Abstract
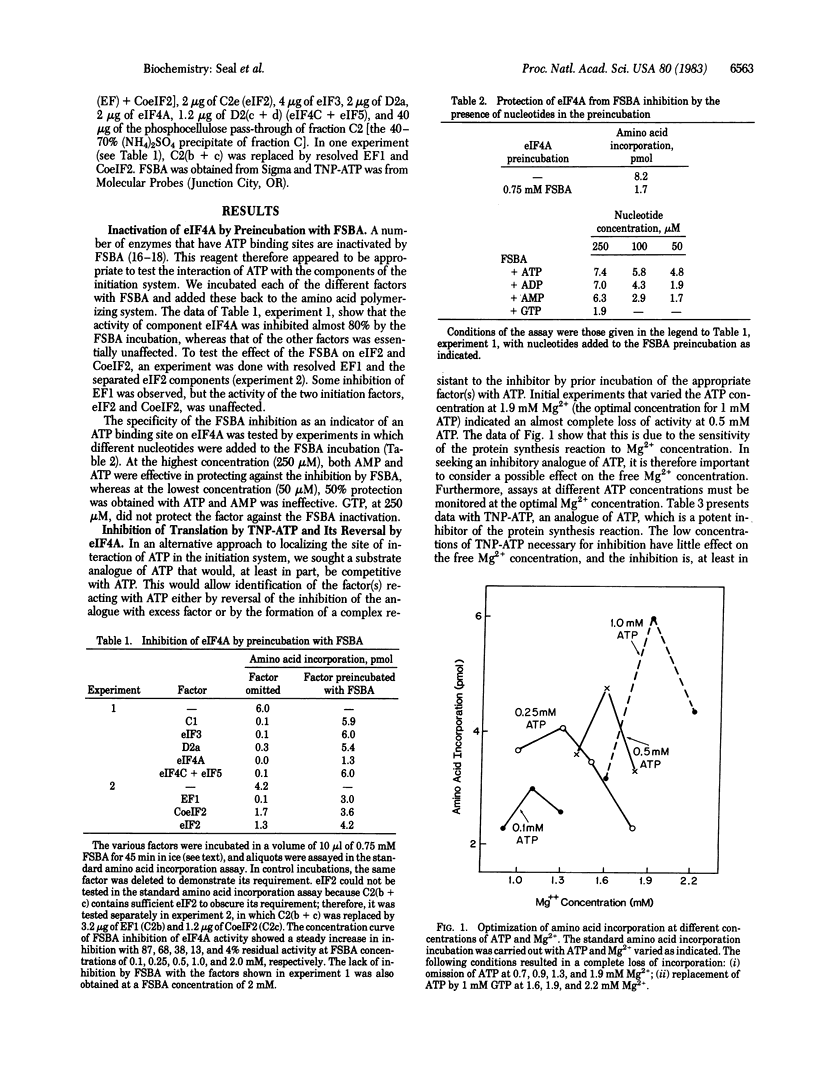
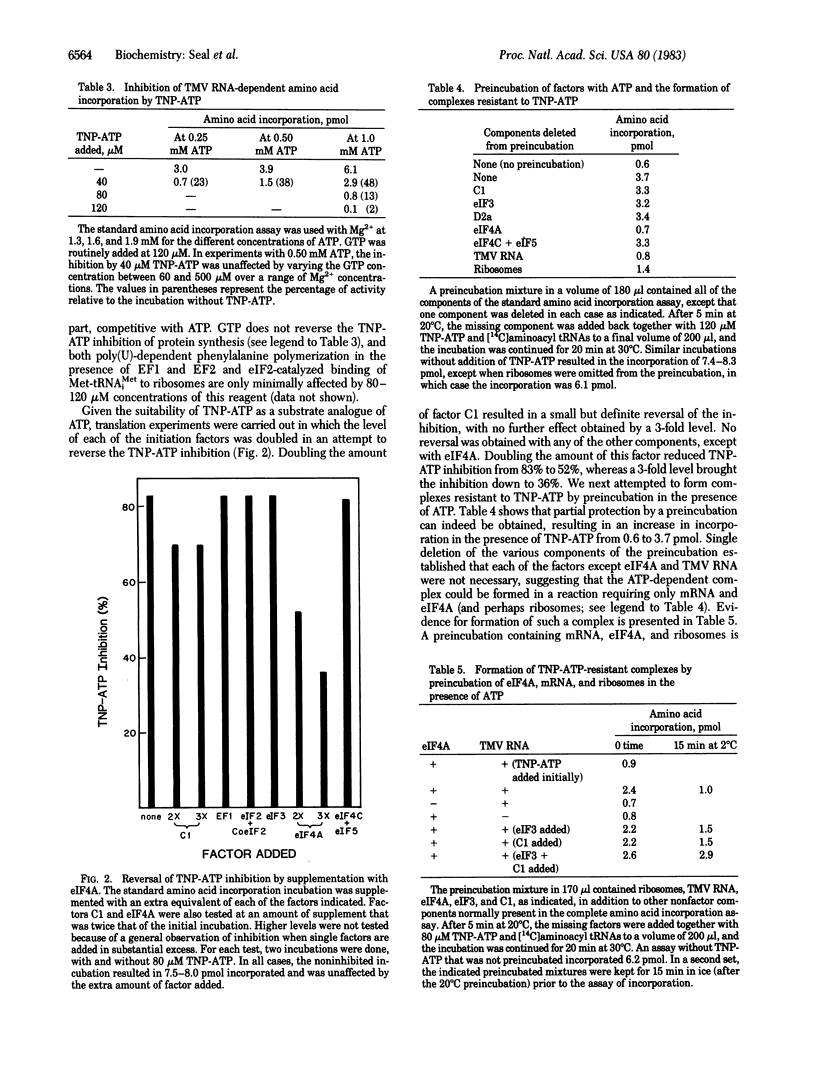
Protein synthesis in a resolved homogenate of wheat germ requires ATP and eight factors functioning at the level of protein chain initiation. To identify the component(s) interacting with ATP, the different factors were treated with the ATP affinity analogue 5'-p-fluorosulfonylbenzoyladenosine (FSBA) and tested for their function in protein synthesis. The activity of eukaryotic initiation factor 4A (eIF4A) was strongly curtailed, whereas all other factors were unaffected. At a concentration of 250 microM, AMP, ADP, and ATP protected eIF4A against FSBA inactivation, whereas at a concentration 50 microM, protection was afforded only by ATP. GTP did not protect at a concentration of 250 microM. In another approach, the substrate analogue 2',3'-O-(2,4,6-trinitrophenyl)adenosine 5'-triphosphate (TNP-ATP) was found to inhibit protein synthesis in a manner, at least in part, competitive with ATP. Supplementing a TNP-ATP inhibited reaction with eIF4A substantially reversed the inhibition. Except for a small effect by factor C1, no reversal was obtained with any other component. Finally, a preincubation of ribosomes with ATP, mRNA, and eIF4A resulted in the formation of a complex capable of TNP-ATP-resistant amino acid incorporation. These data are interpreted to indicate that the primary interaction of ATP is with eIF4A. A model is proposed reconciling this conclusion with other observations relevant to the mRNA . ribosome attachment reaction.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boettcher B. R., Meister A. Covalent modification of the active site of carbamyl phosphate synthetase by 5'-p-fluorosulfonylbenzoyladenosine. Direct evidence for two functionally different ATP-binding sites. J Biol Chem. 1980 Aug 10;255(15):7129–7133. [PubMed] [Google Scholar]
- Colman R. F., Pal P. K., Wyatt J. L. Adenosine derivatives for dehydrogenases and kinases. Methods Enzymol. 1977;46:240–249. doi: 10.1016/s0076-6879(77)46027-0. [DOI] [PubMed] [Google Scholar]
- Esch F. S., Allison W. S. Identification of a tyrosine residue at a nucleotide binding site in the beta subunit of the mitochondrial ATPase with p-fluorosulfonyl[14C]-benzoyl-5'-adenosine. J Biol Chem. 1978 Sep 10;253(17):6100–6106. [PubMed] [Google Scholar]
- Grubmeyer C., Penefsky H. S. The presence of two hydrolytic sites on beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1981 Apr 25;256(8):3718–3727. [PubMed] [Google Scholar]
- Herson D., Schmidt A., Seal S., Marcus A., van Vloten-Doting L. Competitive mRNA translation in an in vitro system from wheat germ. J Biol Chem. 1979 Sep 10;254(17):8245–8249. [PubMed] [Google Scholar]
- Kozak M. Role of ATP in binding and migration of 40S ribosomal subunits. Cell. 1980 Nov;22(2 Pt 2):459–467. doi: 10.1016/0092-8674(80)90356-6. [DOI] [PubMed] [Google Scholar]
- Lee K. A., Guertin D., Sonenberg N. mRNA secondary structure as a determinant in cap recognition and initiation complex formation. ATP-Mg2+ independent cross-linking of cap binding proteins to m7I-capped inosine-substituted reovirus mRNA. J Biol Chem. 1983 Jan 25;258(2):707–710. [PubMed] [Google Scholar]
- Marcus A. Tobacco mosaic virus ribonucleic acid-dependent amino acid incorporation in a wheat embryo system in vitro. Analysis of the rate-limiting reaction. J Biol Chem. 1970 Mar 10;245(5):955–961. [PubMed] [Google Scholar]
- Morgan M. A., Shatkin A. J. Initiation of reovirus transcription by inosine 5'-triphosphate and properties of 7-methylinosine-capped, inosine-substituted messenger ribonucleic acids. Biochemistry. 1980 Dec 23;19(26):5960–5966. doi: 10.1021/bi00567a003. [DOI] [PubMed] [Google Scholar]
- Nygård O., Westermann P. Specific interaction of one subunit of eukaryotic initiation factor eIF-3 with 18S ribosomal RNA within the binary complex, eIF-3 small ribosomal subunit, as shown by cross-linking experiments. Nucleic Acids Res. 1982 Feb 25;10(4):1327–1334. doi: 10.1093/nar/10.4.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier M. H., Erni B., Staehelin T. Initiation of mammalian protein synthesis. I. Purification and characterization of seven initiation factors. J Mol Biol. 1977 Nov;116(4):727–753. doi: 10.1016/0022-2836(77)90268-6. [DOI] [PubMed] [Google Scholar]
- Seal S. N., Schmidt A., Marcus A. A heat-stable protein synthesis initiation factor from wheat germ. J Biol Chem. 1982 Aug 10;257(15):8634–8637. [PubMed] [Google Scholar]
- Seal S. N., Schmidt A., Marcus A. Fractionation and partial characterization of the protein synthesis system of wheat germ. I. Resolution of two elongation factors and five initiation factors. J Biol Chem. 1983 Jan 25;258(2):859–865. [PubMed] [Google Scholar]
- Seal S. N., Schmidt A., Marcus A. Fractionation and partial characterization of the protein synthesis system of wheat germ. II. Initiation factors D1 (eucaryotic initiation factor 3), D2c (eucaryotic initiation factor 5), and D2d (eucaryotic initiation factor 4C). J Biol Chem. 1983 Jan 25;258(2):866–871. [PubMed] [Google Scholar]
- Sonenberg N., Guertin D., Cleveland D., Trachsel H. Probing the function of the eucaryotic 5' cap structure by using a monoclonal antibody directed against cap-binding proteins. Cell. 1981 Dec;27(3 Pt 2):563–572. doi: 10.1016/0092-8674(81)90398-6. [DOI] [PubMed] [Google Scholar]
- Sonenberg N., Shatkin A. J. Reovirus mRNA can be covalently crosslinked via the 5' cap to proteins in initiation complexes. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4288–4292. doi: 10.1073/pnas.74.10.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachsel H., Erni B., Schreier M. H., Staehelin T. Initiation of mammalian protein synthesis. II. The assembly of the initiation complex with purified initiation factors. J Mol Biol. 1977 Nov;116(4):755–767. doi: 10.1016/0022-2836(77)90269-8. [DOI] [PubMed] [Google Scholar]
- Walton G. M., Gill G. N. Nucleotide regulation of a eukaryotic protein synthesis initiation complex;. Biochim Biophys Acta. 1975 May 1;390(2):231–245. doi: 10.1016/0005-2787(75)90344-5. [DOI] [PubMed] [Google Scholar]
- Walton G. M., Gill G. N. Regulation of ternary (Met-tRNAf - GTP - eukaryotic initiation factor 2) protein synthesis initiation complex formation by the adenylate energy charge. Biochim Biophys Acta. 1976 Jan 19;418(2):195–203. doi: 10.1016/0005-2787(76)90069-1. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Inesi G. The use of 2',3'-O-(2,4,6-trinitrophenyl) adenosine 5'-triphosphate for studies of nucleotide interaction with sarcoplasmic reticulum vesicles. J Biol Chem. 1982 Oct 10;257(19):11510–11516. [PubMed] [Google Scholar]


