Abstract
Arsenic a metalloid and environmental contaminated has been found to be associated with public health problems in the affected areas. It is naturally occurred in groundwater and its accumulation in plant and animals leads to toxicity in several tissues most notably hepatic organ. Arsenic exposures (3 mg/kg body weight/day for 30 days) in mice exhibited increased arsenic and Zn levels in hepatocytes associated with enhanced oxidative stress in hepatocytes while there were no significantly changes were observed in Cu level. An increase in the lipid peroxidation and decrease in the levels of reduced glutathione and activity of superoxide dismutase, catalase, and glutathione peroxidase were observed in arsenic treated mice as compared to controls. Arsenic exposure in mice also caused a significant change in serum biomarkers in the SGOT, SGPT and creatinine as compared to the controls. There were no significant changes in the serum levels of total protein in these mice. Co-administration of arsenic and fruit extract of amla (500 mg/kg body weight/day for 30 days) caused a significant reduction of arsenic transference associated with significantly decreases hepatic arsenic levels and balanced the antioxidant enzyme and levels of serum hepatic enzymes like SGOT and SGPT. The results of the present study clearly demonstrate the antioxidant property of amla that could be responsible for its protective efficacy in arsenic induced hepatic toxicity.
Keywords: Arsenic, Emblicaofficinalis, Oxidative stress, Liver dysfunction, Metal estimation, Mice
Introduction
Arsenic is widely distributed in the environment due to its natural existence and anthropogenic activities. Due to the presence of high levels of inorganic arsenic in ground water in many parts of the world, human health related disorders are of major concern [1, 2]. High levels of arsenic has been reported in three districts Ballia, Varansi and Gazipur of Uttar Pradesh in the upper and middle Ganga plain, India [3]. The soluble salts of arsenic including arsenate or arsenite are well absorbed (80 %) through the gastrointestinal tract and cause health effects in individuals. Further, individuals suffers from arsenicosis have high risk to develop other health related disorders including cardiovascular, hepatic, renal, gastrointestinal, neurological and reproductive problems and malignancies [1, 4]. Due to the accumulative properties of arsenic, deposition of high concentrations of arsenic in the liver, kidney, lungs, hair and nails have been well reported as a result of chronic exposure [5]. The trivalent form of arsenic is considered to be more toxic as compared to pentavalent form due to its ability to bind with the sulfhydryl groups of proteins and disrupt the enzyme activity [6]. In view of increasing risk of chronic arsenic toxicity, the World Health Organization has lowered the permissible limit of arsenic in drinking water from 50 μg/l to 10 μg/l [7].
The metabolic function of the liver is primarily responsible for detoxification of toxins and carcinogens. Drug-induced liver injury may manifest as acute hepatitis, cholestasis, and further develop as liver cirrhosis. Reactive oxygen species (ROS) generated by metabolic intermediates of xenobiotics via induction of CYP450 families as well as activated inflammatory cells through NADPH oxidases promote the accumulation of lipid derived oxidation products that cause liver injury, resulting in cell necrosis [8]. Heavy metals have been found to be associated with various effects on blood biochemistry, enzymes and transport protein’s activity [9, 10]. The over production of ROS may overwhelm the antioxidant defense mechanism and cause oxidative damage to cellular ingredients such as lipids, proteins and DNA; this in turn can impair cellular structure and function [11].
Recent research have shown that the plant derived medicines are based upon the premise that they contain natural substances that can promote health and alleviate illness and proved to be safe, better patient tolerance, relatively less expensive plant extracts and phyto-constituents with free-radical scavenging properties could have great importance as therapeutic agents in several diseases associated with enhanced oxidative stress [12, 13]. A number of studies have been carried out to assess the protective profile of herbal or synthetic agents against arsenic induced oxidative stress in animal models [2, 13–15]. Amla, an important fruit of E. officinalis, a well-known medicinal herb, has been widely used as an indigenous medicine in the traditional Indian system of medicine. It is a rich source of vitamin C (478.56 mg/100 ml) and the levels are much higher than those in oranges, tangerines, or lemons [12]. Studies have also reported that deficiencies of vitamin C may results into hyperlipidaemia and also affects the serum cholesterol levels associated with liver dysfunctions [16–18]. Besides, it also contains flavonoids including quercetin, kaempferol 3 O-a-L (600 methyl) rhamnopyranoside and kaempferol 3 O-a-L (600 ethyl) rhamnopyranoside [19, 20] that are associate with its antioxidant potential. Although a number of reports are available on the acute and chronic toxicity of arsenic, not much is known about the sub chronic toxicity of arsenic on various parameters of blood and liver damage. Present study has therefore been carried out to investigate the hepato protective properties of amla in arsenic induced liver damage and oxidative stress in mice.
Materials and Methods
Approval of Protocol
All the experimental procedures and protocols used in the study are reviewed and approved by the institutional animal ethical committee (Ref No. 121 IAH/pharma-11) of King George’s Medical University, Lucknow which is constituted under committee for purpose of control and supervision of experiments on animals, Government of India. Ethical guidelines were strictly followed during all the experiments.
Experimental Design
Adult, Balb/c mice (25 ± 5 g) was purchased from the animal-breeding colony of Indian Institute of Toxicology Research (IITR), Lucknow. Animal was kept in animal house of King George’s Medical University, Lucknow and used for the study. The animals are housed in clean polypropylene cages at 25 ± 2 °C and fed synthetic pellet basal diet and water ad libitum. Animals were divided into following four groups with five animals in each group. Group I were treated with arsenic as sodium arsenite (dissolved in distilled water, 3 mg/kg body weight, p.o., daily for 30 days). In Group II, mice were treated with ethyl acetate extract of amla (dissolved in distilled water, 500 mg/kg body weight, p.o., daily for 30 days). Group III were simultaneously treated with arsenic and fruit of amla in combination identically as in Groups I and II. In Group IV, mice were treated with distilled water p.o. for the duration of the treatment to serve as controls.
Blood Collection/Tissues Preparation
At the end of the experiment period (30 days), all the animals were sacrificed by cervical dislocation. After heart puncher blood was quickly collected in 10% EDTA tubes for the separation of serum. Liver was isolated immediately and placed in ice cold NaCl (0.15 M) solution, perfused with the same solution to remove blood cell, blotted on the filter paper, quickly weight and homogenized by using biochemical examination. Another portion of liver were kept at −20 °C for metals (As, Ca, Cu, Zn) analysis.
Assay of Serum Hepatic Enzyme
Serum collected from the blood samples was subjected to biochemical estimations of glutamic oxaloacetic transaminase (GOT), glutamic pyruvic transaminase (GPT), total protein and creatinine in serum were measured through fully automated biochemical analyzer (CHEMWELL1520,USA)
Biochemical Parameters
Assay of Hepatic Lipid Peroxidation
Lipid per oxidation was analyzed by the method of Esterbauer and Cheeseman [21]. The extent of lipid peroxidation in terms of thiobarbituric acid reactive substances (TBARS) formation was measured The liver tissue homogenate containing 1 mg protein was mixed with 1 ml TCA (20 %), 2 ml TBA (0.67 %) and heated for 1 h at 100 °C. After cooling, the precipitate was removed by centrifugation. The absorbance of the sample was measured at 535 nm using a blank containing all the reagents except the sample. As 99 % TBARS are malondialdehyde (MDA), so TBARS concentrations of the samples were calculated using the extinction coefficient of MDA, which is 1.56 × 105/M/cm.
Assay of Hepatic Reduced Glutathione
Reduced glutathione (GSH) levels in liver tissue were measured spectrophotometrically according to the method of Ellman [22]. The liver tissue homogenates (720 μl) of all the experimental samples were double diluted and 5% TCA was added to precipitate the protein content. After centrifugation (at 10,000×g for 5 min) the supernatant was taken, DTNB solution (Ellman’s reagent) was added to it and the absorbance was measured at 412 nm. A standard curve was drawn using different known concentrations of GSH solution. With the help of this standard curve, GSH contents were calculated.
Assay of Hepatic Catalase (CAT)
Catalase activity was measured by the method of Bonaventura et al. [23]. The enzyme CAT converts H2O2 into water. About 5 μg protein from the liver homogenate was mixed with 2.1 ml of 7.5 mM H2O2 and a time scan was performed for 10 min at 240 nm at 25 °C. The disappearance of peroxide depending on the CAT activity was observed. One unit of CAT activity is defined as the amount of enzyme, which reduces 1 μmol of H2O2 per minute.
Assay of Hepatic Super Oxide Dismutase (SOD)
SOD activity was measured following the method originally developed by Nishikimi et al. [24]. The liver tissue homogenate containing 5 μg protein was mixed with sodium pyrophosphate buffer, PMT and NBT. The reaction was started by the addition of NADH. Reaction mixture was then incubated at 30 °C for 90 s and stopped by the addition of 1 ml of glacial acetic acid. The absorbance of the chromogen formed was measured at 560 nm. One unit of SOD activity is defined as the enzyme concentration required inhibiting chromogen production by 50 % in 1 min under the assay conditions.
Assay of Hepatic Glutathione-S-transferase (GST)
The cytosolic glutathione-s-transferase activity was determined spectrophotometrically by method of Habig et al. [25]. The reaction mixture contained suitable amount of enzyme, KH2PO4 buffer, EDTA, CDNB and GSH. The reaction was carried out at 37 °C and monitored spectrophotometrically at 340 nm for 5 min. A blank was run in the absence of the enzyme. One unit of GST activity is defined as 1 μmol product formation per minute.
Assay of Hepatic Glutathione Peroxides
The activity of glutathione peroxidase was measured by the procedure described by of Flohe and Gunzler [26]. About 50 μl of sample was mixed with 550 μl of 0.1 M KH2PO4 buffer containing 1 mM EDTA (pH 7.5), 50 μl of 2 mM NaN3, 100 μl of GR (2.4 U/ml) and 100 μl of 10 mM GSH. After 10 min of incubation at 37 °C, 100 μl of 1.5 mM NADPH in water was added to the above mixture. The overall reaction was started by adding 100 μl of pre-warmed (37 °C) 1.5 mM H2O2 and the decrease in absorbance at 340 nm were monitored spectrophotometrically for 5 min. The enzyme activity was calculated using molar extinction coefficient of 6.22 × 103/M/cm. One unit of enzyme activity was defined as the amount of enzyme which catalyzes the oxidation of 1 μmol NADPH per minute.
Assay of Hepatic Metal Estimation
Wet acid digestion procedure for measuring As, Cu, Zn, in liver tissue was carried out using conc. HNO3 and HClO4 (6:1) under low heat to complete carbonization. Known volumes of deionized water were added and filtered. The metals were analyzed on Perkin-Elmer AAS at 228.8 nm for Arsenic, 324.7 nm for Cu and 213.9 nm for Zn. Respectively standard were also used.
Statistical Analysis
Statistical analysis was carried out by one-way analysis of variance (ANOVA) involving Newman-Keuls test for post hoc comparisons. All values have been expressed as mean ± SEM. P value up to < 0.05 has been considered significant.
Results
Effect on Body Weight and Liver Weight in Mice
Exposure to arsenic in mice caused a significant decrease in body weight (19 %) and liver weight (25 %) as compared to controls suggesting the general toxic effect of the arsenic and could be associated with decreased food consumption and water intake (Table 1). Co-treatment with arsenic and amla increased the body weight (12 %) and liver weight (28 %) as compared to mice treated with arsenic alone. No significant effect on body weight and liver weight was observed in mice treated with amla alone as compared to controls (Table 1).
Table 1.
Effect on body weight, liver weight in mice exposed to arsenic, amla and their co-treatment for 30 days
| Parameters | CONT | ARS | AMLA | ARS+AMLA |
|---|---|---|---|---|
| Body weight | 31.4 ± 0.812 | 25.40 ± 1.166*a | 31.20 ± 0.969 | 28.60 ± 1.12*b |
| Liver weight | 1.908 ± 0.047 | 1.41 ± 0.096*a | 1.74 ± 0.065 | 1.81 ± 0.0931*b |
Values are mean ± SEM of five animals in each group
* Significantly differs (p < 0.05)
aCompared to control group
bCompared to arsenic treated group
Effect on the Serum Biochemical Enzyme in Mice
Effect of arsenic and co-treatment of arsenic and amla on mice has been presented in Table 2. Exposure to arsenic in mice caused a significant increase SGOT (58 %) and SGPT (61 %) and no significant change in creatinine and total protein as compared to controls suggesting the hepato toxic effect of the arsenic. Co-treatment with arsenic and amla decreases in SGOT (13 %) and SGPT (10 %) as compared to mice treated with arsenic alone. No significant effect on SGOT and SGPT was observed in mice treated with amla alone as compared to controls (Table 2).
Table 2.
Effect on hepatic marker enzymes on mice exposed to arsenic, amla and their co-treatment for 30 days
| Parameters | CONT | ARS | AMLA | ARS+AMLA |
|---|---|---|---|---|
| SGOT (U/L) | 47.58 ± 2.68 | 75.6 ± 3.37*a | 40.6 ± 4.05 | 65.5 ± 2.51*b |
| SGPT (U/L) | 23.68 ± 1.27 | 35.77 ± 1.49*a | 24.01 ± 0.99 | 32.24 ± 1.91*a,b |
| Creatinine (mg/dl) | 0.41 ± 0.02 | 0.48 ± 0.01*a | 0.40 ± 0.016 | 0.45 ± 0.03 |
| Total protein (g/dl) | 3.47 ± 0.20 | 3.71 ± 0.45 | 3.77 ± 0.37 | 3.57 ± 0.23 |
Values are mean ± SEM of five animals in each group
* Significantly differs (p < 0.05)
aCompared to control group
bCompared to arsenic treated group
Effect on the Lipid Peroxidation in Liver of Mice
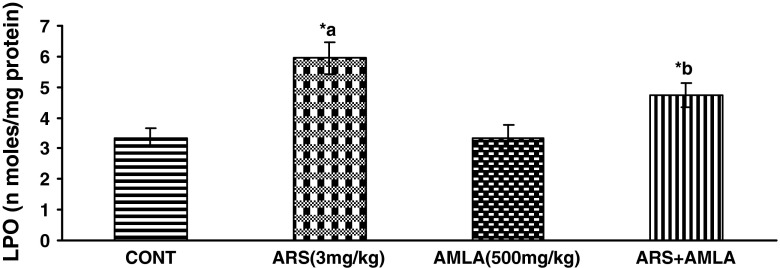
Effect of arsenic and co-treatment of arsenic and amla on mice has been presented in Fig. 1. Exposure to arsenic in mice caused a significant LPO increase (77 %) as compared to controls suggesting the hepato toxic effect of the arsenic. Co-treatment with arsenic and amla decreases in LPO (20 %) as compared to mice treated with arsenic alone. No significant effect on LPO was observed in mice treated with amla alone as compared to controls (Fig. 1).
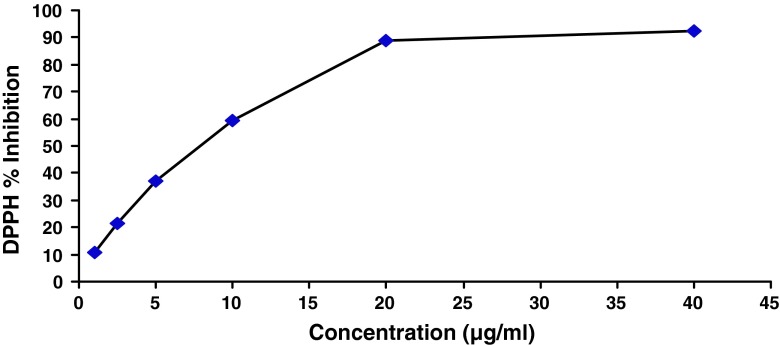
Fig. 1.
DPPH free radical scavenging activity of fruit extract of Emblica officinalis. The concentration of extract above 20 μg/ml showed saturation in the plot indicating the radical scavenging activity of more than 90 %
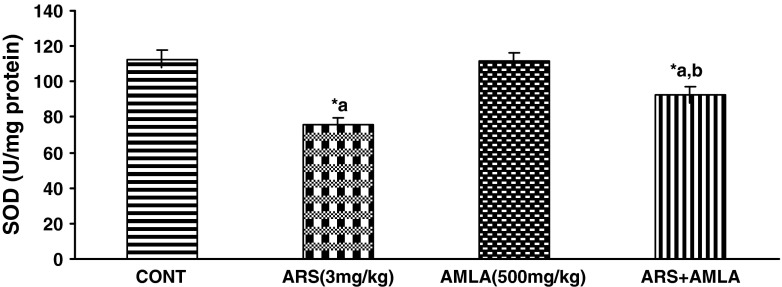
Effect on the Super Oxide Dismutase in Liver of Mice
Effect of arsenic and co-treatment of arsenic and amla on mice has been presented in Fig. 2. Exposure to arsenic in mice caused a significant decrease in SOD (32 %) as compared to controls suggesting the hepato toxic effect of the arsenic. Co-treatment with arsenic and amla increases in SOD (21 %) as compared to mice treated with arsenic alone. No significant effect on SGOT and SGPT was observed in mice treated with amla alone as compared to controls (Fig. 2).
Fig. 2.
Effect of arsenic, amla and their co-treatment on the levels of lipid per oxidation in liver of mice. Values are mean ± SEM of five animals in each group a compared to control group, b compared to arsenic treated group. *Significantly differs (p < 0.05)
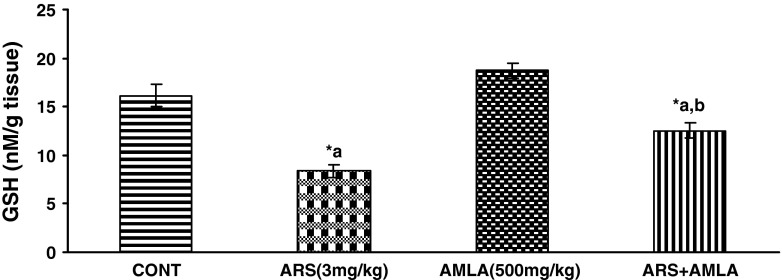
Effect on the GSH Levels in Liver of Mice
Effect of arsenic and co-treatment of arsenic and amla on mice has been presented in Fig. 3. Exposure to arsenic in mice caused a significant decrease in GSH (48 %) as compared to controls suggesting the hepato toxic effect of the arsenic. Co-treatment with arsenic and amla increases in GSH (50 %) as compared to mice treated with arsenic alone. No significant effect on GSH was observed in mice treated with amla alone as compared to controls (Fig. 3).
Fig. 3.
Effect of arsenic, amla and their co-treatment for 30 days on the levels of SOD in liver of mice. Values are mean ± SEM of five animals in each group a compared to control group, b compared to arsenic treated group, *Significantly differs (p < 0.05)
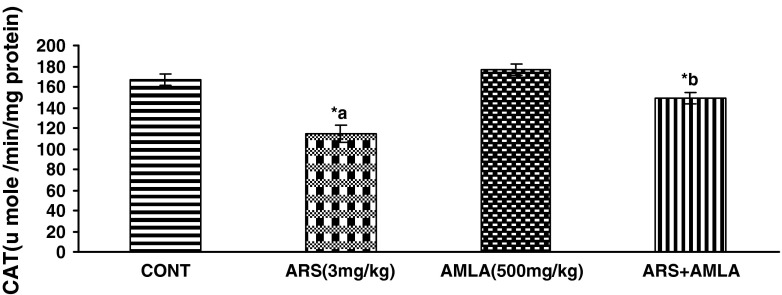
Effect on the Activity of Catalase in Liver of Mice
Effect of arsenic and co-treatment of arsenic and amla on mice has been presented in Fig. 4. Exposure to arsenic in mice caused a significant decrease in catalase (31 %) as compared to controls suggesting the hepato toxic effect of the arsenic. Co-treatment with arsenic and amla increases in catalase (29 %) as compared to mice treated with arsenic alone. No significant effect on catalase was observed in mice treated with amla alone as compared to controls (Fig. 4).
Fig. 4.
Effect of arsenic, amla and their co-treatment for 30 days on the levels of GSH in liver of mice. Values are mean ± SEM of five animals in each group a compared to control group, b compared to arsenic treated group. *Significantly differs (p < 0.05)
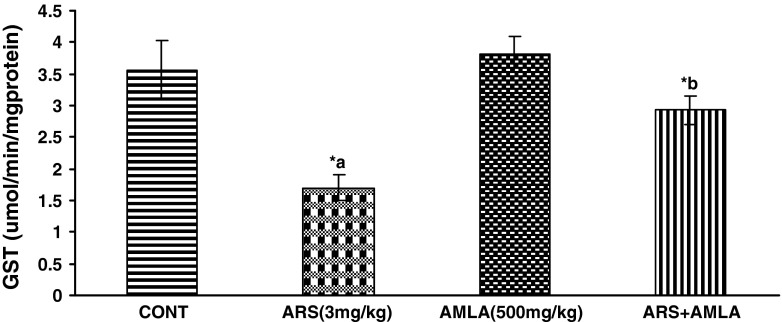
Effect on the Activity of Glutathione-S-transeferase in Liver of Mice
Effect of arsenic and co-treatment of arsenic and amla on mice has been presented in Fig. 5. Exposure to arsenic in mice caused a significant decrease in glutathion-S-transferase (52 %) as compared to controls suggesting the hepato toxic effect of the arsenic. Co-treatment with arsenic and amla increases in glutathion S-transferases (21 %) as compared to mice treated with arsenic alone. No significant effect on glutathion S-transferases was observed in mice treated with amla alone as compared to controls (Fig. 5).
Fig. 5.
Effect of arsenic, amla and their co-treatment for 30 days on the levels of catlase in liver of mice. Values are mean ± SEM of five animals in each group a compared to control group, b compared to arsenic treated group. *Significantly differs (p < 0.05)
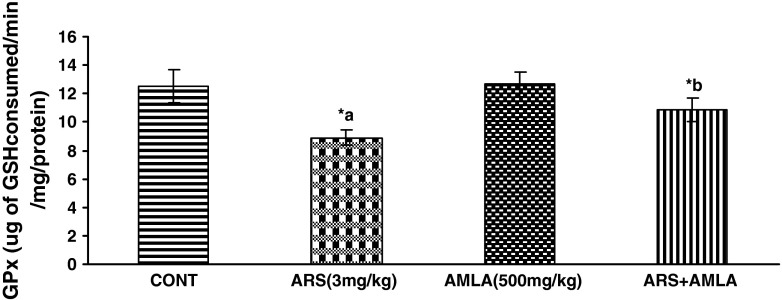
Effect on the Activity of Glutathione Peroxidese in Liver of Mice
Effect of arsenic and co-treatment of arsenic and amla on mice has been presented in Fig. 6. Exposure to arsenic in mice caused a significant decrease in glutathione peroxidese (28 %) as compared to controls suggesting the hepato toxic effect of the arsenic. Co-treatment with arsenic and amla increase in glutathione peroxides (21 %) as compared to mice treated with arsenic alone. No significant effect on glutathione peroxides was observed in mice treated with amla alone as compared to controls (Figs. 6, 7).
Fig. 6.
Effect of arsenic, amla and their co-treatment for 30 days on the levels of GST in liver of mice. Values are mean ± SEM of five animals in each group a compared to control group, b compared to arsenic treated group. *Significantly differs (p < 0.05)
Fig. 7.
Effect of arsenic, amla and their co-treatment for 30 days on the levels of GPx in liver of mice. Values are mean ± SEM of five animals in each group a compared to control group, b compared to arsenic treated group. *Significantly differs (p < 0.05)
Effect on the As, Cu, Zn in Liver of Mice
Exposure to arsenic in mice caused a significant increases hepatic as level in mice (5.3-fold) and Zn (1.3-fold) and no significant change in hepatic Cu as compared to controls suggesting the hepato toxic effect of the arsenic (Table 3). Co-treatment with arsenic and amla decreases in arsenic (2.1-fold) and zinc (1.1-fold) levels as compared to mice treated with arsenic alone. No significant effect on arsenic and micronutrient (Zn, Cu) levels was observed in mice treated with amla alone as compared to controls (Table 3).
Table 3.
Effect of arsenic level in hepatic Cu and Zn on mice exposed to arsenic, amla and their co-treatment for 30 days
| Parameter | CONT | ARS | AMLA | ARS+AMLA |
|---|---|---|---|---|
| As(μg/g) | 0.202 ± .003 | 1.088 ± 0.252*a | 0.016 ± 0.002 | 0.506 ± 178*a,b |
| Cu(μg/g) | 5.368 ± 0.080 | 5.368 ± 0.203 | 5.296 ± 0.190 | 5.45 ± 0.164 |
| Zn(μg/g) | 22.79 ± 0.648 | 30.19 ± 1.039*a | 23.19 ± 0.459 | 25.56 ± 0.681*a,b |
Values are mean ± SEM of five animals in each group
* Significantly differs (p < 0.05)
aCompared to control group
bCompared to arsenic treated group
Discussion
Liver is a versatile organ of the body that regulates the internal chemical environment. Liver injury induced by various hepatotoxins has been recognized as a major toxicological problem for years. Because of its unique metabolic functions and related to the gastrointestinal tract, liver is an important target of toxicity to xenobiotics. Arsenic primarily increased the generation of free radical species and cause an imbalance between pro-oxidation and antioxidant homeostasis in physiological system and cause toxicity due to its attraction towards the sulfhydryl groups of protein and thiols of glutathione. Thus an agent able to reduce the toxic potential of arsenic in liver cell would clearly be a use full compound for arsenical chemotherapy. The liver has long been identified as a target organ of arsenic exposure [27], its importance as an organ arsenic biotransformation is well established [28]. The mechanism by which arsenic directly damages the liver including oxidative stress [29, 30], enhanced inflammation and alteration in cellular methylation status has been investigated [31].
SGOT and SGPT are reliable determinants of liver parenchymal injury. The increment of the activities of SGOT and SGPT in plasma may be mainly due to the leakage of these enzymes from the liver cytosol into the blood stream [31, 32] which gives an indication on the hepato toxic effect of arsenic due to the increased oxidative stress. Thus the association of oxidative stress and biochemical events in apoptosis has bee established and it was confirmed that arsenic trioxide deplete the intracellular GSH and induced apoptosis [33].
The fruit of E. officinalis is a constituent of various liver tonics used against acute viral hepatitis and other liver disorders [34]. Due to multiple pharmacological actions including strong antioxidant effect, fruit of E. officinalis has been suggested to be a good protective herbal agent. The antioxidant activity found in fruit extract of E. officinalis maintain the endogenous antioxidant, thus reduced oxidative stress and alleviate the pathological changes caused by arsenic in liver. Oxidative effects of arsenic have been found to be counteracted by plant extracts and pharmacological agents due to their antioxidant potential and metal binding property [35, 36]. GSH has been considered to be an important intracellular reductant for arsenic methylation and transport [37], which in turn help the removal of arsenic from the body. Depletion of hepatic GSH facilitates accumulation of arsenic in the liver and thus causes oxidative stress.
Recent studies have suggested that the aqueous extract of amla was effective in preventing the invasion of MDAMB-231 cells in the in vitro matrigel invasion assay [38]. The amla phytochemical, kaempferol, inhibited the expression of stromelysin1 (MMP-3) in the MDA-MB-231 breast cancer cell line [39]. The polyphenol gallic acid is also reported to possess inhibitory effects on gastric adenocarcinoma cell migration, decreased expression of MMP-2/9 in vitro [40], It has been shown to exhibit a variety of biological activities including ant-oxidative activity [41]. As it provides a superior source of vitamin C, amla has been particularly indicated for anemia, asthma, bleeding gums, diabetes, cold, chronic lung disease, hyperlipidaemia, yeast infections, scurvy and cancer [42], and inhibits thio acetamide-induced oxidative stress and hyper-proliferation in rat liver [43].
Vitamin C, its act mainly as an antioxidant molecule and beneficial effects could be attributed to its ability to complex with arsenic. Studies have reported that deficiencies of vitamin c may cause hyperlipidaemia and liver dysfunctions [44, 45] Administration with vitamin C a low-molecular mass antioxidant, interacts directly with oxidizing radicals and protect the cell from ROS in vivo and in vitro [46] vitamin C scavenges aqueous ROS by rapid electron transfer that inhibits lipid per oxidation [47]. Co-administration of antioxidant such as vitamins C and E [48] and some essential metal such as zinc [49] during chelating therapy has been found to be beneficial in increasing arsenic mobilization and assisting the recovery of altered biochemical variable, oxidative stress is one of the important mechanisms of arsenic induced toxicity. Micronutrients interact with toxic metals at primary site of action, absorption, transport of toxic metals in the body; binding to target proteins; metabolism, sequestration and excretion of toxic metals and finally in secondary mechanisms of toxicity [50]. Thus it is expected that co-admination of an antioxidant like fruit extract of E. officinalis may be an important component of an effective chelating therapy. The fruit of E. officinalis prevented arsenic induced alterations the hepatic enzyme almost normal. Like vitamin C, fruit of E. officinalis also possesses similar free radical scavenging activity and shows preventive role against arsenic induced hepatic toxicity. These suggest that the essential elements may contribute to the protection of individuals from the effects of heavy metal exposure, while their deficiency may increase toxicity.
Conclusion
In conclusion, we would like to state that fruit extract of E. officinalis plays a protective role against arsenic induced oxidative damage and hepatic toxicity. The mechanistic pathways that the fruit extract of E. officinalis used to exhibit its protective action against that particular toxin is not fully clear except its antioxidant property. Further investigations are necessary to find the active functional group(s) of the compound of E. officinalis.
Acknowledgments
Authors are thankful to the Department of Pharmacology, King George’s Medical University Lucknow for providing research facilities and to the Indian Council of Medical Research (ICMR), New Delhi for providing financial assistance as Senior Research Fellow.
References:
- 1.Brinkel J, Khan MMH, Kraemer A. A systematic review of arsenic exposure and its social and mental health effects with special reference to Bangladesh. Int J Environ Res Public Health. 2009;6:1609–1619. doi: 10.3390/ijerph6051609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yadav RS, Sankhwar ML, Shukla RK, Chandra R, Pant AB, Islam F, et al. Attenuation of arsenic neurotoxicity by curcumin in rats. Toxicol Appl Pharmacol. 2009;240:367–376. doi: 10.1016/j.taap.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 3.Ahamed S, Sengutpa MK, Mukherjee A, Hossain A, Das B, Nayak BA, et al. Arsenic ground water contamination and its health effects in the state of Uttar Pradesh (UP) in upper and middle Ganga plain, India: severe danger. Sci Total Environ. 2006;370:310–322. doi: 10.1016/j.scitotenv.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 4.Kapaj S, Peterson H, Liber K, Bhattacharya P. Human health effects from chronic arsenic poisoning—a review. J Environ Sci Health A. 2006;41:2399–2428. doi: 10.1080/10934520600873571. [DOI] [PubMed] [Google Scholar]
- 5.Klaassen CD. Heavy metals and heavy-metal antagonist. In: Gilman AG, Rall TW, Nies AS, Taylor P, editors. The pharmacological basis of therapeutics. New York: McGraw-Hill; 1996. pp. 1592–1614. [Google Scholar]
- 6.Aposhian HV, Aposhian MM. Newer developments in arsenic toxicity. J Am Coll Toxicol. 1989;8:1297–1305. doi: 10.3109/10915818909009121. [DOI] [Google Scholar]
- 7.WHO, Environmental health criteria 224, arsenic and arsenic compounds. Inter-organization programme for the sound management of chemicals. Geneva 2001.
- 8.Liu J, Liu Y, Goyer RA, Achanzar W, Waalkes MP. Metallothionein-I/II null mice are more sensitive than wild-type mice to the hepatotoxic and nephrotoxic effects of chronic oral or injected inorganic arsenicals. Toxicol Sci. 2000;55:460–467. doi: 10.1093/toxsci/55.2.460. [DOI] [PubMed] [Google Scholar]
- 9.Markovich D, James KM. Heavy metals mercury, cadmium and chromium inhibit the activity of the mammalian liver and kidney sulfate transporter sat-1. Toxicol Appl Pharmacol. 1999;154:181–187. doi: 10.1006/taap.1998.8559. [DOI] [PubMed] [Google Scholar]
- 10.Dash DK, Yeligar VC, Nayak SS, Ghosh T, Rajalingam D, Sengupta P, et al. Evaluation of hepatoprotective and antioxidant activity of Ichnocarpus frutescens (Linn.) R. Br. on paracetamol-induced hepatotoxicity in rats. Trop J Pharm Res. 2007;6:755–765. doi: 10.4314/tjpr.v6i3.14656. [DOI] [Google Scholar]
- 11.Ramirez P, Del Razo LM, Gutierrez-Ruiz MC, Gonsebatt ME. Arsenite induces DNA-protein crosslinks and cytokeratin expression in the WRL-68 human hepatic cell line. Carcinogenesis. 2000;21:701–706. doi: 10.1093/carcin/21.4.701. [DOI] [PubMed] [Google Scholar]
- 12.Khan KH. Roles of Emblica officinalis in medicine—a review. Bot Res Int. 2009;2(4):218–228. [Google Scholar]
- 13.Flora SJS. Arsenic-induced oxidative stress and its reversibility. Free Radical Biol Med. 2011;51:257–281. doi: 10.1016/j.freeradbiomed.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Yadav RS, Sankhwar ML, Shukla RK, Chandravanshi LP, Ansari RW, Shukla PK, et al. Neuroprotective efficacy of curcumin in arsenic induced cholinergic dysfunctions in rats. NeuroToxicology. 2011;32:760–768. doi: 10.1016/j.neuro.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Sharma A, Sharma MK, Kumar M. Modulatory role of emblica officinalis fruit extract against arsenic induced oxidative stress in swiss albino mice. Chemico-Biol interact. 2009;180:20–30. doi: 10.1016/j.cbi.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Sharma P, Pramod J, Sharma PK, Chaturvedi SK, Kothari LK. Effect of vitamin C administration on serum and aortic lipid profile of guinea pigs. Ind J Med Res. 1988;87:283–289. [PubMed] [Google Scholar]
- 17.Kothari LK, Sharma P. Aggravation of cholesterol induced hyperlipidaemia by chronic vitamin C deficiency: an experimental study in guinea pigs. Acta Biologica. 1988;39:49–57. [PubMed] [Google Scholar]
- 18.Kothari LK, Pramod J, Sharma P, Chaturvedi SK. Influence of age and vitamin C status on serum cholesterol. Int J Epidemiol. 1988;17:929–930. doi: 10.1093/ije/17.4.929-a. [DOI] [PubMed] [Google Scholar]
- 19.Habib-ur-Rehman, Yasin KA, Choudhary MA, Khaliq N, Atta-ur-Rahman, Choudhary MI, Malik S. Studies on the chemical constituents of Phyllanthus emblica. Nat Prod Res. 2007;21:775–781. doi: 10.1080/14786410601124664. [DOI] [PubMed] [Google Scholar]
- 20.Krishnaveni M, Mirunalini S. Therapeutic potential of Phyllanthus emblica (amla): the ayurvedic wonder. J Basic Clin Physiol Pharmacol. 2010;21:93–105. doi: 10.1515/jbcpp.2010.21.1.93. [DOI] [PubMed] [Google Scholar]
- 21.Esterbauer H, Cheesman KH. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990;186:407–421. doi: 10.1016/0076-6879(90)86134-H. [DOI] [PubMed] [Google Scholar]
- 22.Ellman GL. Tissue sulfhydryl group. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 23.Bonaventura J, Schroeder WA, Fang S. Human erythrocyte catalase: an improved method of isolation and a revaluation of reported properties. Arch Biochem Biophys. 1972;150:606–617. doi: 10.1016/0003-9861(72)90080-X. [DOI] [PubMed] [Google Scholar]
- 24.Nishikimi M, Rao NA, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulphate and molecular oxygen. Biochem Biophys Res Commun. 1972;46:849–854. doi: 10.1016/S0006-291X(72)80218-3. [DOI] [PubMed] [Google Scholar]
- 25.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 26.Flohe L, Gunzler WA. Assay of glutathione peroxidase. Methods Enzymol. 1984;105:114–121. doi: 10.1016/S0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- 27.Santra A, Maiti A, Das S, Lahiri S, Charkaborty SK, Mazumder DN. Hepatic damage caused by chronic arsenic toxicity in experimental animals. Toxicol Clin Toxicol. 2000;38(4):395–405. doi: 10.1081/CLT-100100949. [DOI] [PubMed] [Google Scholar]
- 28.Rossman TG. Mechanism of arsenic carcinogenesis: an integrated approach. Mutat Res. 2003;533:37–65. doi: 10.1016/j.mrfmmm.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 29.Patrick L. Toxic metal and antioxidants: part II. The role of antioxidant in arsenic and cadmium toxicity. Altern Med Rev. 2003;8:106–128. [PubMed] [Google Scholar]
- 30.Das S, Santra A, Lahiri S, Guha Mazumder DN. Implication of oxidative stress and hepatic cytokine (TNF-alpha and IL-6) response in the pathogenesis of hepatic collagenesis in chronic arsenic toxicity. Toxicol Appl Pharmacol. 2005;204:18–26. doi: 10.1016/j.taap.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 31.Mazumder S, Mukherjee S, Mitra A, Karmakar S, Das AS, Mukherjee M, Nanda A, Mitra C. Folic acid or combined of folic acid and vitamin12 prevent short term arsenic trioxide-induced systemic and mitrochondrial dysfunction and DNA damage. Environ Biophys. 2009;860:277–285. doi: 10.1002/tox.20442. [DOI] [PubMed] [Google Scholar]
- 32.Navarro CM, Montilla PM, Martin A, Jimenez J, Utrilla PM. Free radicals scavenger and anti-hepatotoxic activity of Rosmarinus. Plant Med. 1993;59:312–314. doi: 10.1055/s-2006-959688. [DOI] [PubMed] [Google Scholar]
- 33.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 34.Rehaily AJAI, TAAl Howiriny, Sohaibani MOAI, Rafatullah S. Gastro protective role of (Amla) Emblica-officinalis on in vivo test models in phytomedicine. Phytomedicine. 2002;9:515–522. doi: 10.1078/09447110260573146. [DOI] [PubMed] [Google Scholar]
- 35.Flora SJS, Gupta R. Beneficial effect of Centella asiatica aqueous extract against arsenic induced oxidative stress and essential metal status in rats. Phytother Res. 2007;21:980–988. doi: 10.1002/ptr.2208. [DOI] [PubMed] [Google Scholar]
- 36.Sinha M, Manna P, Sil PC. Protective effect of arjunolic acid against arsenic induced oxidative stress in mouse brain. J Biochem Mol Toxicol. 2008;22(1):15–26. doi: 10.1002/jbt.20209. [DOI] [PubMed] [Google Scholar]
- 37.Carter DE, Aposhian HV, Gandolfi AJ. The metabolism of inorganic arsenic oxides. Gallium arsenide and arsine: a toxicochemical review. Toxicol Appl Pharmacol. 2003;193:309–334. doi: 10.1016/j.taap.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 38.Ngamkitidechakul C, Jaijoy K, Hansakul P, Soonthornchareonnon N, Sireeratawong S. Antitumour effects of Phyllanthus emblica L: induction of cancer cell apoptosis and inhibition of in vivo tumour promotion and in vitro invasion of human cancer cells. Phytother Res. 2010;24:1405–1413. doi: 10.1002/ptr.3127. [DOI] [PubMed] [Google Scholar]
- 39.Phromnoi K, Yodkeeree S, Anuchapreeda S, Limtrakul P. Inhibition of MMP-3 activity and invasion of the MDA-MB-231 human invasive breast carcinoma cell line by bioflavonoids. Acta Pharmacol Sin. 2009;30:1169–1176. doi: 10.1038/aps.2009.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ho HH, Chang CS, Ho WC, Liao SY, Wu CH, Wang CJ. Anti-metastasis effects of gallic acid on gastric cancer cells involves inhibition of NF-kappa B activity and downregulation of PI3K/AKT/small GTPase signals. Food Chem Toxicol. 2010;48:2508–2516. doi: 10.1016/j.fct.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 41.Anila L, Vijayalakshmi NR. Antioxidant action of flavonoid from Mangifera indica and Emblica officanalis in hyper cholesterolemic rats. Foods chem. 2003;83:57–569. [Google Scholar]
- 42.Kim HJ, Yokozawa T, Kim HY, Tohda C, Rao TP, Juneja LR. Influence of Amla (Emblica officinalis) on hypercholesterolemia and lipid peroxidation in cholesterol-fed rats. J Nutr Sci Vitaminol. 2005;51:413–418. doi: 10.3177/jnsv.51.413. [DOI] [PubMed] [Google Scholar]
- 43.Sultana S, Ahmed S, Sharma S, Jahangir T. Emblica officinalis reverses thioacetamide-induced oxidation stress and early promotional events of primary hepatocarcinogenesis. J Pharmacol. 2004;5612:1573–1576. doi: 10.1211/0022357044931. [DOI] [PubMed] [Google Scholar]
- 44.Sharma P, Pramod J, Kothari LK, Ranka R, Sharma S. Hyperlipidaemia in guinea pigs induced by chronic vitamin C deficiency. Ind J Clin Biochem. 1989;4:62–64. doi: 10.1007/BF02867654. [DOI] [Google Scholar]
- 45.Sharma P, Pramod J, Sharma PK, Sapra M, Kothari LK. Effect of vitamin C deficiency and excess on liver: a histopathological and biochemical study in guinea pigs fed normal or high cholesterol diet. Ind J Pathol Microbiol. 1990;33(4):307–313. [PubMed] [Google Scholar]
- 46.Kojo S. Vitamin C: basic metabolism and its function as an index of oxidative stress. Curr Med Chem. 2004;11:1041–1064. doi: 10.2174/0929867043455567. [DOI] [PubMed] [Google Scholar]
- 47.Rana T, Bera A, Das K, Pan S, Bandyopadhyay D, Bhattacharyya S, et al. Effect of ascorbic acid on blood oxidative stress in experimental chronic arsenicosis in rodents. Food Chem Toxicol. 2010;48:1027–1077. doi: 10.1016/j.fct.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 48.Kannan GM, Flora SJS. Chronic arsenic poisoning in the rat: treatment with combined administration of succimers and an antioxidant. Ecotoxicol Environ Saf. 2004;58:37–43. doi: 10.1016/S0147-6513(03)00096-4. [DOI] [PubMed] [Google Scholar]
- 49.Modi M, Kaul RRK, Kannan GM, Flora SJS. Co-administration of zinc and N-acetylcysteine prevents arsenic induced tissue oxidative stress in male rats. J Trace Elem Med Biol. 2005;20:197–204. doi: 10.1016/j.jtemb.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 50.Peraza MA, Ayala-Fierro F, Barber DS, Casarez E, Rael LT. Effects of micronutrients on metal toxicity. Environ Health Perspect. 1998;1:203–216. doi: 10.1289/ehp.98106s1203. [DOI] [PMC free article] [PubMed] [Google Scholar]