrophage function in organogenesis and also supports an emerging link between CSF-1, macrophages and the IGF-1 growth axis.133 Growth hormone (GH) and IGF-1 interact to provide the principle regulatory mechanism of somatic growth in mammals. The findings that hepatic-derived circulating IGF-1 (the principle source of IGF-1) contributes only minimally to body size has highlighted the significance of locally produced IGF-1 acting in an autocrine/paracrine manner during normal growth and organ development.134-136 Macrophages can produce significant amounts of IGF-1, particularly in response to CSF-1, in vitro.137 Interestingly, many of the growth and developmental deficiencies of Csf1op/op mice are common to IGF-1-deficient animals,138 and in the tl/tl rat, dysfunctional CSF-1 production is coupled with decreased macrophages and a failure of the postnatal IGF-1 spike.133
Macrophage polarization, development and perturbation
Improved understanding of the key role of macrophages in organogenesis supports a more thorough examination of macrophage phenotype in governing proper development, and whether developmental perturbation may impact or be impacted upon by phenotypic and functional skewing. For example, in mouse lung, alveolar development is associated with the upregulation of M2 macrophages.61 Examination of macrophage-mediated mechanisms of alveolar development may have particular clinical relevance for addressing lung immaturity. In particular, the alteration of trophic macrophages in bronchopulmonary dysplasia (BPD)-associated arrest of alveolarisation is underexamined, as is the macrophage implications of the ex-utero environmental changes, inflammation and therapeutic interventions in prematurity-associated lung developmental perturbation. Indeed, the inflammatory activation of macrophages during development not only contributes to tissue damage and disruption of organ development through pro-inflammatory injury, but may also skew macrophages away from their trophic functions.111,139,140 Inflammatory activation of fetal lung macrophages through NF-κB signaling was found to upregulate pro-inflammatory mediators such as IL-1β and alter expression of Wnt7b, bone morphogenic protein (BMP)4111 and fibroblast growth factor (FGF)-10.139 And while inflammatory challenges such as lipopolysaccharide (LPS) or IL-1β administration in models of chorioamnionitis promote accelerated maturation, the mechanism is distinct from alveolarisation, characterized by a lung pathology associated with BPD.141 Today, corticosteroids remain one of the most important advancements in reducing the mortality associated with preterm birth, however they are associated with precocious maturation and significant side effects. Glucocorticoids are known M2 polarizing stimuli and in a microarray study of glucocorticoid-responsive genes, the gene most significantly reduced in embryonic glucocorticoid receptor deficient mice is the M2 polarization-associated gene Chi3l3.142 They are particularly associated with the M2a subset, which aside from Th2 responses is also linked to negative aspects including ECM production and allergy.48,51 The effect of such medications on developmental macrophages requires examination to ascertain if inappropriate skewing of macrophage phenotype may contribute to some of the negative side effects of glucocorticoid treatment. Indeed, immunological research shows that the M1/M2 balance is a two-edged sword and that both provide important functions in their appropriate context.7 Dysregulation of M2 macrophages is also associated with enhanced matrix production and pathological implications observed in disorders such as in asthma143 and the immunosuppressive functions of M2s and CSF-1 is linked to tumor growth and metastasis.144,145 CSF-1 has also been linked to the formation of atherosclerotic plaques.146,147 Furthermore, CSF-1 is implicated in Paneth cell development and CSF-1 hyperstimulation may be associated with hyperproliferative disorders of the small intestine.86
Resident Macrophages and Regeneration
Post development, the preservation of a tissue resident population of macrophages is important in homeostatic maintenance and responding to pathogenic challenge. Virtually all organs contain a resident population, comprising up to 15%, which reside independent of inflammatory or immune stimulus.20,83 The heterogeneity of macrophages is demonstrated by the diversity of these resident populations.3,46 In response to microenvironmental cues, macrophages display specific phenotypes and tissue-specific functions that allow them to respond to the distinct requirements relevant to the organ. While recruitment and infiltration of circulating precursors is an important strategy, particularly in host defense, whether the tissue resident population is maintained by infiltration or local proliferation remains debated.7,148 Also of interest is the ontogeny of these resident macrophage populations. It is suggested that fetal macrophages which colonise organs during embryonic development may constitute the resident population of macrophages maintained by local proliferation in adulthood.149,150
One area where this hypothesis has been strengthened is microglia of the brain.151 Elegant lineage-tracing studies, using both Runx and Myb-mediated fate mapping, have demonstrated that microglia are derived from early yolk sac macrophages.23,150,151 Ginhoux et al. demonstrated through neonatal bone marrow transplantation and parabiosis that resident populations are maintained independently of circulating monocytes. Furthermore, activation of yellow fluorescent protein (YFP) in leukocytes at E7.5 contributed significantly to adult microglia, whereas activation later in development, when definitive hemopoiesis was predominant, resulted in little YFP observed in the brain resident macrophage population.150 An essential role for CSF-1 in the development of yolk sac macrophages and subsequent resident macrophage populations, but not monocytes was also reported.150 Highlighting that Runx labeling also labels a small population of HSC, Schulz et al. demonstrated the appropriateness of Myb-dependency as defining yolk sac and HSC-derived populations.23 Myb−/− mice displayed normal levels of tissue macrophages in the brain, as well as the skin, spleen, pancreas, kidney and lung at E16.5.23 Indeed, these F4/80bright yolk sac-derived macrophages displayed the characteristic spindle-shape morphology in close association with the developing kidney and lung epithelium as described previously.13,60 Furthermore, through pulse labeling of CSF-1R+ yolk sac-derived macrophages at E8, they demonstrated that at 4 weeks of age the resident populations within these organs contains macrophages derived from this early yolk sac lineage.23
While there is a capacity for bone marrow-derived replacement of cells in settings of depletion and injury, it remains of interest whether resident populations are maintained through in situ proliferation. Again, microglia are one resident population where such local proliferation rather than recruitment from the circulation is key.148,152 To assess the contribution of local proliferation vs. infiltration of circulatory cells in maintaining resident populations, chimerism was induced through conditional Myb deletion and subsequent bone marrow transplantation. Although circulatory-derived donor cells contributed to replenishment in several tissues, resident macrophages in the brain remained of host origin.23 Indeed, resident in situ proliferation has also been demonstrated in Langerhans cells of this skin.153-155 Hoeffel et al. also indicated that Langerhans cells are of embryonic origin; initially derived from early colonisation of yolk sac-derived macrophage with precursors subsequently largely replaced by fetal liver-derived precursors.149
Differential ontogeny is also suggested to contribute to the alternate functions of macrophages in response to injury. Fetal macrophages possess intriguing wound healing abilities. In embryonic and PU.1-deficient neonatal mice wound healing is enhanced, with a lack of fibrotic scarring that is not associated with an inflammatory response.156 Evidence is also increasing for a differential immune response by resident macrophage populations. Jenkins et al.157 demonstrated that resident plural macrophages proliferate in situ in an IL-4 dependent manner and take on a more regulatory or reparative phenotype, in comparison to infiltrating cells which contribute to a more pro-inflammatory response. Similarly in the brain, proliferating resident microglia and infiltrating circulatory monocytes were shown to represent distinct populations with differential function in an experimental autoimmune encephalomyelitis (EAE) model of neuroinflammatory injury.148 While circulatory monocyte infiltration correlated with EAE disease progression, blockade of these recruited cells reduced disease severity. Furthermore, circulatory invasion was shown to be a transitory event and these cells did not remain and contribute to the resident microglial pool.148 The unique nature of embryonic macrophages and the suggestion that they may retain their beneficial functions as the adult resident population highlights them as potentially useful target not only in organogenesis but also in tissue repair and regeneration.
In summary, macrophages represent an important developmental cell-type essential for the regulation of normal fetal and organ development. It is clear that trophic macrophages are a versatile and potentially useful target for utilization in settings of developmental impairment, and that it will be through careful understanding of macrophage biology that safe and efficacious therapies may be devised. Furthermore, improved understanding of embryonic macrophages has implications beyond organogenesis, with these trophic macrophages in development informing ontogeny and activation important in tissue repair and regeneration.
Glossary
Abbreviations:
- Colony-stimulating factor-1 (CSF-1)
embryonic day (E), multipotent haemopoietic stem cells (HSCs), aorto-gonado-mesonephron (AGM), platelet-derived growth factors (PDGFs) transforming growth factors (TGFs), insulin-like growth factor-1 (IGF-1), chemokine (C-C motif) ligand (CCL), interleukin (IL), interferon gamma (IFN-γ) and tumour necrosis factor (TNF), extracellular matrix (ECM), arginase 1 (Arg1), resistin-like molecule alpha (Retnla
- Fizz1)
chitinase 3-like 3 (Chi3l3
- Ym1)
mannose receptor 1 (Mrc1), macrophage scavenger receptor 2 (Msr2), complement component 1q (C1q), triggering receptor expressed on myeloid cells 2 (Trem 2), tumour-associated macrophages (TAMS), CSF-1 receptor (CSF-1R), CSF-1-deficient mice (mutation osteopetrotic
- Csf1op/op)
granulocyte-macrophage colony-stimulating factor (GM-CSF), nerve growth factor (NGF), matrix metalloproteinases (MMPs), growth hormone (GH), bone morphogenic protein (BMP) and fibroblast growth factor (FGF), lipopolysaccharide (LPS), bronchopulmonary dysplasia (BPD), yellow fluorescent protein (YFP), experimental autoimmune encephalomyelitis (EAE)
Disclosure of Potential Conflicts of Interest
The authors have a published patent related to CSF-1 and development
Footnotes
Previously published online: www.landesbioscience.com/journals/organogenesis/article/25676
References
- 1.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–61. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hume DA. The mononuclear phagocyte system. Curr Opin Immunol. 2006;18:49–53. doi: 10.1016/j.coi.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Stefater JA, 3rd, Ren S, Lang RA, Duffield JS. Metchnikoff’s policemen: macrophages in development, homeostasis and regeneration. Trends Mol Med. 2011;17:743–52. doi: 10.1016/j.molmed.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol. 2009;9:259–70. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ricardo SD, van Goor H, Eddy AA. Macrophage diversity in renal injury and repair. J Clin Invest. 2008;118:3522–30. doi: 10.1172/JCI36150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gordon S. The macrophage: past, present and future. Eur J Immunol. 2007;37(Suppl 1):S9–17. doi: 10.1002/eji.200737638. [DOI] [PubMed] [Google Scholar]
- 7.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–37. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mirza R, DiPietro LA, Koh TJ. Selective and specific macrophage ablation is detrimental to wound healing in mice. Am J Pathol. 2009;175:2454–62. doi: 10.2353/ajpath.2009.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–44. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–69. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamilton JA. Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol. 2008;8:533–44. doi: 10.1038/nri2356. [DOI] [PubMed] [Google Scholar]
- 13.Alikhan MA, Jones CV, Williams TM, Beckhouse AG, Fletcher AL, Kett MM, et al. Colony-stimulating factor-1 promotes kidney growth and repair via alteration of macrophage responses. Am J Pathol. 2011;179:1243–56. doi: 10.1016/j.ajpath.2011.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okazaki T, Ebihara S, Asada M, Yamanda S, Saijo Y, Shiraishi Y, et al. Macrophage colony-stimulating factor improves cardiac function after ischemic injury by inducing vascular endothelial growth factor production and survival of cardiomyocytes. Am J Pathol. 2007;171:1093–103. doi: 10.2353/ajpath.2007.061191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamilton JA. Colony stimulating factors and macrophage heterogeniety. Inflam Regen. 2011;31:228–36. doi: 10.2492/inflammregen.31.228. [DOI] [Google Scholar]
- 16.MacDonald KP, Palmer JS, Cronau S, Seppanen E, Olver S, Raffelt NC, et al. An antibody against the colony-stimulating factor 1 receptor depletes the resident subset of monocytes and tissue- and tumor-associated macrophages but does not inhibit inflammation. Blood. 2010;116:3955–63. doi: 10.1182/blood-2010-02-266296. [DOI] [PubMed] [Google Scholar]
- 17.Zhang MZ, Yao B, Yang S, Jiang L, Wang S, Fan X, et al. CSF-1 signaling mediates recovery from acute kidney injury. J Clin Invest. 2012;122:4519–32. doi: 10.1172/JCI60363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bertrand JY, Jalil A, Klaine M, Jung S, Cumano A, Godin I. Three pathways to mature macrophages in the early mouse yolk sac. Blood. 2005;106:3004–11. doi: 10.1182/blood-2005-02-0461. [DOI] [PubMed] [Google Scholar]
- 19.Ovchinnikov DA. Macrophages in the embryo and beyond: much more than just giant phagocytes. Genesis. 2008;46:447–62. doi: 10.1002/dvg.20417. [DOI] [PubMed] [Google Scholar]
- 20.Ovchinnikov DA, van Zuylen WJ, DeBats CE, Alexander KA, Kellie S, Hume DA. Expression of Gal4-dependent transgenes in cells of the mononuclear phagocyte system labeled with enhanced cyan fluorescent protein using Csf1r-Gal4VP16/UAS-ECFP double-transgenic mice. J Leukoc Biol. 2008;83:430–3. doi: 10.1189/jlb.0807585. [DOI] [PubMed] [Google Scholar]
- 21.Bertrand JY, Chi NC, Santoso B, Teng S, Stainier DY, Traver D. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature. 2010;464:108–11. doi: 10.1038/nature08738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boisset JC, van Cappellen W, Andrieu-Soler C, Galjart N, Dzierzak E, Robin C. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature. 2010;464:116–20. doi: 10.1038/nature08764. [DOI] [PubMed] [Google Scholar]
- 23.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 24.Cecchini MG, Dominguez MG, Mocci S, Wetterwald A, Felix R, Fleisch H, et al. Role of colony stimulating factor-1 in the establishment and regulation of tissue macrophages during postnatal development of the mouse. Development. 1994;120:1357–72. doi: 10.1242/dev.120.6.1357. [DOI] [PubMed] [Google Scholar]
- 25.Wiktor-Jedrzejczak W, Bartocci A, Ferrante AW, Jr., Ahmed-Ansari A, Sell KW, Pollard JW, et al. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc Natl Acad Sci U S A. 1990;87:4828–32. doi: 10.1073/pnas.87.12.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshida H, Hayashi S, Kunisada T, Ogawa M, Nishikawa S, Okamura H, et al. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990;345:442–4. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]
- 27.Dai XM, Ryan GR, Hapel AJ, Dominguez MG, Russell RG, Kapp S, et al. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood. 2002;99:111–20. doi: 10.1182/blood.V99.1.111. [DOI] [PubMed] [Google Scholar]
- 28.Pollard JW, Stanley ER. Pleiotropic roles for CSF-1 in development defined by the mouse mutant osteopetrotic (op) Adv Dev Biochem. 1996;4:153–93. doi: 10.1016/S1064-2722(08)60060-2. [DOI] [Google Scholar]
- 29.Hibbs ML, Quilici C, Kountouri N, Seymour JF, Armes JE, Burgess AW, et al. Mice lacking three myeloid colony-stimulating factors (G-CSF, GM-CSF, and M-CSF) still produce macrophages and granulocytes and mount an inflammatory response in a sterile model of peritonitis. J Immunol. 2007;178:6435–43. doi: 10.4049/jimmunol.178.10.6435. [DOI] [PubMed] [Google Scholar]
- 30.Nucera S, Biziato D, De Palma M. The interplay between macrophages and angiogenesis in development, tissue injury and regeneration. Int J Dev Biol. 2011;55:495–503. doi: 10.1387/ijdb.103227sn. [DOI] [PubMed] [Google Scholar]
- 31.Henson PM, Hume DA. Apoptotic cell removal in development and tissue homeostasis. Trends Immunol. 2006;27:244–50. doi: 10.1016/j.it.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 32.Li MO, Sarkisian MR, Mehal WZ, Rakic P, Flavell RA. Phosphatidylserine receptor is required for clearance of apoptotic cells. Science. 2003;302:1560–3. doi: 10.1126/science.1087621. [DOI] [PubMed] [Google Scholar]
- 33.Lang RA, Bishop JM. Macrophages are required for cell death and tissue remodeling in the developing mouse eye. Cell. 1993;74:453–62. doi: 10.1016/0092-8674(93)80047-I. [DOI] [PubMed] [Google Scholar]
- 34.Marín-Teva JL, Dusart I, Colin C, Gervais A, van Rooijen N, Mallat M. Microglia promote the death of developing Purkinje cells. Neuron. 2004;41:535–47. doi: 10.1016/S0896-6273(04)00069-8. [DOI] [PubMed] [Google Scholar]
- 35.Lobov IB, Rao S, Carroll TJ, Vallance JE, Ito M, Ondr JK, et al. WNT7b mediates macrophage-induced programmed cell death in patterning of the vasculature. Nature. 2005;437:417–21. doi: 10.1038/nature03928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rao S, Lobov IB, Vallance JE, Tsujikawa K, Shiojima I, Akunuru S, et al. Obligatory participation of macrophages in an angiopoietin 2-mediated cell death switch. Development. 2007;134:4449–58. doi: 10.1242/dev.012187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pang C, Gao Z, Yin J, Zhang J, Jia W, Ye J. Macrophage infiltration into adipose tissue may promote angiogenesis for adipose tissue remodeling in obesity. Am J Physiol - Endoc M. 2008;295:E313–E22. doi: 10.1152/ajpendo.90296.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nacu N, Luzina IG, Highsmith K, Lockatell V, Pochetuhen K, Cooper ZA, et al. Macrophages produce TGF-beta-induced (beta-ig-h3) following ingestion of apoptotic cells and regulate MMP14 levels and collagen turnover in fibroblasts. J Immunol. 2008;180:5036–44. doi: 10.4049/jimmunol.180.7.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu H, Huang D, Saederup N, Charo IF, Ransohoff RM, Zhou L. Macrophages recruited via CCR2 produce insulin-like growth factor-1 to repair acute skeletal muscle injury. FASEB J. 2011;25:358–69. doi: 10.1096/fj.10-171579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boulter L, Govaere O, Bird TG, Radulescu S, Ramachandran P, Pellicoro A, et al. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med. 2012;18:572–9. doi: 10.1038/nm.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin SL, Li B, Rao S, Yeo EJ, Hudson TE, Nowlin BT, et al. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci U S A. 2010;107:4194–9. doi: 10.1073/pnas.0912228107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stefater JA, 3rd, Lewkowich I, Rao S, Mariggi G, Carpenter AC, Burr AR, et al. Regulation of angiogenesis by a non-canonical Wnt-Flt1 pathway in myeloid cells. Nature. 2011;474:511–5. doi: 10.1038/nature10085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Geutskens SB, Otonkoski T, Pulkkinen MA, Drexhage HA, Leenen PJ. Macrophages in the murine pancreas and their involvement in fetal endocrine development in vitro. J Leukoc Biol. 2005;78:845–52. doi: 10.1189/jlb.1004624. [DOI] [PubMed] [Google Scholar]
- 44.Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, et al. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010;116:829–40. doi: 10.1182/blood-2009-12-257832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–64. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 47.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 48.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–61. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 49.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–86. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 50.Laskin DL, Sunil VR, Gardner CR, Laskin JD. Macrophages and tissue injury: agents of defense or destruction? Annu Rev Pharmacol Toxicol. 2011;51:267–88. doi: 10.1146/annurev.pharmtox.010909.105812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williams TM, Little MH, Ricardo SD. Macrophages in renal development, injury, and repair. Semin Nephrol. 2010;30:255–67. doi: 10.1016/j.semnephrol.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 52.Verreck FA, de Boer T, Langenberg DM, Hoeve MA, Kramer M, Vaisberg E, et al. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc Natl Acad Sci U S A. 2004;101:4560–5. doi: 10.1073/pnas.0400983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nathan C, Shiloh MU. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc Natl Acad Sci U S A. 2000;97:8841–8. doi: 10.1073/pnas.97.16.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duffield JS. The inflammatory macrophage: a story of Jekyll and Hyde. Clin Sci (Lond) 2003;104:27–38. doi: 10.1042/CS20020240. [DOI] [PubMed] [Google Scholar]
- 55.Szekanecz Z, Koch AE. Macrophages and their products in rheumatoid arthritis. Curr Opin Rheumatol. 2007;19:289–95. doi: 10.1097/BOR.0b013e32805e87ae. [DOI] [PubMed] [Google Scholar]
- 56.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 57.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–83. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 58.Katakura T, Miyazaki M, Kobayashi M, Herndon DN, Suzuki F. CCL17 and IL-10 as effectors that enable alternatively activated macrophages to inhibit the generation of classically activated macrophages. J Immunol. 2004;172:1407–13. doi: 10.4049/jimmunol.172.3.1407. [DOI] [PubMed] [Google Scholar]
- 59.Gong D, Shi W, Yi SJ, Chen H, Groffen J, Heisterkamp N. TGFβ signaling plays a critical role in promoting alternative macrophage activation. BMC Immunol. 2012;13:31. doi: 10.1186/1471-2172-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rae F, Woods K, Sasmono T, Campanale N, Taylor D, Ovchinnikov DA, et al. Characterisation and trophic functions of murine embryonic macrophages based upon the use of a Csf1r-EGFP transgene reporter. Dev Biol. 2007;308:232–46. doi: 10.1016/j.ydbio.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 61.Jones CV, Williams TM, Walker KA, Dickinson H, Sakkal S, Rumballe BA, et al. M2 macrophage polarisation is associated with alveolar formation during postnatal lung development. Respir Res. 2013;14:41. doi: 10.1186/1465-9921-14-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–55. doi: 10.1016/S1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 63.Pucci F, Venneri MA, Biziato D, Nonis A, Moi D, Sica A, et al. A distinguishing gene signature shared by tumor-infiltrating Tie2-expressing monocytes, blood “resident” monocytes, and embryonic macrophages suggests common functions and developmental relationships. Blood. 2009;114:901–14. doi: 10.1182/blood-2009-01-200931. [DOI] [PubMed] [Google Scholar]
- 64.Ojalvo LS, King W, Cox D, Pollard JW. High-density gene expression analysis of tumor-associated macrophages from mouse mammary tumors. Am J Pathol. 2009;174:1048–64. doi: 10.2353/ajpath.2009.080676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kodelja V, Müller C, Tenorio S, Schebesch C, Orfanos CE, Goerdt S. Differences in angiogenic potential of classically vs alternatively activated macrophages. Immunobiology. 1997;197:478–93. doi: 10.1016/S0171-2985(97)80080-0. [DOI] [PubMed] [Google Scholar]
- 66.Gratchev A, Guillot P, Hakiy N, Politz O, Orfanos CE, Schledzewski K, et al. Alternatively activated macrophages differentially express fibronectin and its splice variants and the extracellular matrix protein betaIG-H3. Scand J Immunol. 2001;53:386–92. doi: 10.1046/j.1365-3083.2001.00885.x. [DOI] [PubMed] [Google Scholar]
- 67.Loke P, Nair MG, Parkinson J, Guiliano D, Blaxter M, Allen JE. IL-4 dependent alternatively-activated macrophages have a distinctive in vivo gene expression phenotype. BMC Immunol. 2002;3:7. doi: 10.1186/1471-2172-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Anderson CF, Gerber JS, Mosser DM. Modulating macrophage function with IgG immune complexes. J Endotoxin Res. 2002;8:477–81. doi: 10.1179/096805102125001118. [DOI] [PubMed] [Google Scholar]
- 69.Varga G, Ehrchen J, Tsianakas A, Tenbrock K, Rattenholl A, Seeliger S, et al. Glucocorticoids induce an activated, anti-inflammatory monocyte subset in mice that resembles myeloid-derived suppressor cells. J Leukoc Biol. 2008;84:644–50. doi: 10.1189/jlb.1107768. [DOI] [PubMed] [Google Scholar]
- 70.Haas CS, Gleason B, Lin S, Tramonti G, Kanwar YS. Matrix metalloproteinases in renal development. Connect Tissue Res. 2004;45:73–85. doi: 10.1080/03008200490442644. [DOI] [PubMed] [Google Scholar]
- 71.Kheradmand F, Rishi K, Werb Z. Signaling through the EGF receptor controls lung morphogenesis in part by regulating MT1-MMP-mediated activation of gelatinase A/MMP2. J Cell Sci. 2002;115:839–48. doi: 10.1242/jcs.115.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wiseman BS, Sternlicht MD, Lund LR, Alexander CM, Mott J, Bissell MJ, et al. Site-specific inductive and inhibitory activities of MMP-2 and MMP-3 orchestrate mammary gland branching morphogenesis. J Cell Biol. 2003;162:1123–33. doi: 10.1083/jcb.200302090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lelongt B, Trugnan G, Murphy G, Ronco PM. Matrix metalloproteinases MMP2 and MMP9 are produced in early stages of kidney morphogenesis but only MMP9 is required for renal organogenesis in vitro. J Cell Biol. 1997;136:1363–73. doi: 10.1083/jcb.136.6.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arnould C, Lelièvre-Pégorier M, Ronco P, Lelongt B. MMP9 limits apoptosis and stimulates branching morphogenesis during kidney development. J Am Soc Nephrol. 2009;20:2171–80. doi: 10.1681/ASN.2009030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Allavena P, Chieppa M, Monti P, Piemonti L. From pattern recognition receptor to regulator of homeostasis: the double-faced macrophage mannose receptor. Crit Rev Immunol. 2004;24:179–92. doi: 10.1615/CritRevImmunol.v24.i3.20. [DOI] [PubMed] [Google Scholar]
- 77.Durante W, Johnson FK, Johnson RA. Arginase: a critical regulator of nitric oxide synthesis and vascular function. Clin Exp Pharmacol Physiol. 2007;34:906–11. doi: 10.1111/j.1440-1681.2007.04638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pixley FJ, Stanley ER. CSF-1 regulation of the wandering macrophage: complexity in action. Trends Cell Biol. 2004;14:628–38. doi: 10.1016/j.tcb.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 79.Stanley ER, Chen DM, Lin HS. Induction of macrophage production and proliferation by a purified colony stimulating factor. Nature. 1978;274:168–70. doi: 10.1038/274168a0. [DOI] [PubMed] [Google Scholar]
- 80.Chitu V, Stanley ER. Colony-stimulating factor-1 in immunity and inflammation. Curr Opin Immunol. 2006;18:39–48. doi: 10.1016/j.coi.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 81.Sherr CJ. Colony-stimulating factor-1 receptor. Blood. 1990;75:1–12. [PubMed] [Google Scholar]
- 82.Shadle PJ, Aldwin L, Nitecki DE, Koths K. Human macrophage colony-stimulating factor heterogeneity results from alternative mRNA splicing, differential glycosylation, and proteolytic processing. J Cell Biochem. 1989;40:91–107. doi: 10.1002/jcb.240400110. [DOI] [PubMed] [Google Scholar]
- 83.Sasmono RT, Oceandy D, Pollard JW, Tong W, Pavli P, Wainwright BJ, et al. A macrophage colony-stimulating factor receptor-green fluorescent protein transgene is expressed throughout the mononuclear phagocyte system of the mouse. Blood. 2003;101:1155–63. doi: 10.1182/blood-2002-02-0569. [DOI] [PubMed] [Google Scholar]
- 84.Visvader J, Verma IM. Differential transcription of exon 1 of the human c-fms gene in placental trophoblasts and monocytes. Mol Cell Biol. 1989;9:1336–41. doi: 10.1128/mcb.9.3.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huynh D, Akçora D, Malaterre J, Chan CK, Dai XM, Bertoncello I, et al. CSF-1 receptor-dependent colon development, homeostasis and inflammatory stress response. PLoS One. 2013;8:e56951. doi: 10.1371/journal.pone.0056951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huynh D, Dai XM, Nandi S, Lightowler S, Trivett M, Chan CK, et al. Colony stimulating factor-1 dependence of paneth cell development in the mouse small intestine. Gastroenterology. 2009;137:136–44. doi: 10.1053/j.gastro.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nandi S, Gokhan S, Dai X-M, Wei S, Enikolopov G, Lin H, et al. The CSF-1 receptor ligands IL-34 and CSF-1 exhibit distinct developmental brain expression patterns and regulate neural progenitor cell maintenance and maturation. Dev Biol. 2012;367:100–13. doi: 10.1016/j.ydbio.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Menke J, Iwata Y, Rabacal WA, Basu R, Yeung YG, Humphreys BD, et al. CSF-1 signals directly to renal tubular epithelial cells to mediate repair in mice. J Clin Invest. 2009;119:2330–42. doi: 10.1172/JCI39087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stanley E, Lieschke GJ, Grail D, Metcalf D, Hodgson G, Gall JA, et al. Granulocyte/macrophage colony-stimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proc Natl Acad Sci U S A. 1994;91:5592–6. doi: 10.1073/pnas.91.12.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wiktor-Jedrzejczak W, Urbanowska E, Szperl M. Granulocyte-macrophage colony-stimulating factor corrects macrophage deficiencies, but not osteopetrosis, in the colony-stimulating factor-1-deficient op/op mouse. Endocrinology. 1994;134:1932–5. doi: 10.1210/en.134.4.1932. [DOI] [PubMed] [Google Scholar]
- 91.Roth P, Stanley ER. Colony stimulating factor-1 expression is developmentally regulated in the mouse. J Leukoc Biol. 1996;59:817–23. doi: 10.1002/jlb.59.6.817. [DOI] [PubMed] [Google Scholar]
- 92.Svensson J, Jenmalm MC, Matussek A, Geffers R, Berg G, Ernerudh J. Macrophages at the fetal-maternal interface express markers of alternative activation and are induced by M-CSF and IL-10. J Immunol. 2011;187:3671–82. doi: 10.4049/jimmunol.1100130. [DOI] [PubMed] [Google Scholar]
- 93.Fleetwood AJ, Lawrence T, Hamilton JA, Cook AD. Granulocyte-macrophage colony-stimulating factor (CSF) and macrophage CSF-dependent macrophage phenotypes display differences in cytokine profiles and transcription factor activities: implications for CSF blockade in inflammation. J Immunol. 2007;178:5245–52. doi: 10.4049/jimmunol.178.8.5245. [DOI] [PubMed] [Google Scholar]
- 94.Lichanska AM, Browne CM, Henkel GW, Murphy KM, Ostrowski MC, McKercher SR, et al. Differentiation of the mononuclear phagocyte system during mouse embryogenesis: the role of transcription factor PU.1. Blood. 1999;94:127–38. [PubMed] [Google Scholar]
- 95.Walton NM, Sutter BM, Laywell ED, Levkoff LH, Kearns SM, Marshall GP, 2nd, et al. Microglia instruct subventricular zone neurogenesis. Glia. 2006;54:815–25. doi: 10.1002/glia.20419. [DOI] [PubMed] [Google Scholar]
- 96.Bessis A, Béchade C, Bernard D, Roumier A. Microglial control of neuronal death and synaptic properties. Glia. 2007;55:233–8. doi: 10.1002/glia.20459. [DOI] [PubMed] [Google Scholar]
- 97.Pow DV, Perry VH, Morris JF, Gordon S. Microglia in the neurohypophysis associate with and endocytose terminal portions of neurosecretory neurons. Neuroscience. 1989;33:567–78. doi: 10.1016/0306-4522(89)90409-0. [DOI] [PubMed] [Google Scholar]
- 98.Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–8. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 99.Michaelson MD, Bieri PL, Mehler MF, Xu HH, Arezzo JC, Pollard JW, et al. CSF-1 deficiency in mice results in abnormal brain development. Development. 1996;122:2661–72. doi: 10.1242/dev.122.9.2661. [DOI] [PubMed] [Google Scholar]
- 100.Erblich B, Zhu L, Etgen AM, Dobrenis K, Pollard JW. Absence of colony stimulation factor-1 receptor results in loss of microglia, disrupted brain development and olfactory deficits. PLoS One. 2011;6:e26317. doi: 10.1371/journal.pone.0026317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ingman WV, Wyckoff J, Gouon-Evans V, Condeelis J, Pollard JW. Macrophages promote collagen fibrillogenesis around terminal end buds of the developing mammary gland. Dev Dyn. 2006;235:3222–9. doi: 10.1002/dvdy.20972. [DOI] [PubMed] [Google Scholar]
- 102.Gouon-Evans V, Rothenberg ME, Pollard JW. Postnatal mammary gland development requires macrophages and eosinophils. Development. 2000;127:2269–82. doi: 10.1242/dev.127.11.2269. [DOI] [PubMed] [Google Scholar]
- 103.Van Nguyen A, Pollard JW. Colony stimulating factor-1 is required to recruit macrophages into the mammary gland to facilitate mammary ductal outgrowth. Dev Biol. 2002;247:11–25. doi: 10.1006/dbio.2002.0669. [DOI] [PubMed] [Google Scholar]
- 104.Pollard JW, Hennighausen L. Colony stimulating factor 1 is required for mammary gland development during pregnancy. Proc Natl Acad Sci U S A. 1994;91:9312–6. doi: 10.1073/pnas.91.20.9312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Charré S, Rosmalen JG, Pelegri C, Alves V, Leenen PJ, Drexhage HA, et al. Abnormalities in dendritic cell and macrophage accumulation in the pancreas of nonobese diabetic (NOD) mice during the early neonatal period. Histol Histopathol. 2002;17:393–401. doi: 10.14670/HH-17.393. [DOI] [PubMed] [Google Scholar]
- 106.Banaei-Bouchareb L, Gouon-Evans V, Samara-Boustani D, Castellotti MC, Czernichow P, Pollard JW, et al. Insulin cell mass is altered in Csf1op/Csf1op macrophage-deficient mice. J Leukoc Biol. 2004;76:359–67. doi: 10.1189/jlb.1103591. [DOI] [PubMed] [Google Scholar]
- 107.Banaei-Bouchareb L, Peuchmaur M, Czernichow P, Polak M. A transient microenvironment loaded mainly with macrophages in the early developing human pancreas. J Endocrinol. 2006;188:467–80. doi: 10.1677/joe.1.06225. [DOI] [PubMed] [Google Scholar]
- 108.Frade JM, Barde YA. Microglia-derived nerve growth factor causes cell death in the developing retina. Neuron. 1998;20:35–41. doi: 10.1016/S0896-6273(00)80432-8. [DOI] [PubMed] [Google Scholar]
- 109.Huang T, Cui J, Li L, Hitchcock PF, Li Y. The role of microglia in the neurogenesis of zebrafish retina. Biochem Biophys Res Commun. 2012;421:214–20. doi: 10.1016/j.bbrc.2012.03.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Smith SJ, Mohun TJ. Early cardiac morphogenesis defects caused by loss of embryonic macrophage function in Xenopus. Mech Dev. 2011;128:303–15. doi: 10.1016/j.mod.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Blackwell TS, Hipps AN, Yamamoto Y, Han W, Barham WJ, Ostrowski MC, et al. NF-κB signaling in fetal lung macrophages disrupts airway morphogenesis. J Immunol. 2011;187:2740–7. doi: 10.4049/jimmunol.1101495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Morris L, Graham CF, Gordon S. Macrophages in haemopoietic and other tissues of the developing mouse detected by the monoclonal antibody F4/80. Development. 1991;112:517–26. doi: 10.1242/dev.112.2.517. [DOI] [PubMed] [Google Scholar]
- 113.Roth P, Dominguez MG, Stanley ER. The effects of colony-stimulating factor-1 on the distribution of mononuclear phagocytes in the developing osteopetrotic mouse. Blood. 1998;91:3773–83. [PubMed] [Google Scholar]
- 114.Shibata Y, Zsengeller Z, Otake K, Palaniyar N, Trapnell BC. Alveolar macrophage deficiency in osteopetrotic mice deficient in macrophage colony-stimulating factor is spontaneously corrected with age and associated with matrix metalloproteinase expression and emphysema. Blood. 2001;98:2845–52. doi: 10.1182/blood.V98.9.2845. [DOI] [PubMed] [Google Scholar]
- 115.Lin H, Lee E, Hestir K, Leo C, Huang M, Bosch E, et al. Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science. 2008;320:807–11. doi: 10.1126/science.1154370. [DOI] [PubMed] [Google Scholar]
- 116.Ma X, Lin WY, Chen Y, Stawicki S, Mukhyala K, Wu Y, et al. Structural basis for the dual recognition of helical cytokines IL-34 and CSF-1 by CSF-1R. Structure. 2012;20:676–87. doi: 10.1016/j.str.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 117.Wei S, Nandi S, Chitu V, Yeung Y-G, Yu W, Huang M, et al. Functional overlap but differential expression of CSF-1 and IL-34 in their CSF-1 receptor-mediated regulation of myeloid cells. J Leukoc Biol. 2010;88:495–505. doi: 10.1189/jlb.1209822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Greter M, Lelios I, Pelczar P, Hoeffel G, Price J, Leboeuf M, et al. Stroma-derived interleukin-34 controls the development and maintenance of langerhans cells and the maintenance of microglia. Immunity. 2012;37:1050–60. doi: 10.1016/j.immuni.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang Y, Szretter KJ, Vermi W, Gilfillan S, Rossini C, Cella M, et al. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat Immunol. 2012;13:753–60. doi: 10.1038/ni.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Foucher ED, Blanchard S, Preisser L, Garo E, Ifrah N, Guardiola P, et al. IL-34 induces the differentiation of human monocytes into immunosuppressive macrophages. antagonistic effects of GM-CSF and IFNγ. PLoS One. 2013;8:e56045. doi: 10.1371/journal.pone.0056045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hume DA, MacDonald KP. Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling. Blood. 2012;119:1810–20. doi: 10.1182/blood-2011-09-379214. [DOI] [PubMed] [Google Scholar]
- 122.Pollard JW, Bartocci A, Arceci R, Orlofsky A, Ladner MB, Stanley ER. Apparent role of the macrophage growth factor, CSF-1, in placental development. Nature. 1987;330:484–6. doi: 10.1038/330484a0. [DOI] [PubMed] [Google Scholar]
- 123.Bartocci A, Pollard JW, Stanley ER. Regulation of colony-stimulating factor 1 during pregnancy. J Exp Med. 1986;164:956–61. doi: 10.1084/jem.164.3.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Arceci RJ, Shanahan F, Stanley ER, Pollard JW. Temporal expression and location of colony-stimulating factor 1 (CSF-1) and its receptor in the female reproductive tract are consistent with CSF-1-regulated placental development. Proc Natl Acad Sci U S A. 1989;86:8818–22. doi: 10.1073/pnas.86.22.8818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gorivodsky M, Torchinsky A, Shepshelovich J, Savion S, Fein A, Carp H, et al. Colony-stimulating factor-1 (CSF-1) expression in the uteroplacental unit of mice with spontaneous and induced pregnancy loss. Clin Exp Immunol. 1999;117:540–9. doi: 10.1046/j.1365-2249.1999.00986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Piccinni MP, Scaletti C, Vultaggio A, Maggi E, Romagnani S. Defective production of LIF, M-CSF and Th2-type cytokines by T cells at fetomaternal interface is associated with pregnancy loss. J Reprod Immunol. 2001;52:35–43. doi: 10.1016/S0165-0378(01)00111-5. [DOI] [PubMed] [Google Scholar]
- 127.Katano K, Matsumoto Y, Ogasawara M, Aoyama T, Ozaki Y, Kajiura S, et al. Low serum M-CSF levels are associated with unexplained recurrent abortion. Am J Reprod Immunol. 1997;38:1–5. doi: 10.1111/j.1600-0897.1997.tb00268.x. [DOI] [PubMed] [Google Scholar]
- 128.Cohen PE, Nishimura K, Zhu L, Pollard JW. Macrophages: important accessory cells for reproductive function. J Leukoc Biol. 1999;66:765–72. doi: 10.1002/jlb.66.5.765. [DOI] [PubMed] [Google Scholar]
- 129.Salmassi A, Mettler L, Jonat W, Buck S, Koch K, Schmutzler AG. Circulating level of macrophage colony-stimulating factor can be predictive for human in vitro fertilization outcome. Fertil Steril. 2010;93:116–23. doi: 10.1016/j.fertnstert.2008.09.083. [DOI] [PubMed] [Google Scholar]
- 130.Nishimura K, Tanaka N, Kawano T, Matsuura K, Okamura H. Changes in macrophage colony-stimulating factor concentration in serum and follicular fluid in in-vitro fertilization and embryo transfer cycles. Fertil Steril. 1998;69:53–7. doi: 10.1016/S0015-0282(97)00433-0. [DOI] [PubMed] [Google Scholar]
- 131.Takasaki A, Ohba T, Okamura Y, Honda R, Seki M, Tanaka N, et al. Clinical use of colony-stimulating factor-1 in ovulation induction for poor responders. Fertil Steril. 2008;90:2287–90. doi: 10.1016/j.fertnstert.2007.10.043. [DOI] [PubMed] [Google Scholar]
- 132.Roth P, Stanley ER. Colony-stimulating factor-1 expression in the human fetus and newborn. J Leukoc Biol. 1995;58:432–7. doi: 10.1002/jlb.58.4.432. [DOI] [PubMed] [Google Scholar]
- 133.Gow DJ, Sester DP, Hume DA. CSF-1, IGF-1, and the control of postnatal growth and development. J Leukoc Biol. 2010;88:475–81. doi: 10.1189/jlb.0310158. [DOI] [PubMed] [Google Scholar]
- 134.Stratikopoulos E, Szabolcs M, Dragatsis I, Klinakis A, Efstratiadis A. The hormonal action of IGF1 in postnatal mouse growth. Proc Natl Acad Sci U S A. 2008;105:19378–83. doi: 10.1073/pnas.0809223105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B, et al. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci U S A. 1999;96:7324–9. doi: 10.1073/pnas.96.13.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sjögren K, Liu JL, Blad K, Skrtic S, Vidal O, Wallenius V, et al. Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. Proc Natl Acad Sci U S A. 1999;96:7088–92. doi: 10.1073/pnas.96.12.7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Arkins S, Rebeiz N, Biragyn A, Reese DL, Kelley KW. Murine macrophages express abundant insulin-like growth factor-I class I Ea and Eb transcripts. Endocrinology. 1993;133:2334–43. doi: 10.1210/en.133.5.2334. [DOI] [PubMed] [Google Scholar]
- 138.Liu JL, Yakar S, LeRoith D. Conditional knockout of mouse insulin-like growth factor-1 gene using the Cre/loxP system. Proc Soc Exp Biol Med. 2000;223:344–51. doi: 10.1046/j.1525-1373.2000.22349.x. [DOI] [PubMed] [Google Scholar]
- 139.Benjamin JT, Carver BJ, Plosa EJ, Yamamoto Y, Miller JD, Liu JH, et al. NF-kappaB activation limits airway branching through inhibition of Sp1-mediated fibroblast growth factor-10 expression. J Immunol. 2010;185:4896–903. doi: 10.4049/jimmunol.1001857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Moreira S, Stramer B, Evans I, Wood W, Martin P. Prioritization of competing damage and developmental signals by migrating macrophages in the Drosophila embryo. Curr Biol. 2010;20:464–70. doi: 10.1016/j.cub.2010.01.047. [DOI] [PubMed] [Google Scholar]
- 141.Kramer BW, Kallapur S, Newnham J, Jobe AH. Prenatal inflammation and lung development. Semin Fetal Neonatal Med. 2009;14:2–7. doi: 10.1016/j.siny.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bird AD, Tan KH, Olsson PF, Zieba M, Flecknoe SJ, Liddicoat DR, et al. Identification of glucocorticoid-regulated genes that control cell proliferation during murine respiratory development. J Physiol. 2007;585:187–201. doi: 10.1113/jphysiol.2007.136796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Moreira AP, Hogaboam CM. Macrophages in allergic asthma: fine-tuning their pro- and anti-inflammatory actions for disease resolution. J Interferon Cytokine Res. 2011;31:485–91. doi: 10.1089/jir.2011.0027. [DOI] [PubMed] [Google Scholar]
- 144.Mantovani A, Germano G, Marchesi F, Locatelli M, Biswas SK. Cancer-promoting tumor-associated macrophages: new vistas and open questions. Eur J Immunol. 2011;41:2522–5. doi: 10.1002/eji.201141894. [DOI] [PubMed] [Google Scholar]
- 145.Qualls JE, Murray PJ. Tumor macrophages protective and pathogenic roles in cancer development. Curr Top Dev Biol. 2011;94:309–28. doi: 10.1016/B978-0-12-380916-2.00010-3. [DOI] [PubMed] [Google Scholar]
- 146.Irvine KM, Andrews MR, Fernandez-Rojo MA, Schroder K, Burns CJ, Su S, et al. Colony-stimulating factor-1 (CSF-1) delivers a proatherogenic signal to human macrophages. J Leukoc Biol. 2009;85:278–88. doi: 10.1189/jlb.0808497. [DOI] [PubMed] [Google Scholar]
- 147.Wilson HM. Macrophages heterogeneity in atherosclerosis - implications for therapy. J Cell Mol Med. 2010;14:2055–65. doi: 10.1111/j.1582-4934.2010.01121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci. 2011;14:1142–9. doi: 10.1038/nn.2887. [DOI] [PubMed] [Google Scholar]
- 149.Hoeffel G, Wang Y, Greter M, See P, Teo P, Malleret B, et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J Exp Med. 2012;209:1167–81. doi: 10.1084/jem.20120340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–5. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gomez Perdiguero E, Schulz C, Geissmann F. Development and homeostasis of “resident” myeloid cells: The case of the microglia. Glia. 2013;61:112–20. doi: 10.1002/glia.22393. [DOI] [PubMed] [Google Scholar]
- 152.Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–43. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- 153.Chorro L, Sarde A, Li M, Woollard KJ, Chambon P, Malissen B, et al. Langerhans cell (LC) proliferation mediates neonatal development, homeostasis, and inflammation-associated expansion of the epidermal LC network. J Exp Med. 2009;206:3089–100. doi: 10.1084/jem.20091586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Merad M, Manz MG, Karsunky H, Wagers A, Peters W, Charo I, et al. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat Immunol. 2002;3:1135–41. doi: 10.1038/ni852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chorro L, Geissmann F. Development and homeostasis of ‘resident’ myeloid cells: the case of the Langerhans cell. Trends Immunol. 2010;31:438–45. doi: 10.1016/j.it.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 156.Martin P, D’Souza D, Martin J, Grose R, Cooper L, Maki R, et al. Wound healing in the PU.1 null mouse--tissue repair is not dependent on inflammatory cells. Curr Biol. 2003;13:1122–8. doi: 10.1016/S0960-9822(03)00396-8. [DOI] [PubMed] [Google Scholar]
- 157.Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332:1284–8. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]