Abstract
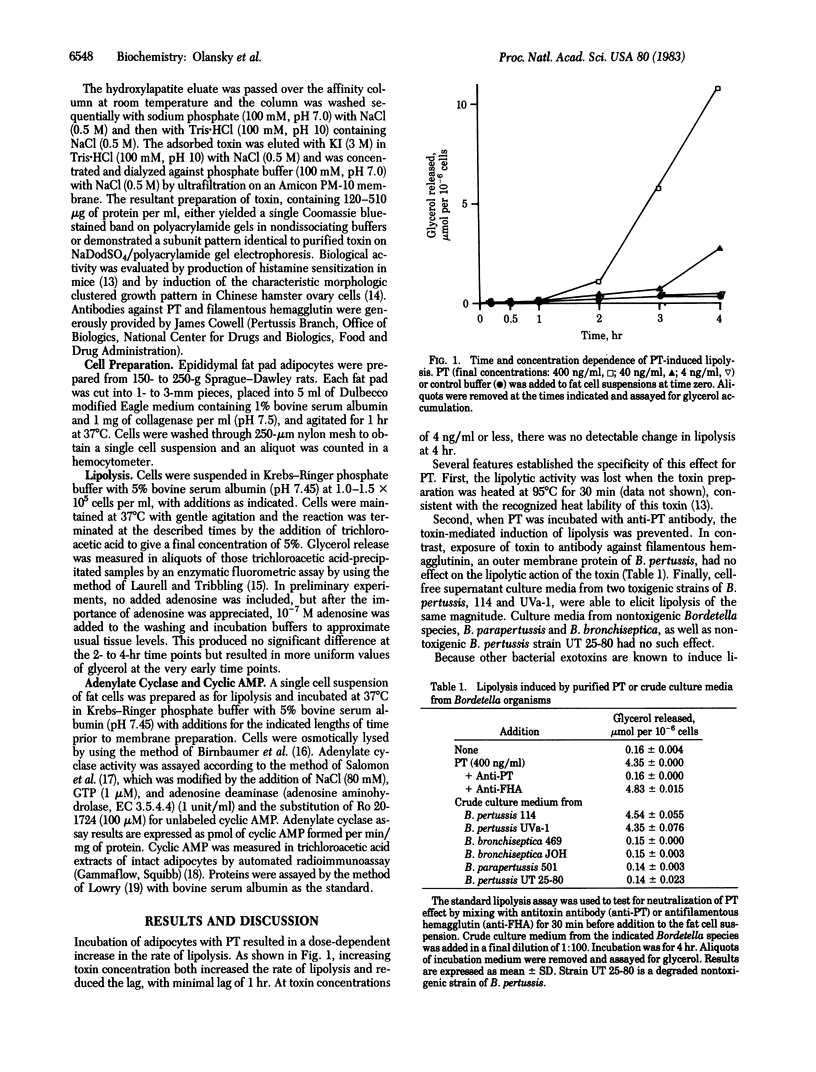
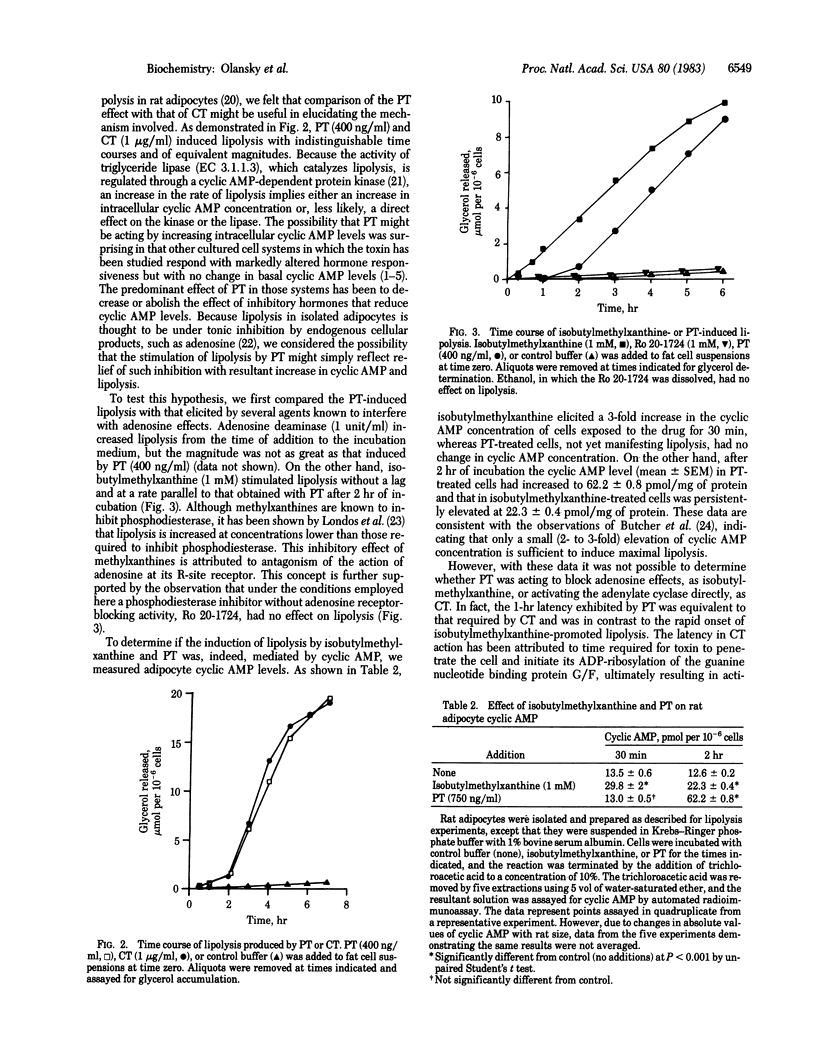
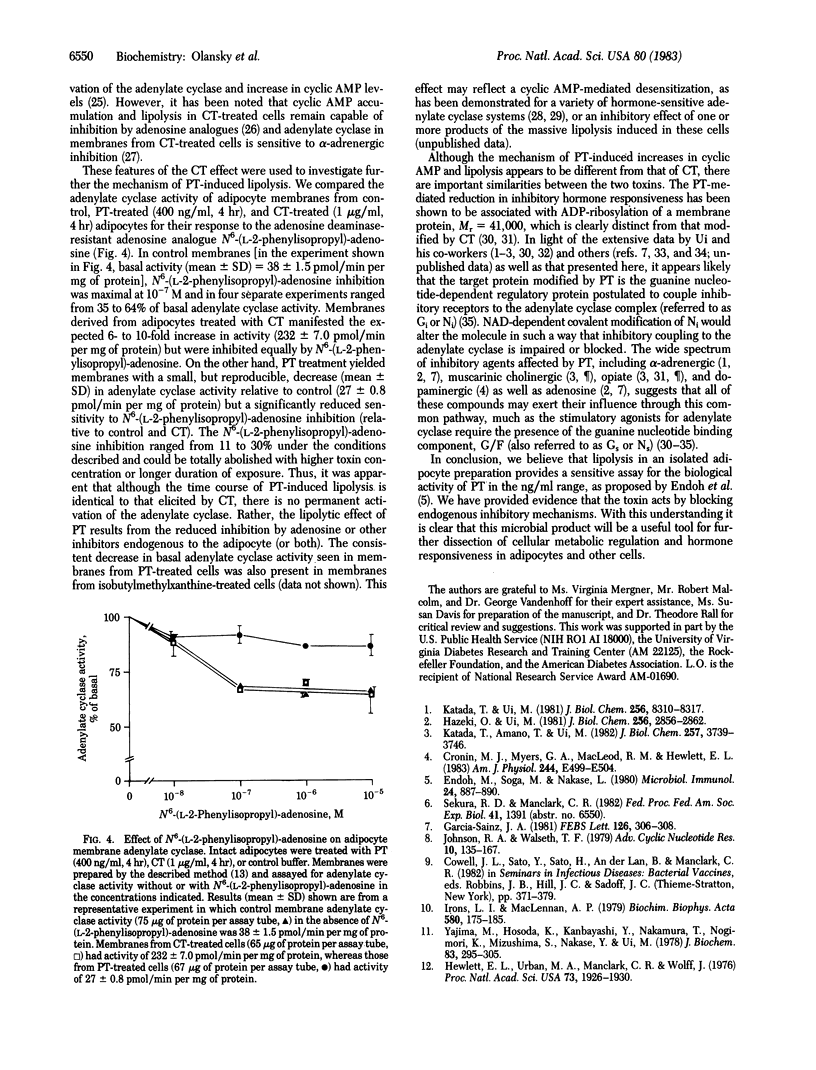
Pertussis toxin (PT), a protein produced by Bordetella pertussis, was studied for its effect on lipolysis in isolated rat epididymal adipocytes. Exposure of adipocytes to pertussis toxin resulted in a significant increase in cyclic AMP levels and lipolysis after a lag of 1-2 hr. Both the maximal rate of lipolysis and the time lag (beyond 1 hr) were PT concentration-dependent. Heat treatment (95 degrees C, 30 min) or incubation with specific antibody directed against PT eliminated the ability of toxin to increase lipolysis. Cell-free culture medium from B. pertussis, but not from nontoxigenic Bordetella species, had the same effect on lipolysis as purified toxin. Comparison of the PT effect with the known lipolytic effect of cholera toxin (CT) revealed that the two toxins elicited responses that were indistinguishable in time course and magnitude. In contrast, the adenylate cyclase (EC 4.6.1.1) activities in membranes prepared from PT- or CT-treated adipocytes were different. Adenylate cyclase activity in membranes from control (untreated) adipocytes was inhibited 35-64% by the adenosine analogue N6-(L-2-phenylisopropyl)-adenosine. As expected from previous studies, membranes from CT-treated adipocytes demonstrated an increased basal activity but showed the same proportional inhibition by N6-(L-2-phenylisopropyl)-adenosine as controls. On the other hand, membranes from adipocytes exposed to PT (400 ng/ml for 4 hr) showed no increase in basal adenylate cyclase activity but had reduced sensitivity to N6-(L-2-phenylisopropyl)-adenosine inhibition, with the maximal effect ranging from 11 to 30% at 10(-6) M N6-(L-2-phenylisopropyl)-adenosine. These data support the hypothesis that PT promotes cyclic AMP-dependent lipolysis in a manner quantitatively equivalent to CT, but by a different mechanism involving increased cyclic AMP levels resulting from loss of responsiveness to endogenous inhibitors such as adenosine.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnbaumer L., Pohl S. L., Rodbell M. Adenyl cyclase in fat cells. 1. Properties and the effects of adrenocorticotropin and fluoride. J Biol Chem. 1969 Jul 10;244(13):3468–3476. [PubMed] [Google Scholar]
- Bokoch G. M., Katada T., Northup J. K., Hewlett E. L., Gilman A. G. Identification of the predominant substrate for ADP-ribosylation by islet activating protein. J Biol Chem. 1983 Feb 25;258(4):2072–2075. [PubMed] [Google Scholar]
- Brooker G., Terasaki W. L., Price M. G. Gammaflow: a completely automated radioimmunoassay system. Science. 1976 Oct 15;194(4262):270–276. doi: 10.1126/science.184530. [DOI] [PubMed] [Google Scholar]
- Burns D. L., Hewlett E. L., Moss J., Vaughan M. Pertussis toxin inhibits enkephalin stimulation of GTPase of NG108-15 cells. J Biol Chem. 1983 Feb 10;258(3):1435–1438. [PubMed] [Google Scholar]
- Butcher R. W., Ho R. J., Meng H. C., Sutherland E. W. Adenosine 3',5'-monophosphate in biological materials. II. The measurement of adenosine 3',5'-monophosphate in tissues and the role of the cyclic nucleotide in the lipolytic response of fat to epinephrine. J Biol Chem. 1965 Nov;240(11):4515–4523. [PubMed] [Google Scholar]
- Clark R. B., Butcher R. W. Desensitization of adenylate cyclase in cultured fibroblasts with prostaglandin E1 and epinephrine. J Biol Chem. 1979 Oct 10;254(19):9373–9378. [PubMed] [Google Scholar]
- Cronin M. J., Myers G. A., MacLeod R. M., Hewlett E. L. Pertussis toxin uncouples dopamine agonist inhibition of prolactin release. Am J Physiol. 1983 May;244(5):E499–E504. doi: 10.1152/ajpendo.1983.244.5.E499. [DOI] [PubMed] [Google Scholar]
- Endoh M., Soga M., Nakase Y. In vitro assay for histamine-sensitizing factor of Bordetella pertussis. Microbiol Immunol. 1980;24(9):887–890. doi: 10.1111/j.1348-0421.1980.tb02893.x. [DOI] [PubMed] [Google Scholar]
- Fredrikson G., Strålfors P., Nilsson N. O., Belfrage P. Hormone-sensitive lipase of rat adipose tissue. Purification and some properties. J Biol Chem. 1981 Jun 25;256(12):6311–6320. [PubMed] [Google Scholar]
- García-Sáinz J. A. Decreased sensitivity to alpha 2 adrenergic amines, adenosine and prostaglandins in white fat cells from hamsters treated with pertussis vaccine. FEBS Lett. 1981 Apr 20;126(2):306–308. doi: 10.1016/0014-5793(81)80267-0. [DOI] [PubMed] [Google Scholar]
- Hazeki O., Ui M. Modification by islet-activating protein of receptor-mediated regulation of cyclic AMP accumulation in isolated rat heart cells. J Biol Chem. 1981 Mar 25;256(6):2856–2862. [PubMed] [Google Scholar]
- Hewlett E. L., Guerrant R. L., Evans D. J., Jr, Greenough W. B., 3rd Toxins of Vibrio cholerae and Escherichia coli stimulate adenyl cyclase in rat fat cells. Nature. 1974 May 24;249(455):371–373. doi: 10.1038/249371a0. [DOI] [PubMed] [Google Scholar]
- Hewlett E. L., Sauer K. T., Myers G. A., Cowell J. L., Guerrant R. L. Induction of a novel morphological response in Chinese hamster ovary cells by pertussis toxin. Infect Immun. 1983 Jun;40(3):1198–1203. doi: 10.1128/iai.40.3.1198-1203.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlett E. L., Urban M. A., Manclark C. R., Wolff J. Extracytoplasmic adenylate cyclase of Bordetella pertussis. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1926–1930. doi: 10.1073/pnas.73.6.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irons L. I., MacLennan A. P. Isolation of the lymphocytosis promoting factor-haemagglutinin of Bordetella pertussis by affinity chromatography. Biochim Biophys Acta. 1979 Sep 29;580(1):175–185. doi: 10.1016/0005-2795(79)90208-3. [DOI] [PubMed] [Google Scholar]
- Jakobs K. H., Schultz G. Different inhibitory effect of adrenaline on platelet adenylate cyclase in the presence of GTP plus cholera toxin and of stable GTP analogues. Naunyn Schmiedebergs Arch Pharmacol. 1979 Dec;310(2):121–127. doi: 10.1007/BF00500276. [DOI] [PubMed] [Google Scholar]
- Johnson R. A., Walseth T. F. The enzymatic preparation of [alpha-32P]ATP, [alpha-32P]GTP, [32P]cAMP, and [32P]cGMP, and their use in the assay of adenylate and guanylate cyclases and cyclic nucleotide phosphodiesterases. Adv Cyclic Nucleotide Res. 1979;10:135–167. [PubMed] [Google Scholar]
- Katada T., Amano T., Ui M. Modulation by islet-activating protein of adenylate cyclase activity in C6 glioma cells. J Biol Chem. 1982 Apr 10;257(7):3739–3746. [PubMed] [Google Scholar]
- Katada T., Ui M. Direct modification of the membrane adenylate cyclase system by islet-activating protein due to ADP-ribosylation of a membrane protein. Proc Natl Acad Sci U S A. 1982 May;79(10):3129–3133. doi: 10.1073/pnas.79.10.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katada T., Ui M. Islet-activating protein. A modifier of receptor-mediated regulation of rat islet adenylate cyclase. J Biol Chem. 1981 Aug 25;256(16):8310–8317. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laurell S., Tibbling G. An enzymatic fluorometric micromethod for the determination of glycerol. Clin Chim Acta. 1966 Mar;13(3):317–322. doi: 10.1016/0009-8981(66)90210-5. [DOI] [PubMed] [Google Scholar]
- Londos C., Cooper D. M., Schlegel W., Rodbell M. Adenosine analogs inhibit adipocyte adenylate cyclase by a GTP-dependent process: basis for actions of adenosine and methylxanthines on cyclic AMP production and lipolysis. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5362–5366. doi: 10.1073/pnas.75.11.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J., Vaughan M. Activation of adenylate cyclase by choleragen. Annu Rev Biochem. 1979;48:581–600. doi: 10.1146/annurev.bi.48.070179.003053. [DOI] [PubMed] [Google Scholar]
- Murayama T., Ui M. Loss of the inhibitory function of the guanine nucleotide regulatory component of adenylate cyclase due to its ADP ribosylation by islet-activating protein, pertussis toxin, in adipocyte membranes. J Biol Chem. 1983 Mar 10;258(5):3319–3326. [PubMed] [Google Scholar]
- Rodbell M. The role of hormone receptors and GTP-regulatory proteins in membrane transduction. Nature. 1980 Mar 6;284(5751):17–22. doi: 10.1038/284017a0. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Schimmel R. J., McMahon K. K. Inhibition of lipolysis and cyclic AMP accumulation by adenosine analogues in hamster epididymal adipocytes exposed to cholera toxin. Biochim Biophys Acta. 1980 Dec 1;633(2):237–244. doi: 10.1016/0304-4165(80)90409-2. [DOI] [PubMed] [Google Scholar]
- Schwabe U., Ebert R., Erbler H. C. Adenosine release from fat cells: effect on cyclic AMP levels and hormone actions. Adv Cyclic Nucleotide Res. 1975;5:569–584. [PubMed] [Google Scholar]
- Wessels M. R., Mullikin D., Lefkowitz R. J. Selective alteration in high affinity agonist binding: a mechanism of beta-adrenergic receptor desensitization. Mol Pharmacol. 1979 Jul;16(1):10–20. [PubMed] [Google Scholar]
- Yajima M., Hosoda K., Kanbayashi Y., Nakamura T., Nogimori K., Mizushima Y., Nakase Y., Ui M. Islets-activating protein (IAP) in Bordetella pertussis that potentiates insulin secretory responses of rats. Purification and characterization. J Biochem. 1978 Jan;83(1):295–303. doi: 10.1093/oxfordjournals.jbchem.a131904. [DOI] [PubMed] [Google Scholar]


