Abstract
Species from Entoloma subg. Entoloma are commonly recorded from both the Northern and Southern Hemispheres and, according to literature, most of them have at least Nearctic-Palearctic distributions. However, these records are based on morphological analysis, and studies relating morphology, molecular data and geographical distribution have not been reported. In this study, we used phylogenetic species recognition criteria through gene genealogical concordance (based on nuclear ITS, LSU, rpb2 and mitochondrial SSU) to answer specific questions considering species limits in Entoloma subg. Entoloma and their geographic distribution in Europe, North America and Australasia. The studied morphotaxa belong to sect. Entoloma, namely species like the notorious poisonous E. sinuatum (E. lividum auct.), E. prunuloides (type-species of sect. Entoloma), E. nitidum and the red-listed E. bloxamii. With a few exceptions, our results reveal strong phylogeographical partitions that were previously not known. For example, no collection from Australasia proved to be conspecific with the Northern Hemisphere specimens. Almost all North American collections represent distinct and sister taxa to the European ones. And even within Europe, new lineages were uncovered for the red-listed E. bloxamii, which were previously unknown due to a broad morphological species concept. Our results clearly demonstrate the power of the phylogenetic species concept to reveal evolutionary units, to redefine the morphological limits of the species addressed and to provide insights into the evolutionary history of key morphological characters for Entoloma systematics. New taxa are described, and new combinations are made, including E. fumosobrunneum, E. pseudoprunuloides, E. ochreoprunuloides and E. caesiolamellatum. Epitypes are selected for E. prunuloides and E. bloxamii. In addition, complete descriptions are given of some other taxa used in this study for which modern descriptions are lacking, viz. E. subsinuatum, E. whiteae, E. flavifolium, E. luridum, E. bloxamii, E. madidum, E. corneri, E. callidermum and E. coeruleoviride.
Keywords: biodiversity, DNA barcoding, Entolomataceae, fungal biogeography, molecular systematics, phylogenetic species
INTRODUCTION
The introduction of phylogenetic methods in systematic studies and the refinement of species concepts have reshaped our understanding of species limits, their relationships and distribution patterns. In the past decade many studies have been published from several genera spanning a variety of trophic guilds of macrofungi, such as ectomycorrhizal (ECM) (e.g., Geml et al. 2008, Li et al. 2009), saprobic (e.g., Hibbett 2001, Geml et al. 2005a, Moncalvo & Buchanan 2008), parasitic (e.g., Coetzee et al. 2000, Mueller et al. 2001) and lichenised fungi (e.g., Kroken & Taylor 2001, Geml et al. 2012), showing the utility of phylogenetics to establish objective limits to species and their phylogeography. However, so far this has not been done in the large and morphologically diverse genus Entoloma.
The genus Entoloma s.l. (Entolomataceae, Agaricales, Basidiomycota) contains c. 1 500 described species globally (Kirk et al. 2008, Noordeloos & Gates 2012). In traditional morphology-based systematics, some authors interpret the genus as one species-rich entity with extensive infrageneric classification (e.g., Romagnesi 1974, 1978, Noordeloos 1992, 2004), while others recognize and segregate multiple genera from it (e.g., Largent & Baroni 1988, Largent 1994, Baroni & Matheny 2011, Baroni et al. 2011). Most of these genera, however, were the same entities as the infrageneric taxa of the broad-concept Entoloma. The recent study of Co-David et al. (2009) addressed the molecular phylogeny of the Entolomataceae, using 3 independent genetic markers, shed phylogenetic light in this dispute and showed that Entoloma s.l. is monophyletic with the inclusion of Rhodogaster and Richoniella, the sequestrate taxa of the Entolomataceae. Furthermore, this study pointed out that most of the supraspecific groups within Entoloma s.l. defined based on morphological characters (e.g., Noordeloos 1992, 2004, Largent 1994) are not monophyletic, with few exceptions (e.g., subg. Pouzarella), placing in relevance the key role of phylogenetic data to study the systematics in this group.
Entoloma has a worldwide distribution, ranging from arctic regions to the tropics (Horak 1980, Noordeloos & Gulden 1989, Noordeloos 2004, Manimohan et al. 2006, Noordeloos & Gates 2012), and species from Entoloma subg. Entoloma are commonly recorded from both the Northern and Southern Hemispheres and, according to several authors (e.g., Miller & Farr 1975, Horak 1980, Noordeloos 2004, Baroni et al. 2011), most of them have, at least, a Nearctic-Palearctic distribution. Diagnostic morphological features are usually found in basidioma habit and colours, pileus hygrophaneity, spore shape and size and the type of structure and pigmentation of the pileipellis. Only recently molecular studies have been performed in Entoloma systematics (Co-David et al. 2009, Kasuya et al. 2010, Baroni et al. 2011, Baroni & Matheny 2011, Xiao-Lan et al. 2012), however, none of these studies focused on biogeographical distribution patterns, and knowledge of their actual distribution still remains hindered by uncertainties in morphological species recognition and lack of taxon sampling.
In this study most sections of E. subg. Entoloma are represented, but the main focus is on species of E. sect. Entoloma (sensu Noordeloos 2004) that have been recorded from a wide range of geographical regions. The selection includes:
1) Entoloma sinuatum (Bull.: Fr.) P. Kumm., a notorious poisonous ECM species (Agerer 1997) described initially from Europe and commonly recorded also from North America (e.g., Graham 1944, Roody 2003, Matheny et al. 2006);
2) Entoloma bloxamii (Berk. & Broome) Sacc., a taxon with purple-blue coloured tricholomatoid basidioma, that was initially described from Europe and is also commonly recorded in North America (e.g., Hesler 1967, Largent 1994, Roody 2003, Baroni & Matheny 2011). Entoloma bloxamii is included in the red list of European fungi (Dahlberg & Croneborg 2006). Similar species have been collected in Australasia and are also included in this study. This complex also comprises similar taxa without blue pigments, viz. E. rubellum.
3) Entoloma prunuloides (Fr.: Fr.) Quél., the type species of the subgenus, that was initially described from Europe and often reported from North America (e.g., Largent 1994, Matheny et al. 2006). The infraspecific variety E. prunuloides var. obscurum Arnolds & Noordel. is also included.
4) Entoloma nitidum Quél., an ECM fungus (Montecchio et al. 2006) initially described from Europe and recorded from North America and Australasia (Miller & Farr 1975, Horak 1980, Baroni et al. 2011).
The focus of this study is on the systematics and phylogeography of taxa in E. section Entoloma and aims 1) to delimit phylogenetic species in these presumed species complexes and define their biogeography with molecular phylogenetic methods across three major geographical regions (Europe, North America and Australasia); and 2) to determine which morphological characters are useful for species circumscription.
MATERIAL AND METHODS
Isolates and DNA extraction
Sixty-one collections were analysed from the four morphospecies (and possible species complexes), representing different regions of putative distribution. The collections included in this study, their geographic origin, collection reference and public database accession number are summarized in Table 1. DNA extraction protocols have been described previously (Co-David et al. 2009).
Table 1.
Species used in the phylogenetic analyses. The sequences with KC in the accession number were generated for this study, the remaining were downloaded from GenBank and UNITE database. Unless otherwise indicated, the voucher specimens are deposited in L. Leg.: EU: Europe; AA: Australasia; NA: North America.
| Phylogram reference | Species | Public database accession number |
Collection reference | Remarks | Geographic origin of collection | |||
|---|---|---|---|---|---|---|---|---|
| mtSSU | rpb2 | LSU | ITS | |||||
| Clitopilus hirneolus 263 | Clitopilus hirneolus | GQ289352 | GQ289278 | GQ289211 | KC710132 | MEN 199956 | Italy, EU | |
| Clitopilus fallax 37 | Clitopilus fallax | GQ289350 | GQ289276 | GQ289210 | MEN 200367 | Slovakia, EU | ||
| Clitopilus nitellinus 400 | Clitopilus nitellinus | GQ289355 | GQ289282 | GQ289215 | MEN 200435 | Austria, EU | ||
| E. albidum 620 | E. albidum | KC710180 | KC710151 | KC710102 | Y. Lamoureux 3218 (CMMF) | Québec, Canada, NA | ||
| E. albomagnum 427 | E. albomagnum | KC710165 | KC710137 | KC710065 | G. Gates E2030 | Tasmania, AA | ||
| E. alcedicolor 210 | E. alcedicolor | GQ289292 | GQ289224 | GQ289152 | KC710123 | E. Arnolds 02-760276 (holotype) | The Netherlands, EU | |
| E. araneosum 14 | E. araneosum | GQ289293 | GQ289225 | GQ289153 | KC710056 | MEN 200314 | Belgium, EU | |
| E. baronii L644 | E. baronii | KC710093 | G. Gates E2292 (holotype) | Tasmania, Australia, AA | ||||
| E. bloxamii 219 | E. bloxamii | GQ289294 | GQ289226 | GQ289154 | KC710087 | MEN 200442 | Austria, EU | |
| E. bloxamii 8003 | E. bloxamii | KC710083 | MEN 02165 | Italy, EU | ||||
| E. bloxamii 171211 | E. bloxamii | KC710077 | LNM 171211 | Portugal, EU | ||||
| E. bloxamii L646 | E. bloxamii | KC710098 | S. Baireuther, 1-10-2010 | Germany, EU | ||||
| E. bloxamii UDB003233 | E. bloxamii | UDB003233 | TU101448 | Estonia, EU | ||||
| E. bloxamii v. rubellum 13 | E. bloxamii v. rubellum | KC710082 | J.C.Vegeau, 01-10-2000 | France, EU | ||||
| E. bloxamii v. rubellum 14 | E. bloxamii v. rubellum | KC710068 | M.Maletii, 28-10-1998 | Italy, EU | ||||
| E. bloxamii v. rubellum 619 | E. bloxamii v. rubellum | KC710166 | KC710066 | G. Wölfel E20/04 | Germany, EU | |||
| E. bloxamii v. rubellum L645 | E. bloxamii v. rubellum | KC710070 | S.Baireuther, 1-10-2001 | Germany, EU | ||||
| E. bloxamii-NA 628 | Entoloma sp. nov. 1 | KC710189 | KC710159 | E.C. Vellinga | California, USA, NA | |||
| E. bloxamii-NA 53901 | Entoloma sp. nov. 1 | KC710168 | KC710139 | KC710071 | H.D.Thiers 53901 (NY) | California, USA, NA | ||
| E. bloxamii-NA OSC 1064185 | Entoloma sp. nov. 1 | EU526002 | OSC1064185 | GenBank ID: E. madidum | Oregon, USA, NA | |||
| E. bloxamii-NA TJB7876 | Entoloma sp. nov. 1 | KC710081 | TJB7876 (CORT) | California, NA | ||||
| E. bloxamii-NA UBC F16248 | Entoloma sp. nov. 1 | EF530938 | UBCF16248 | GenBank ID: E. bloxamii | Canada, NA | |||
| E. caccabus 17 | E. caccabus | GQ289295 | GQ289227 | GQ289155 | KC710063 | MEN 200324 | Belgium, EU | |
| E. caesiolamellatum 626 | E. caesiolamellatum | KC710187 | KC710157 | KC710126 | Wölfel, 20.2.2000 (holotype) | Canary Islands, Spain, EU | ||
| E. callidermum 512 | E. callidermum | KC710183 | KC710153 | KC710115 | Stubbe 06252 (GENT) | Malaysia, AA | ||
| E. clypeatum 41 | E. clypeatum | KC710164 | KC710136 | KC710059 | MEN 198302 | The Netherlands, EU | ||
| E. coeruleogracilis 214 | E. coeruleogracilis | GQ289307 | GQ289238 | GQ289167 | KC710107 | MEN 2004055 | Tasmania, Australia, AA | |
| E. coeruleogracilis E1777 | E. coeruleogracilis | GQ289308 | GQ289239 | GQ289168 | KC710069 | G. Gates E1777 | GenBank ID: E. haastii | Tasmania, Australia, AA |
| E. coeruleoviride 609 | E. coeruleoviride | KC710162 | KC710134 | KC710057 | Stubbe 06236 (GENT) | Malaysia, AA | ||
| E. conferendum 6 | E. conferendum | GQ289300 | GQ289231 | GQ289160 | MEN 200313 | Belgium, EU | ||
| E. conferendum 30 | E. conferendum | KC710161 | KC710191 | KC710133 | KC710055 | MEN 200330 | Slovakia, EU | |
| E. corneri 607 | E. corneri | KC710163 | KC710135 | KC710058 | D. Stubbe 06241 (GENT) | Malaysia, AA | ||
| E. cretaceum 213 | E. cretaceum | GQ289302 | GQ289233 | GQ289162 | KC710064 | G. Gates E1181 (holotype) | Tasmania, AA | |
| E. cretaceum 2010039 | E. cretaceum | KC710090 | MEN 2010039 | Newfoundland, Canada, NA | ||||
| E. cretaceum 2011022 | E. cretaceum | KC710074 | MEN 2011022 | Tasmania, AA | ||||
| E. flavifolium 621 | E. flavifolium | KC710179 | KC710150 | KC710097 | Y. Lamoureux 2846 (CMMF) | Québec, Canada, NA | ||
| E. flavifolium TB6215 | Entoloma aff. flavifolium | GU384644 | AF261301 | TB 6215 | GenBank ID: E. flavifolium | USA, NA | ||
| E. fumosobrunneum 2005113 | E. fumosobrunneum | KC710185 | KC710155 | KC710124 | MEN2005113 | Newfoundland, Canada, NA | ||
| E. fumosobrunneum 2005120 | E. fumosobrunneum | KC710186 | KC710156 | KC710125 | MEN2005120 (holotype) | Newfoundland, Canada, NA | ||
| E. gelatinosum E792 | E. gelatinosum | GQ289305 | GQ289236 | GQ289165 | KC710103 | G. Gates E792 | Tasmania, Australia, AA | |
| E. gracilior 2011043 | E. gracilior | KC710079 | MEN 2011043 | Tasmania, AA | ||||
| E. gracilior E1220 | E. gracilior | GQ289309 | GQ289240 | GQ289169 | KC710112 | G. Gates E1220 | Tasmania, Australia, AA | |
| E. aff. griseoluridum 15 | Entoloma aff. griseopruinatum | KC710118 | LNM 221111 | Portugal, EU | ||||
| E. haastii 126 | E. haastii | KC710173 | KC710144 | KC710086 | MEN 2004055/53 | Tasmania, Australia, AA | ||
| E. haastii 190506 | E. haastii | KC710174 | KC710145 | KC710089 | MEN 2006617 | New Zealand, AA | ||
| E. haastii 2010034 | E. haastii | KC710113 | MEN 2010034 | Tasmania, Australia, AA | ||||
| E. haastii 2011045 | E. haastii | KC710101 | MEN 2011045 | Tasmania, Australia, AA | ||||
| E. indigoticoumbrinum 83 | E. indigoticoumbrinum | GQ289311 | GQ289242 | GQ289171 | MEN 200406 | Tasmania, Australia, AA | ||
| E. kermandii 222 | E. kermandii | GQ289313 | GQ289244 | GQ289173 | G. Gates E227(holotype) | Tasmania, Australia, AA | ||
| E. kermandii L703 | E. kermandii | KC710075 | G. Gates E227 (isotype) | Tasmania, AA | ||||
| E. lividoalbum 233 | E. lividoalbum | KC710182 | KC710152 | KC710114 | MEN 200328 | Belgium, EU | ||
| E. luridum 634 | E. luridum | KC710170 | KC710141 | KC710080 | MEN 2005634 | Newfoundland, Canada, NA | ||
| E. luridum 3952 | E. luridum | KC710177 | KC710148 | KC710094 | Y. Lamoureux 3952 (CMMF) | Québec, Canada, NA | ||
| E. luridum 2005108 | E. luridum | KC710175 | KC710192 | KC710146 | KC710091 | MEN 2005108 | Newfoundland, Canada, NA | |
| E. madidum 221 | E. madidum | KC710188 | KC710158 | KC710127 | MEN 2004030 | The Netherlands, EU | ||
| E. madidum 985 | E. madidum | JF907990 | 985 | Italy, EU | ||||
| E. madidum 1702 | E. madidum | KC710131 | E.C. Vellinga & | The Netherlands, EU | ||||
| E. madidum 2165 | E. madidum | KC710129 | MEN 2165 | Italy, EU | ||||
| E. madidum 67195 | E. madidum | KC710130 | 67195 (O) | Norway, EU | ||||
| E. manganaense 215 | E. manganaense | KC710172 | KC710143 | KC710085 | G. Gates E369 (isotype) | Tasmania, Australia, AA | ||
| E. myrmecophilum 231 | E. myrmecophilum | GQ289314 | GQ289245 | GQ289174 | KC710120 | G. Tjallingii-Beukers 1981-10-30 | The Netherlands, EU | |
| E. nitidum 24 | E. nitidum | GQ289315 | GQ289246 | GQ289175 | KC710122 | MEN 200324 | Slovakia, EU | |
| E. nitidum 287 | E. nitidum | JF907989 | 287 | Italy, EU | ||||
| E. nitidum 8376 | E. nitidum | KC710076 | MEN 8376 | Scotland, EU | ||||
| E. nitidum 2006201 | E. nitidum | KC710100 | Hausknecht 2006201 | Austria, EU | ||||
| E. nitidum F14054UBC | E. nitidum | AF335449 | F14054(UBC) | Canada, NA | ||||
| E. nitidum F14288UBC | E. nitidum | AY228340 | F14288 (UBC) | Canada, NA | ||||
| E. nitidum TB7526 | E. nitidum | GU384655 | GU384626 | TB 7526 | New York, USA, NA | |||
| E. nitidum UDB011837 | E. nitidum | UDB011837 | TU106631 | Estonia, EU | ||||
| E. ochreoprunuloides 5 | E. ochreoprunuloides | KC710106 | D. Harries, 3-10-2010 | UK, EU | ||||
| E. ochreoprunuloides 632 | E. ochreoprunuloides | KC710176 | KC710147 | KC710092 | E. Arnolds 01-142 (holotype) | Germany, EU | ||
| E. ochreoprunuloides 15721 | E. ochreoprunuloides | KC710111 | R.A. Maas-Geesteranus 15721 | Corsica, France, EU | ||||
| E. ochreoprunuloides v. hyacinthinum 6 | E. ochreoprunuloides f. hyacinthinum | KC710105 | D. Harries, 25-9-2010 | UK, EU | ||||
| E. perbloxamii 71 | E. perbloxamii | GQ289318 | GQ289249 | GQ289178 | KC710117 | MEN 2004071 (holotype) | Tasmania, Australia, AA | |
| E. perbloxamii 2010037 | E. perbloxamii | KC710095 | MEN 2010037 | Tasmania, Australia, AA | ||||
| E. prunuloides 11 | E. prunuloides | KC710099 | R. Kristiansen, 27-7-1980 | Norway, EU | ||||
| E. prunuloides 40 | E. prunuloides | GQ289324 | GQ289255 | GQ289184 | KC710073 | MEN 200340 | Slovakia, EU | |
| E. prunuloides 99202 | E. prunuloides | KC710072 | MEN 99202 | UK, EU | ||||
| E. prunuloides UDB011485 | E. prunuloides | UDB011485 | TU106063 | Estonia, EU | ||||
| E. pseudoprunuloides 627 | E. pseudoprunuloides | KC710169 | KC710140 | KC710078 | MEN 2005115 | Newfoundland, Canada, EU | ||
| E. sinuatum 10 | E. sinuatum | KC710110 | MEN 09-10-2001 | Austria, EU | ||||
| E. sinuatum 45 | E. sinuatum | KC710108 | A.Reijenders 45 | The Netherlands, EU | ||||
| E. sinuatum 50 | E. sinuatum | GQ289333 | GQ289264 | GQ289193 | KC710109 | J.Wisman 2003-09-19 | The Netherlands, EU | |
| E. sinuatum 182 | E. sinuatum | KC710184 | KC710154 | KC710116 | J.Vauras 8181F (TUR) | Finland, EU | ||
| E. sinuatum H6003960 | E. sinuatum | GU373512 | H:6003960 | Finland, EU | ||||
| E. sordidulum 1 | E. sordidulum | GQ289334 | GQ289265 | GQ289194 | KC710062 | Co-David 2003 | Belgium, EU | |
| E. sordidulum L700 | E. sordidulum | KC710119 | LNM 191211 | Portugal, EU | ||||
| E. sp. 128736 | Entoloma sp. 2 | EU784208 | RBG Kew K(M)128736 | GenBank ID: E. bloxamii | UK, EU | |||
| E. sp. AFTOL ID 524 | Entoloma aff. subsinuatum | AY691891 | DQ486700 | TB 5349 | GenBank ID: E. sinuatum | USA, NA | ||
| E. sp. BHS2009 07 | Entoloma sp. 1 | BHS2009-07 | BHS2009-07 | GenBank ID: E. sinuatum | Massachusetts, USA, NA | |||
| E. sp. BY21 | Entoloma sp. 6 | AF261309 | BY21 | GenBank ID: E. haastii | Texas, USA, NA | |||
| E. sp. MLS007 | Entoloma sp. 1 | GQ397994 | MLS007 | GenBank ID: E. sinuatum | Massachusetts, USA, NA | |||
| E. sp. TB5034 | Entoloma aff. subsinuatum | AF261294 | TB 5034 | GenBank ID: E. lividum | USA, NA | |||
| E. sp. TB6117 | E. caesiolamellatum | AF261289 | KC710128 | TB 6117 E. Weeda1702 | GenBank ID: E. bloxamii | California, USA, NA | ||
| E. sp. TB6807 | Entoloma aff. subsinuatum | AF261295 | TB 6807 | GenBank ID: E. eulividum | USA, NA | |||
| E. sp. TJB4765 | Entoloma aff. whiteae | DQ385883 | DQ206983 | TJB4765 | GenBank ID: E. prunuloides isolate AFTOL-ID523 | New York, USA, NA | ||
| E. sp. TM02 134 | Entoloma sp. 4 | EU522735 | TM 02 134 | GenBank ID: E. sinuatum | Canada, NA | |||
| E. sp. TM02 335 | Entoloma sp. 3 | EU522771 | TM 02 335 | GenBank ID: E. sinuatum | Canada, NA | |||
| E. sp. TM03 429 | Entoloma sp. 5 | EU522811 | TM 03 429 | GenBank ID: E. sinuatum | Canada, NA | |||
| E. sp. TRTC156542 | Entoloma aff. myrmecophilum | JN021020 | TRTC156542 | Québec, Canada, NA | ||||
| E. sp. TRTC156546 | Entoloma aff. myrmecophilum | JN021019 | TRTC156546 | Quebec, NA | ||||
| E. sphagneti 209 | E. sphagneti | GQ289335 | GQ289195 | KC710061 | C. Bas 6.86 | The Netherlands, EU | ||
| E. subsinuatum 624 | E. subsinuatum | KC710167 | KC710138 | KC710067 | MEN 2005624 | Newfoundland, Canada, NA | ||
| E. subsinuatum 2269 | E. subsinuatum | KC710178 | KC710149 | KC710096 | Y. Lamoureux 2269 (CMMF) | Québec, Canada, NA | ||
| E. subsinuatum 2005100 | E. subsinuatum | KC710181 | KC710104 | MEN 2005100 | Newfoundland, Canada, NA | |||
| E. aff. subsinuatum 633 | Entoloma aff. subsinuatum | KC710190 | KC710160 | MEN 84321 | New York, USA, NA | |||
| E. trachyosporum 405 | E. trachyosporum | GQ289338 | GQ289198 | KC710088 | H. den Bakker 1153 | Canada, NA | ||
| E. trachyosporum 414 | E. trachyosporum | GQ289339 | GQ289199 | KC710121 | H. den Bakker 1901 | Canada, NA | ||
| E. trachyosporum DAVFP 28111 | E. trachyosporum | JF899553 | DAVFP:28111 | GenBank ID: Rhodocybe trachyospora | Canada, NA | |||
| E. trachyosporum TB5856 | E. trachyosporum | GU384658 | GU384629 | TB 5856 | GenBank ID: Rhodocybe trachyospora | USA, NA | ||
| E. turbidum 27 | E. turbidum | GQ289341 | GQ289269 | GQ289201 | KC710060 | MEN 200351 | Slovakia, EU | |
| E. whiteae 629 | E. whiteae | KC710171 | KC710142 | KC710084 | Y. Lamoureux 1430 (CMMF) | Québec, Canada, NA | ||
| E. zuccherellii 242 | E. zuccherellii | GQ289346 | GQ289206 | A.Zuccherelli, 1996-01-25 | Italy, EU | |||
| Lyophyllum leucophaeatum Hae251.97 | Lyophyllum leucophaeatum | AF357101 | DQ367434 | AF223202 | AF357032 | Hae 251.97 | ||
| Rugosomyces carneus CBS552.50 | Rugosomyces carneus | AF357097 | DQ825407 | AF223175 | AF357028 | CBS 552.50 | ||
PCR and DNA sequencing
DNA sequences were obtained from four loci: internal transcribed spacer (ITS) (including ITS1, 5.8S and ITS2), nuclear large ribosomal subunit (LSU), nuclear RNA polymerase II second largest subunit (rpb2), and mitochondrial ribosomal small subunit gene (mtSSU). The primers, PCR and sequencing protocols have been described previously (Co-David et al. 2009), with the exception of the primer pair used for ITS, which was ITS1F and ITS4 (White et al. 1990). The newly generated sequences for this study have been submitted to GenBank (Table 1). Additionally, all relevant and available homologous sequences from public databases, GenBank and UNITE were included in the phylogenetic analyses (Table 1).
Phylogenetic analysis
Sequences were aligned, for each region independently using MUSCLE (Edgar 2004), MAFFT (Katoh & Toh 2008), Kalign (Lassmann & Sonnhammer 2005) and ClustalW2 (Larkin et. al. 2007). MUMSA (Lassmann & Sonnhammer 2006), a tool for automatic scoring of alignments, was used to choose the best alignment. MUSCLE had invariably the best score and therefore was used for the following steps. The alignments were visually checked for conspicuous errors (comparing the alignments of the most closely related sequences) with the software Se-Al v2.0a11 Carbon (Rambaut 2002). Homologous sequences of Lyophyllum leucophaetum and Rugosomyces carneus were also included in the sequence alignment step and used to root all trees. For each locus we reconstructed a maximum-likelihood (ML) phylogeny using Garli 2.0 (Zwickl 2006) using a general-time-reversible model with a proportion of invariable sites and gamma distribution rate of variable sites. Gaps were scored as ‘missing data’. The bootstrap (BS) test (Felsenstein 1985) was calculated with 500 replicates. Bootstrap values of 70 % or greater were considered significant. ML phylogenies of individual loci were compared for their concordance, taking into account phylogenetic placement and internal nodes with significant BS support. Since there was no discordance among the gene trees, the alignments were concatenated in a combined dataset in Geneious Pro 5.5.7 (Drummond et al. 2011). The combined dataset was then used to conduct a final phylogenetic reconstruction using ML in Garli 2.0 with the same settings as above. We performed also Bayesian inference (BI) using MrBayes 3.2 (Ronquist et al. 2012), with two independent runs of 5 million generations, sampling every 1 000th generation. Although likelihood values converged already after 30 000 generations, a majority rule consensus tree was calculated on the last 2 500 sampled trees (from the last 2 500 000 generations) to obtain Bayesian posterior probability (BPP) values for the branches. Both ML and BI were run using a general-time-reversible model with estimated nucleotide substitution rates and estimated proportion of invariable sites.
The High Performance Computing cluster maintained by the University of Alaska Fairbanks (UAF), Biotechnology Computing Research Group (http://biotech.inbre.alaska.edu/) was used to run MUSCLE, MAFFT, Clustal W2, Garli 2.0 and MrBayes. The European Molecular Biology Laboratory - European Bioinformatics Institute (EMBL-EBI), was used to run the online version of Kalign. The Center Stockholm Bioinformatics was used to run the online version of MUMSA.
Morphological analysis
The macroscopic descriptions and notes on the ecology of the basidioma were made based on freshly collected material, and photos were taken whenever possible. Colour notations follow Kornerup & Wanscher (1978). Microscopic features were observed on dried material following standard methods with a Leica DM1000 microscope and drawn with the help of a drawing tube. For microscopic measures an Olympus BH2 microscope was used, and pictures were taken with a Soft Imaging System Colorview I CCD-camera with Olympus Cell^D software. For basidiospore measurements the hylar appendage was excluded, and the following abbreviations were used: Q = quotient between the measures of length and width; Qav = average value of Q values.
Entoloma bloxamii (Berk. & Broome) Sacc. and E. madidum (Fr.) Gillet are nowadays accepted as conspecific taxa (e.g., Largent 1974, Noordeloos 1992) and considered synonyms by contemporary authors (Moser 1978, Noordeloos 1984, 1992, Largent 1994). However, during the previous century several authors recognised them as distinct taxa (either at inter- or intraspecific rank) based on spore size and odour (Konrad & Maublanc 1924–1932, Kühner & Romagnesi 1953, Largent 1974). Therefore, all the collections belonging to the clade of E. bloxamii were tested for statistical differences in spore size (length and width) range between the detected phylogenetic species. Six-hundred-and-thirty measurements (30 per collection) were made and graphically confirmed that the data followed a normal distribution. Then the phylogenetic species (recovered from the results of the phylogeny) were tested for significance on the size of spores (width and length) using a general linear mixed model (GLMM) with phylogenetic species as explanatory variables and collections as a random factor (to account for multiple sampling within a collection). Pairwise comparisons between all pairs of species were performed with Tukey range test. All statistical analyses were carried out in R (R development core team 2012).
Biogeographic analysis
All sequences included in the phylogeny were tested for statistical differences between the three geographical areas, Australasia, Europe and North America. The ML phylogenetic tree generated for inferring species limits was used to carry out Fast UniFrac analysis (Hamady et al. 2010). UniFrac Significance and P-test (Martin 2002) were calculated to assess differences among the geographic areas sampled. The UniFrac significance is calculated as the percentage of branch length leading to descendants from only one of the environments represented in the phylogenetic tree, and reflects differences in the phylogenetic lineages in one geographic area versus the others. The P-test estimates similarity between geographic areas as the number of parsimony changes (transitions from one geographic area to another on the phylogeny) to explain the distribution of sequences between the different geographical areas in the phylogeny. As a remark, we initially tested whether or not there is any phylogeographic structure between Eastern and Western North America, but because there was no significant difference, we combined them for the final analysis.
Species recognition criterion
To delimit species boundaries we applied the phylogenetic species (PS) concept. The PS concept comprises more than one definition, and Taylor et al. (2000) provided a comprehensive discussion with study cases from the fungal kingdom. In our study we followed the genealogical concordance method for species delimitation that minimizes subjectivity from the recognition of species boundaries (Avise & Ball 1990, Taylor et al. 2000) and that has already been widely applied to study fungal species delimitations (e.g., Taylor et al. 2000, Dettman et al. 2003, Geml et al. 2005b, Taylor et al. 2006, van de Putte et al. 2012). This concept is based on the comparison of gene geneologies and follows the assumption that the recombination between individuals of the same species can create conflict among gene genealogies, and the transition between concordance and discordance demarks the species limits (Taylor et al. 2000). We distinguished morphological varieties and forms within phylogenetic species based on morphological differences.
RESULTS
Molecular analysis
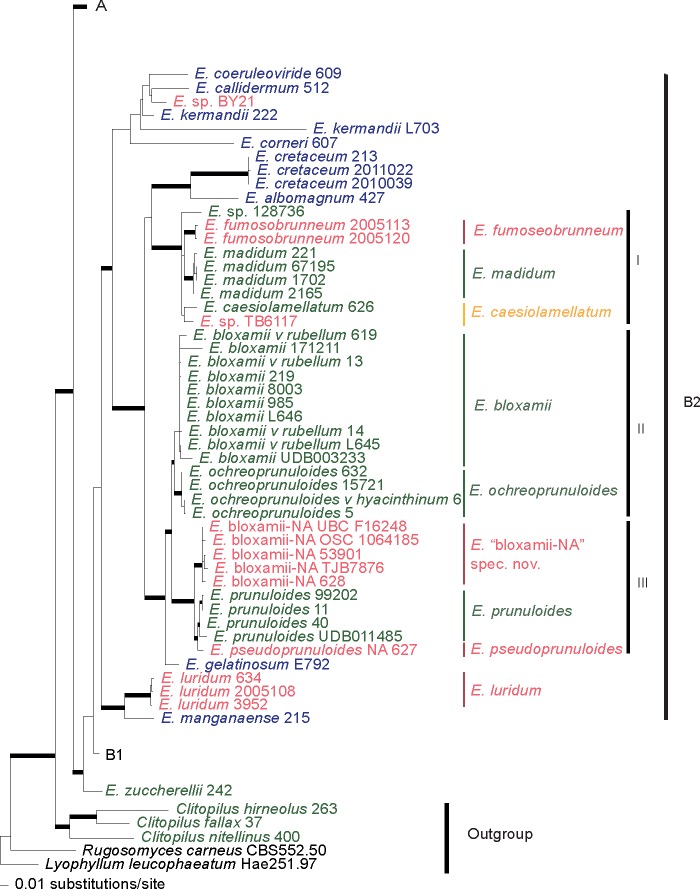
In this study, a total of 138 new sequences were generated, 78 from the ITS, 28 from LSU, 30 from mtSSU, and 2 from rpb2. The ITS sequences ranged from 364 to 998 bp in length, the LSU from 403 to 1 057 bp, the mtSSU from 220 to 614 bp and the rpb2 from 566 to 907 bp. The ITS, LSU, mtSSU, rpb2 and the combined dataset alignment consisted of 1 738, 1 129, 650, 926 and 4 434 characters, respectively, including gaps, of which, 680, 319, 117 and 1 444 were parsimony-informative characters, respectively. ML analysis of the concatenated dataset resulted in a single tree (-ln L = 34597.8078) (Fig. 1, 2, 3, 4).
Fig. 1.
Layout of the phylogram of maximum-likelihood, with major clades colapsed. Thicker branches have significant support (bootstrap (BS) ≥ 70 % and Bayesian posterior probability (PP) ≥ 0.95).
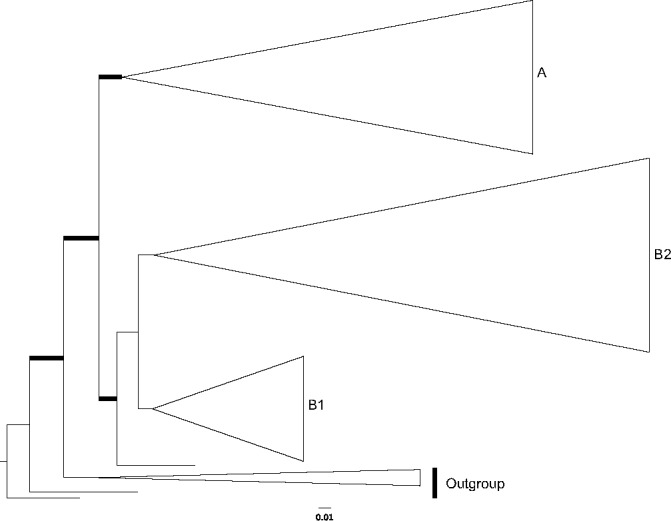
Fig. 2.
Clade B1 of the phylogram of maximum-likelihood of the combined dataset (ITS, LSU, rpb2 and mtSSU). Thicker branches have significant support (bootstrap (BS) ≥ 70 % and Bayesian posterior probability (PP) ≥ 0.95). In green colour are collections from Europe, in red from North America and in blue from Australasia, in yellow are species considered to occur in both Europe and North America.
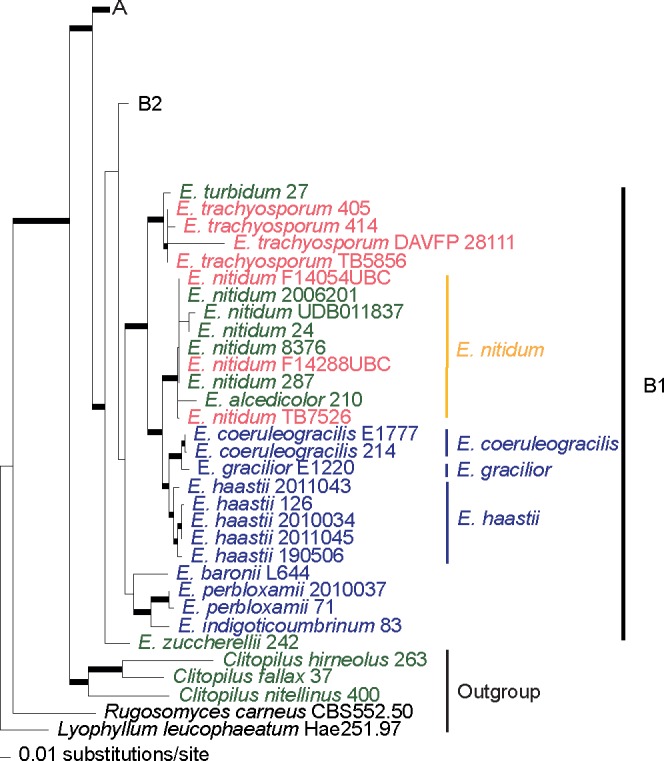
Fig. 3.
Clade B2 of the phylogram of maximum-likelihood of the combined dataset (ITS, LSU, rpb2 and mtSSU). Thicker branches have significant support (bootstrap (BS) ≥ 70 % and Bayesian posterior probability (PP) ≥ 0.95). In green colour are collections from Europe, in red from North America and in blue from Australasia, in yellow are species considered to occur in both Europe and North America.
Fig. 4.
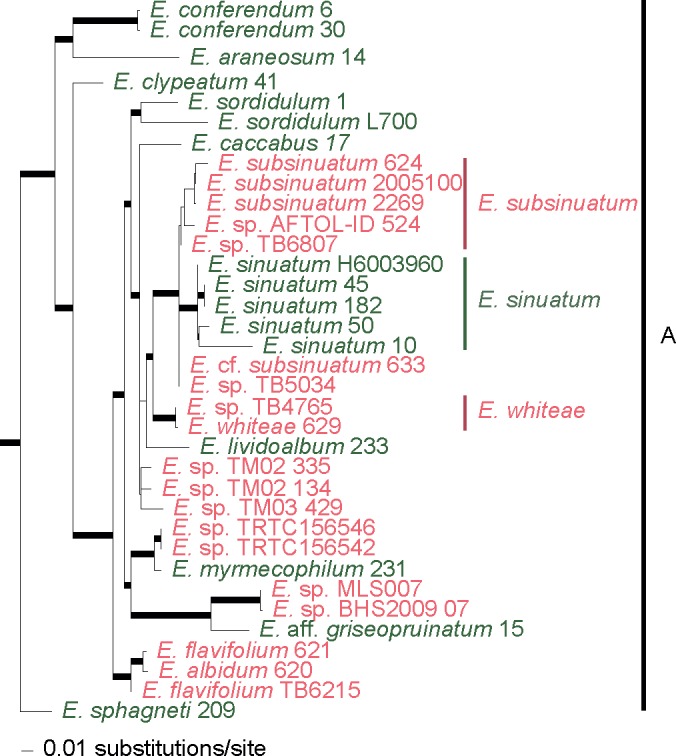
Clade A of the phylogram of maximum-likelihood of the combined dataset (ITS, LSU, rpb2 and mtSSU). Thicker branches have significant support (bootstrap (BS) ≥ 70 % and Bayesian posterior probability (PP) ≥ 0.95). In green colour are collections from Europe and in red from North America.
Phylogenetic diversity in the geographical areas studied
UniFrac analysis revealed that the taxa sampled differed significantly in phylogenetic constituency among the tree major geographical areas tested, Europe (EU), North America (NA) and Australasia (AA). In the P-test, the pairwise comparison of these geographical areas indicated that each region is significantly different (0.01 > P > 0.001) from each other (AA vs EU < 0.002, AA vs NA < 0.002, NA vs EU < 0.002), while the UniFrac Significance indicated AA as significantly different from EU and NA, and EU and NA only suggestively different (0.05 > P > 0.01) (AA vs EU < 0.002, AA vs NA < 0.002, NA vs EU = 0.084).
Statistical analysis of spore measurements
Spore size (length and width) differed significantly (p < 0.05) between some of the phylogenetic species marked in grey in Table 2, namely a closely related species pair (E. ochreoprunuloides and E. bloxamii), and a morphologically similar pair (E. bloxamii and E. madidum). The resulting box plot with range, mean and variance of spore length is provided in Fig. 5 and P values of the pairwise comparison are summarized in Table 2.
Table 2.
P values of Tukey range test, of the phylospecies in the bloxamii/prunuloides clade, with highlighted values for close related species with no significant difference. Leg.: blox: Entoloma bloxamii, bloxNA: E. sp. nov., caesio: E. caesiolamellatum, fumoso: E. fumosobrunneum, madidum: E. madidum, ochreo: E. ochreoprunuloides, prunuEU: E. prunuloides, pseudoprunu: E. pseudoprunuloides, grey: significantly different.
| blox | bloxNA | caesio | fumoso | madidum | ochreo | prunuEU | |
|---|---|---|---|---|---|---|---|
| blox | |||||||
| bloxNA | 0.5905 | ||||||
| caesio | < 0.01 | 0.4272 | |||||
| fumoso | < 0.01 | 0.1148 | 0.9691 | ||||
| madidum | < 0.01 | 0.4284 | 1 | 0.84641 | |||
| ochreo | < 0.01 | 0.059 | 0.9991 | 0.9974 | 0.9547 | ||
| prunuEU | 0.4858 | 1 | 0.5131 | 0.1474 | 0.529 | 0.0883 | |
| pseudoprunu | 0.0152 | 0.6165 | 1 | 0.9928 | 1 | 1 | 0.6868 |
Fig. 5.
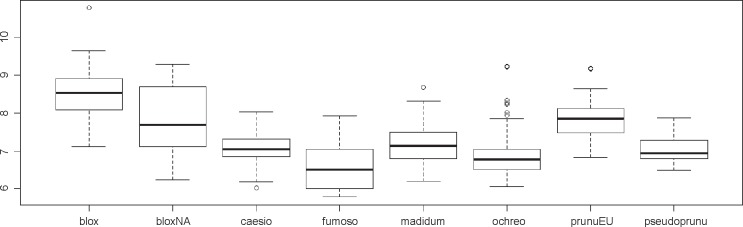
Box-plot of length measures of spores in the E. bloxamii morphospecies and clade. Leg.: blox: Entoloma bloxamii; bloxNA: E. cf. bloxamii, sp. nov.; caesio: E. caesiolamellatum; fumoso: E. fumosobrunneum; madidum: E. madidum; ochreo: E. ochreoprunuloides; prunuEU: E. prunuloides; pseudoprunu: E. pseudoprunuloides.
Species identification
With the combination of molecular phylogenetic and morphological tools, we were able to uncover consistent morphological patterns between the collections that formed phylogenetic species. The species complexes analysed here are placed in two major clades, one of which is further divided in two clades (Fig. 1, 2, 3, 4).
Clade A (Fig. 4), the ‘rhodopolioid’ clade in Co-David et al. (2009), mainly includes collections from Europe and North America. Almost all collections of the E. sinuatum complex are restricted to this well-supported clade (100/0.99) with the exception of E. luridum. All European (EU) collections labelled ‘E. sinuatum’ clustered in a monophyletic and highly supported clade (95/1.0), while the North American (NA) collections (both ours and the publicly available sequences) formed several sister clades. The more closely related clusters from North America represent distinct taxa, namely E. subsinuatum Murrill and E. whiteae Murrill. They differ morphologically mainly in pileus colour, which is obvious at least in the young basidiomata, and in the slightly hygrophanous pileus in the latter species. The remaining sequences from North America designated as E. sinuatum available in public databases, did not cluster in the above groups, are of unknown identity. Entoloma flavifolium, a NA species that shares the character of yellow lamellae with E. sinuatum, appears sister to E. albidum (which does not have yellow lamellae). Entoloma albidum and E. flavifolium differ on several morphological characters, viz. pileus and lamellae colour, spore size range and presence or absence of caulocystidia. Taking into account that this clade receives significant support for every locus and that a collection labelled as E. flavifolium TB6215 (from Moncalvo et al. 2002) appears as a minor distinct branch to the above pair, this clade likely represents a putative species complex, perhaps, with origin on the taxonomic ‘over-reliance’ on the yellowish colour of the lamellae. Entoloma luridum Hesler, which macroscopically resembles E. sinuatum due to the tricholomatoid habit, pale pileus and strong yellow lamellae, is an independent and phylogenetically distant species, belonging to clade B2 of our study. It appears to be closely related to E. manganaense, a yellow-gilled species recently described from Tasmania (Gates & Noordeloos 2007).
Clades B1 and B2 (Fig. 2, 3), the ‘prunuloides’ clade in Co-David et al. (2009), includes the E. nitidum, E. bloxamii and E. prunuloides complexes. This clade comprises collections from Europe, North America and Australasia. In the E. nitidum complex, (clade B1), EU and NA collections showed little phylogenetic divergence. Therefore, they are considered conspecific. Resembling Australian collections are distinct and represent several well-delimited species, namely E. haastii G. Stev., E. coeruleogracilis G.M. Gates & Noordel. and E. gracilior Noordel. & G.M. Gates. The European E. alcedicolor Arnolds & Noordel. is only known from the type locality and morphologically similar to E. nitidum, differing however in the smaller stature and a more pronouncedly trichodermal pileipellis. Since there is no phylogenetic divergence from the remaining E. nitidum populations, the taxa are considered conspecific.
The E. prunuloides complex, in clade B2, is not monophyletic because the European E. ochreoprunuloides (previously known as E. prunuloides var. obscurum Arnolds & Noordel.) is placed in a different clade than the type variety. Only one collection from NA appears closely related to the EU ones, but it is placed in a sister position to them with maximum BS support and BPP. Because it also shows slight morphological differences in pileus colour and spore size it is considered a sister species and described as a distinct taxon, E. pseudoprunuloides, in the taxonomy section below. Two other collections from Canada with smoky-grey basidiomata have a distinct and well-supported phylogenetic placement and represent a novel species that is described as E. fumosobrunneum below.
The E. bloxamii species complex, in clade B2, is paraphyletic and comprises three distinct lineages (clade I, II and III, Fig. 3), representing five phylogenetic species. The main morphological differences are spore size, basidioma colours and colour change during maturation. At least three different species occur in Europe: E. bloxamii (clade II), E. caesiolamellatum (comb. nov.) (clade I), and E. madidum (clade I). Small spored collections with persistent colours are considered to represent E. madidum. Sister to the previous species appears a clade composed of two collections that do not receive significant support. One represents the type collection of E. caesiolamellatum (previously known as E. bloxamii var. caesiolamellatum Wölfel & Noordel.) from the Canary Islands while the other is a similar collection from California. The morphology of both collections is similar, therefore, they seem to be conspecific.
In clade III, sister to E. prunuloides and E. pseudoprunuloides, appears a cluster of several collections solely from western North America labelled (in Fig. 3) as E. bloxamii-NA that receives maximal BS support and PP, which represents a distinct and most likely undescribed species. However, since the data on the macroscopic features of these collections is incomplete, we refrain from describing it as a new species at this time.
Clade II is a cluster of EU collections that splits in two phylogenetic species. In both lineages there is a considerable variation in colour of the basidioma. In the E. ochreoprunuloides cluster, there is one collection that has ochre to light brown colours, two with pink-ochre and one with violaceous-blue pileus. Microscopically the collections are similar. Therefore, they are considered conspecific with distinct infraspecific colour varieties. The second lineage in clade II, which does not receive significant support, contains a cluster of collections, which have either a blue or a pink pileus. This clade represents E. bloxamii in the strict sense, since the micromorphology of the spores agrees with the type. As the holotype is in poor condition and unsuitable for DNA analysis, an epitype is designated below. The pink coloured collections fit well with the concept of E. rubellum, which is phylogenetically conspecific with E. bloxamii and will be distinguished on variety level. Since no type material is available of Agaricus rubellus, a neotype is selected here. Further details are provided in the taxonomic part below.
DISCUSSION
Phylogeographic trends
The Fast UniFrac analyses indicate that composition of Entoloma species is significantly influenced by geographical regions. The lineages from Australasia showed the strongest divergence and are all placed in clade B of the phylogeny, where they show a general pattern of having representatives in virtually all the sub-clades with basal positions to their closest relatives in the Holarctic. This pattern suggests that Australasia might be an ancestral area in this clade.
Clade A solely contains collections from the Holarctic. Most sub-clades have representatives from both continents that show phylogenetic segregation, and most likely reproductive isolation. This pattern supports the hypothesis of allopatric speciation which has been noted in other fungal biogeographic studies (e.g., Taylor et al. 2006, Geml et al. 2008, Giraud et al. 2008, Moncalvo & Buchanan 2008).
The UniFrac analyses (P-test) showed that there is a significant difference in phylogenetic structure between Europe and North America. This result supports our general interpretation of the species distribution limits. From our interpretation of the species, we argue that NA collections of ‘E. sinuatum’ do not appear to be conspecific with the EU ones, and therefore we assume that this taxon is not present in NA. On the other hand, several closely related taxa, viz. E. subsinuatum and E. whiteae, to our knowledge, are restricted to North America. Entoloma prunuloides follows the same pattern with the species itself apparently being absent from North America, where the resembling collections represent several related, well delimited species, viz. E. pseudoprunuloides and E. fumosobrunneum that are restricted to North America. Entoloma bloxamii is restricted to Europe, were three other related and morphologically similar species occur, E. caesiolamellatum, E. madidum and E. obscureoprunuloides. In sharp contrast with the rest of the ‘bloxamii look-alikes’, there are preliminary data to raise the hypothesis that the later mentioned taxon is also present in western North America, although more collections are needed to test this hypothesis. Interestingly, most of the ‘bloxamii look-alikes’ from North America included in this study represent a distinct and undescribed species, E. bloxamii-NA, which appears to be restricted to western North America and seems to occur in sympatry with E. caesiolamellatum.
On the other hand, the EU and NA collections of E. nitidum appear to be conspecific. That implies this taxon has a wide distribution in the Northern Hemisphere unlike most species addressed in this study. The similar taxa from Australasia are distinct and represent several related and morphologically well-delimited species that have not been found outside Australasia. Entoloma nitidum is an ECM fungi (Montecchio et al. 2006) that seems to have a broad range of hosts (Noordeloos 1992). To our knowledge the earliest record of this species for North America is from 1963 by Morten & Farr (in Miller & Farr 1975), however, it is absent from the main taxonomical revisions dealing with North American Entoloma and satellite genera (Hesler 1967, Largent 1994), making it difficult to raise hypothesis to justify the observed pattern.
Morphological characters useful for species delimitation
In this study, the phylogenetic species detected by the genealogical concordance species recognition criteria are all supported by morphological characters and no real cryptic species seem to occur. In a broad overview, for the taxa included in this study, most can be diagnosed with the combination of the following characters: spore morphology, basidioma habit, colours and their evolution in the basidioma development and hygrophaneity of the pileus.
The diagnostic morphological characters for distinguishing taxa around E. sinuatum are the combination of the following characters: spore morphology, colour of the pileus of the young basidioma, colour of the lamellae and its persistency during the basidioma maturation and the hygrophaneity of the pileus. Interestingly, the NA sister species, E. subsinuatum appears to be equally toxic as the EU E. sinuatum, with a few intoxication cases during the last two decades in Québec (A. Voitk pers. comm. and Y. Lamoureux pers. obs.). For the E. prunuloides group, the diagnostic characters are the spore size and pileus colour. For E. nitidum and look-alikes, the diagnostic characters are the combination of spore morphology, basidioma habit and pileus colour, while for E. bloxamii and related taxa, the combination of spore morphology, basidioma colour and its persistency, especially in the pileus and stipe apex can be considered diagnostic.
Phylogenetically informative characters
Even though the previously mentioned characters are crucial for species delimitation, by itself, only spore morphology can be regarded as phylogenetically reliable in the group of taxa studied. This feature was already discussed by Co-David et al. (2009), who divided Entoloma spore types in 3 groups: 1) regular entolomatoid spores, that have completely interconnecting ridges to form facets and that have no isolated bumps; 2) irregular entolomatoid spores with at least one facet not delineated by ridges, and without bumps; and 3) very irregular entolomatoid spores with bumps.
Our study includes the 3 spore types and the results support the distinction between regular entolomatoid spores and the latter two types. However, the phylogenetic divergence between species with irregular and very irregular entolomatoid spores is not significantly supported by BS and PP, because E. zuccherellii, that possesses very irregular spores, did not form a monophyletic group with other species that also have very irregular entolomatoid spores, such as E. nitidum and the related taxa. Therefore, our results confirm the findings of Co-David et al. (2009) as opposed to the work of Baroni et al. (2011), that suggested this feature as monophyletic with a basal position in the genus Entoloma, that they used to separate the species with very irregular spores as a new genus.
As for the other morphological characters that received varying amount of emphasis in the traditional systematics of these groups of species, it seems that interpretation of single characters have been overly emphasized. The presence of yellow colour in the lamellae apparently evolved independently at least two times, as it is found in both major clades (clade A, in E. sinuatum, and clade B in E. luridum and E. manganaense).
Entolomatoid species with irregular entolomatoid spores, tricholomatoid habit and purple-bluish colours represent more distinct and diverse taxonomic units than previously assumed. Species with these characteristics are restricted to clade B. Even though they were not statistically tested, and that our study was only partially directed to these taxa, it seems plausible to raise the hypothesis that these features could represent a synapomorphy for the clade.
CONCLUDING REMARKS
Allopatric divergence has long been thought to be the main process of speciation (Mayr 1963), since extrinsic geographic barriers are impediments to gene flow. Finlay (2002) suggested that fungi could be exceptions since eukaryotic microorganisms have long been considered to have little dispersal limitations and, thus, global geographic ranges. Our study provides evidence for strong phylogeographic and biogeographic partitions in the genus Entoloma that is in agreement with already observed trends in many fungal species (Taylor et al. 2006).
The integration of molecular phylogenies in systematic studies has reshaped our understanding of morphological features in an evolutionary context. Since Fayod (1889), spore morphology in Entolomataceae has received increasing emphasis in systematic studies. Lately this trend has been reinforced by studies involving molecular tools, e.g., Co-David et al. (2009), Baroni et al. (2011) and Baroni & Matheny (2011). Our results provide an example from the genus Entoloma for the importance of spore morphology as a key character in the systematics of the Entolomataceae.
Global climate change and habitat loss are main threats to biodiversity. Fungi form one of the most diverse groups of organisms, yet their conservation has attracted very little attention. In this paper, E. bloxamii, an endangered species in Europe (Dalhberg & Croneborg 2006), is revealed to be a complex of several distinct phylogenetic species and is a good example of the current knowledge on the diversity and distribution of fungi and the importance of employing phylogenetic methods in studies dealing with systematics, biogeographic distribution and conservation biology.
TAXONOMIC PART
I. Entoloma sinuatum complex, characterized by robust tricholomatoid basidiomes with yellow lamellae, at least when young
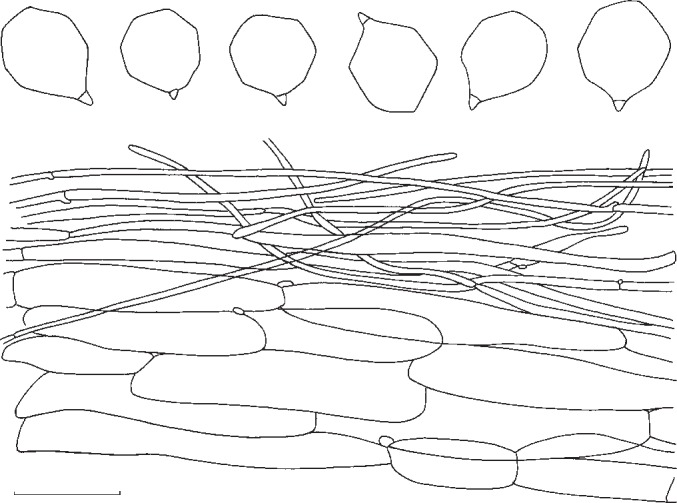
1. Entoloma subsinuatum Murrill, N. Amer. Fl. 10, 2: 125. 1917. — Fig. 6a, 7
Fig. 6.
Habit of the following species: a. Entoloma subsinuatum (Y. Lamoureux 3218); b. E. whiteae (photo Y. Lamoureux 1430); c. E. flavifolium (Y. Lamoureux 3218); d. E. luridum (photo MEN2005108); e. E. luridum (left) and E. subsinuatum (right), resp. MEN 2005108 and MEN 2005624; f. E. pseudoprunuloides (holotype, photo Noordeloos); g. E. ochreoprunuloides (1 = holotype; 2 = R.A Maas Geesteranus 15721); h. E. ochreoprunuloides var. hyacinthinum (photo D. Harries).
Fig. 7.
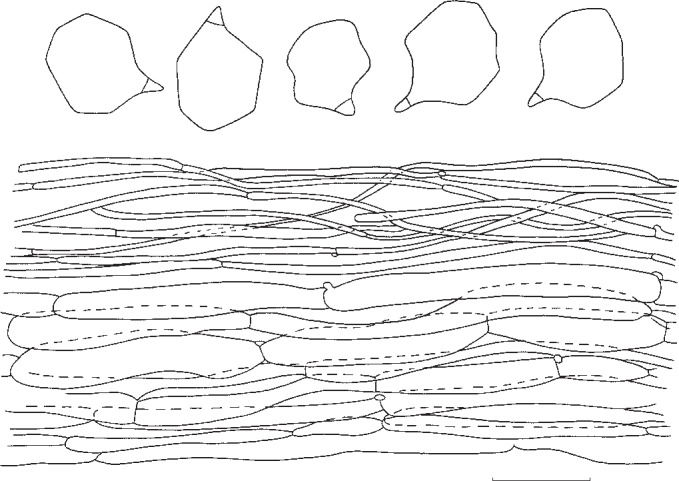
Entoloma subsinuatum. Spores and pileipellis (all: Halling & Noordeloos, 14 Sept. 1984). — Scale bar = 10 μm.
Pileus 50–150 mm broad, conical to hemispherical when young, expanding to convex or almost flat with or without low umbo, with inflexed margin when young, then straight, and slightly extending over the gills, finally irregularly undulating and splitting, not hygrophanous, not translucently striate, very pale cream-colour sometimes mixed with grey to almost white (Muns. 10 YR 8/2–3, 8/4 to 2.5 Y 8/4, 7/4), when old often with yellow tinge (towards 2.5 Y 8/6), smooth, shining when young then innately fibrillose to subtomentose (hand lens) when old splitting up in rather coarse ‘pseudo’ squamules. Lamellae moderately distant, deeply emarginated-adnate, yellowish when young (2.5 Y 8/4 to 10 YR 8/4), then pink (10 YR 7/3 to 7.5 YR 7/4), often retaining the yellow tinge near the margin of pileus. With very eroded, concolorous edge, often veined on sides in large specimens. Stipe 50–120 × 10–25 mm (apex), solid, full then spongy within, often with enlarged, bulbous base (−35 mm), white then sordid yellow in places, strongly and coarsely fibrillose to flocculose, especially in upper part. Context firm, white. Smell strong, farinaceous especially when cut, spontaneously often weak and less distinctly farinaceous. Taste nasty, rancid. Spores 7.3–8.6(−9.4) × 6.7–8.0 μm, average 8.2 × 7.3 μm, Q = 1.0–1.26, Qav = 1.13, subisiodiametric, 5–7-angled in side view, with pronounced angles and relatively thick walls. Basidia 33–45 × 10–12 μm, clavate, 4-spored, clamped. Lamella edge fertile. Cystidia absent. Hymenophoral trama regular, made up of cylindrical hyphae, elements ranging from 55–155 × 8–19 μm, vascular hyphae abundant. Pileipellis an ixocutis of narrow, cylindrical hyphae, 2–8 μm wide, with pale, intracellular pigment; subpellis not really differentiated from underlying trama, made up of cylindrical hyphae, elements 42–90 × 9–15 μm, gradually passing into regular pileitrama, made up of cylindrical elements, 40–100 × 7–26 μm, mixed with narrow, cylindrical connective hyphae, 4–8 μm wide, with abundant vascular hyphae. Stipitipellis a cutis of narrow, cylindrical hyphae, 5–10 μm wide with abundant loose terminal elements (caulocystidia), 24–56 × 5–10 μm, with rounded, sometimes subcapitate apex. Clamp-connections present in all tissues.
Habitat & Distribution — In groups close to coniferous and deciduous trees, including Pinus, Abies, Picea, Quercus and Betula, probably ectomycorrhizal. North-eastern North America, Canada and USA. Type collection from Bar Harbor, Maine, USA deposited at NY.
Collections examined. Canada, Québec, Saint-Herménégilde, 23 Aug. 1998, Y. Lamoureux 2269 (CMMF); Newfoundland, Gros Morne National Park, Killdevil Camp, 2 Sept. 2005, M.E. Noordeloos 2005624. – USA, New York State, Allegany State Park near Red House, 15 Sept. 1984 (L), M.E. Noordeloos 84321; same location and date, Halling & Noordeloos 3848 (NY).
Notes — We present here a full description of the North American E. subsinuatum to facilitate a comparison with the European E. sinuatum. Distinctive features of this species are the fairly robust basidiocarps with firm flesh, the pale colour, the lamellae tinged yellow when young and a rather unpleasant smell. This North American taxon appears to be equally poisonous as the European sister species E. sinuatum. The original description of E. subsinuatum (Murrill 1917) fits well. Unfortunately, we were not able to locate the holotype for comparison. Entoloma flavifolium Peck has a smaller stature, brittle flesh, and darker coloured fruit bodies and is distantly related. Entoloma luridum Hesler has deeper yellow lamellae, often persistent in older specimens, a stuffed then hollow stipe, and differs by the slightly smaller, thin-walled, multiangular spores.
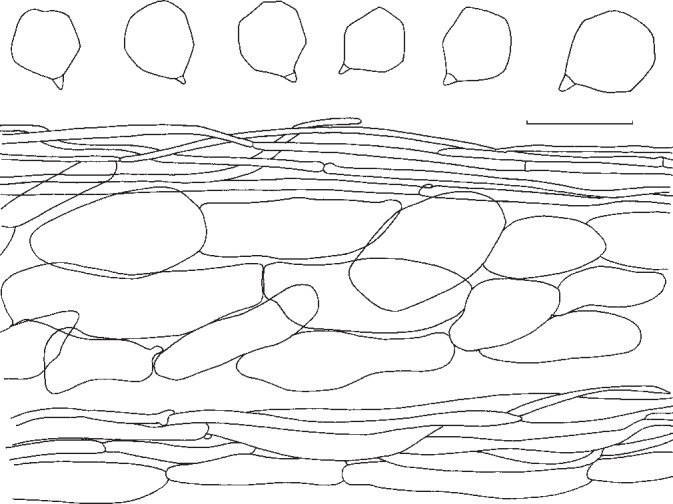
2. Entoloma whiteae Murrill, N. Amer. Fl. 10, 2: 126. 1917. — Fig. 6b, 8
Fig. 8.
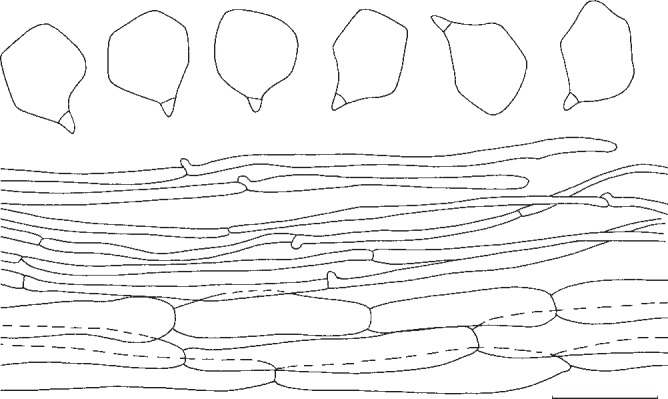
Entoloma whiteae. Spores and pileipellis (all: Y. Lamoureux 1430). — Scale bar = 10 μm.
Pileus 70–200 mm broad, campanulate then convex, finally expanded, often with large, broad umbo, with deflexed margin, finally with undulating marginal zone, slightly hygrophanous, glabrous or sometimes radially grooved or rugulose in marginal zone, greyish brown to brownish yellow at first, somewhat paler, beige or pale brown with age. Lamellae sinuate, moderately distant, broadly ventricose, pale yellow then with pink tinge, with concolorous, eroded edge. Stipe 50–150 × 10–35 mm, cylindrical, equal or with broadened base, solid, full then spongy within, white to pale brown, striate-fibrillose all over to somewhat squamulose. Context firm, thick, white. Smell strongly farinaceous-rancid or somewhat alkaline. Taste unpleasant, farinaceous-rancid. Spores 7–9.6 × 6.5–8.5 μm, average 8.5 × 7.4 μm, Q = 1.1–1.4, Qav = 1.15, subheterodiametrical, 5–6-angled in side-view. Basidia 22–40 × 7–12 μm, 4-spored, clamped. Cystidia absent. Hymenophoral trama regular, made up of medium sized, cylindrical elements, 60–15 μm wide. Pileipellis a cutis of cylindrical hyphae 4–11 μm wide, sometimes with ascending terminal elements, 60–120 × 8–15 μm, forming subtrichodermal tufts; subpellis not well differentiated from pileitrama. Pigment pale brown, intracellular. Pileitrama regular, made up of cylindrical to slightly inflated elements, 50–120 × 6–22 μm. Stipitipellis a cutis of cylindrical hyphae, 5–17 μm wide, sometimes with trichodermal tufts of inflated terminal elements, 60–120 × 5–22 μm. Clamp-connections abundant in all tissues.
Habitat & Distribution — In mixed deciduous forest (Quercus, Fagus, Picea) on various soils. North-eastern North America, Canada and USA. Type from Bar Harbor, Maine, USA deposited at NY.
Collections examined. Canada, Québec, City of Mascouche, 23 Aug. 1991, Y. Lamoureux 1430 (CMMF); City of Mont-Saint-Hilaire, 9 Aug. 1993, Y. Lamoureux 1959 (CMMF).
Notes — Entoloma whiteae is well characterized by the slightly hygrophanous aspect and the relatively dark colours in the pileus and pale yellow lamellae when young. Unfortunately, we were not able to study the type. It belongs to a group of closely related and similar species, that are fairly poorly known, such as E. griseum Peck and E. grande Peck, both lacking yellow tinges in the lamellae, and with a more grey tinged pileus (Noordeloos 2008). Detailed studies in subg. Rhodopolia, both in North America and Europe, are needed to come to a stable species concept.
3. Entoloma flavifolium Peck, Bull. New York State Mus. 105: 21. 1906. — Fig. 6c, 9
Fig. 9.
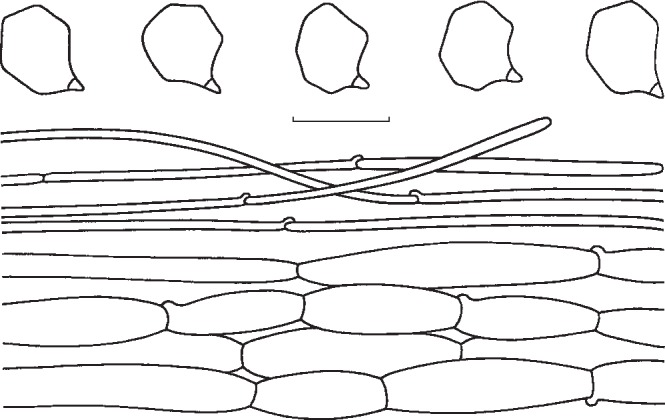
Entoloma flavifolium. Spores and pileipellis (all: holotype (NYS)). — Scale bar = 10 μm.
Pileus 20–60 mm broad, campanulate to broadly convex or nearly plane, often with large umbo, with undulating marginal zone, hygrophanous, not or shortly translucently striate, olivaceous-brown in the beginning, quickly pallescent with age then yellow-brown or yellow-olivaceous, pallescent on drying to greyish whitish, glabrous. Lamellae moderately distant to distant, adnexed, broad, ventricose, yellow-ochre to yellow-olivaceous when young, then pink, with concolorous, eroded edge. Stipe 35–90 × 4–10 mm, cylindrical with clavate base, mostly fragile stuffed then hollow, whitish glabrous, finely fibrillose-striate. Context concolorous, brittle. Smell and taste slightly farinaceous, unpleasant. Spores 7.0–9.0 × 6.5–7.5(−8.0) μm, average 8.0–8.5 × 6.5–7.0 μm, Q = 1.1–1.3, Qav = 1.2, 5–7-angled in side-view with pronounced angles and thick walls. Basidia 37–48 × 9–11 μm, 4-spored, clavate, clamped. Lamella edge fertile. Cystidia absent. Hymenophoral trama regular, made up of cylindrical to inflated elements, 40–85 × 5–8 μm. Pileipellis 2-layered, suprapellis an ixocutis of 2–4 μm wide, cylindrical hyphae; subpellis made up of inflated elements, 27–74 × 8–21 μm; gradually passing to pileitrama, which is regular, made up of inflated or cylindrical hyphae, 35–100 × 13–21 μm. Pigment brownish, intracellular and diffuse in pileipellis and upper trama, the most pronounced in suprapellis; in addition some minute incrustations have been observed in suprapellis. Stipitipellis a cutis of cylindrical hyphae, 4–10 μm wide, bearing dense clusters of caulocystidia, especially in upper part of stipe; caulocystidia cylindrical with rounded to subcapitate apex, 40–85 × 5–8 μm. Clamp-connections abundant in all tissues.
Habitat & Distribution — On clayey soil of Quercus woods. North-eastern North America, Canada and USA.
Collections examined. Canada, Québec, City of Longueuil. 13 July 1992, Y. Lamoureux 1663 (CMMF); Québec, City of Mont-Saint-Hilaire, Y. Lamoureux 3218 (CMMF). – USA, New York, Essex City, Post Henry, 8 Aug. 1885, C.H. Peck (holotype NYS).
Notes — Entoloma flavifolium has a distinctly pigmented pileus, brittle flesh, and relatively thick-walled, pronouncedly angled spores. It is easy to recognize in the field. The present description is based on many observations by YL on the same spot for almost 15 years. Although Peck (1906) originally described it as a rather pale species, it is assumed that his specimens were not fresh but dried out when he studied them. Noordeloos (2008) published a detailed type study. Entoloma flavifolium belongs to (section) Rhodopolia, where it is distinct because of the often intensely yellow tinged lamellae, particularly when young. Entoloma sinuatum and E. luridum are paler, and more robust species with a less pronounced hygrophanous pileus. In addition, the yellow tinges in the lamellae of E. sinuatum are less intense. Entoloma luridum has brightly yellow lamellae, and smaller, thin-walled, less pronouncedly angled spores. Judging from the description and photograph in Largent (1994), E. pseudolividum Largent is very similar to E. flavifolium. We have not yet studied material of this species. Entoloma cerinum E. Horak from New Zealand also has yellowish lamellae when young, which turn dark-pink, however, with age, the pileus is very dark brown, and the hyphae are clampless (Horak 2008).
4. Entoloma luridum Hesler, Beih. Nova Hedwigia 23: 66. 1967. — Fig. 6d, e, 10
Fig. 10.
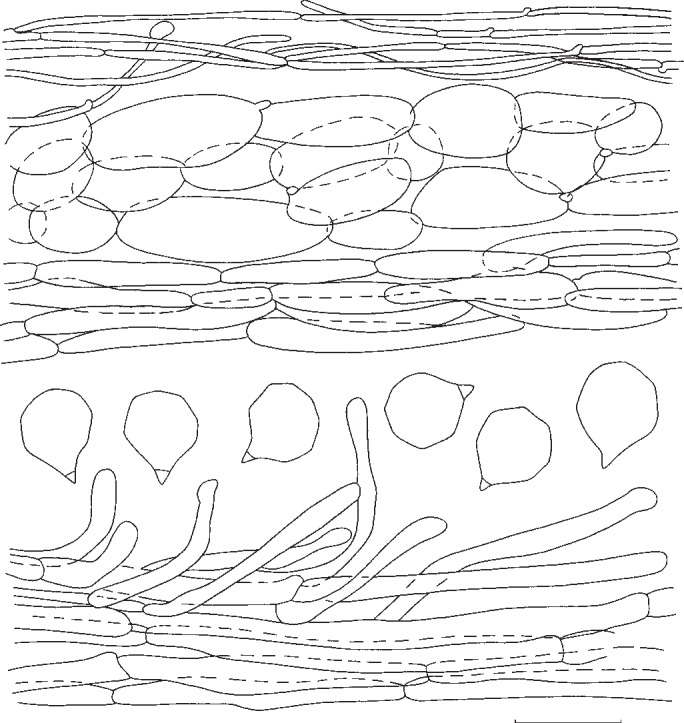
Entoloma luridum. Spores, pileipellis, and stipitipellis (all: MEN 2005108). — Scale bar = 10 μm.
Pileus 40–120 mm, conical then conico-convex finally irregularly applanate with or without conical umbo, with regular, deflexed margin, distinctly hygrophanous, hardly translucently striate at margin only, very pale creamy white, ivory or very pale brownish, pallescent on drying to almost white, glabrous somewhat greasy to touch but not viscid. Lamellae rather crowded, adnate-emarginate, segmentiform to subventricose, persistently bright citrine-yellow (about 5 × 8/6–7/6) when mature with pink tinge, with irregularly eroded, concolorous edge. Stipe 30–75 × 10–16 (apex) and 20–30 mm (at base), cylindrical with clavate base, mostly fragile stuffed then hollow, white, innately fibrillose, glabrous. Context thick, white. Smell more a less indistinct, somewhat sweetish. Taste rather unpleasant. Spores 6.5–8.5 × 6.0–8.5 μm, average 7.2–7.5 × 7.2 μm, Q = 1.0–1.1, Qav = 1.05, rounded, thin-walled, many-angled with weak angles. Basidia (31−)35–43(−47) × 8.5–14 μm, 2- and 4-spored, clavate, clamped. Lamella edge fertile. Cystidia absent. Hymenophoral trama regular, made up of cylindrical to inflated hyphae, 40–150 × 9–20 μm, with diffuse yellowish intracellular pigment. Pileipellis 2-layered, suprapellis an ixocutis with transitions to an ixotrichoderm, made up of cylindrical 3–9 μm wide hyphae; subpellis well differentiated from trama, made up of inflated elements, 25–90 × 8–26 μm. Pigment very pale, intracellular in pileipellis. Pileitrama regular, made up of cylindrical elements, 22–90 × 4–20 μm with pale intracellular pigment. Vascular hyphae present. Stipitipellis a cutis of narrow, cylindrical hyphae, 4–11 μm wide, caulocystidia sometimes present at apex of stipe, 27–60 × 3–8 μm, cylindrical with rounded or slightly swollen apex. Stipititrama regular, made up of cylindrical to slightly inflated elements, 27–200 × 6–15 μm with very pale diffuse intracellular pigment. Clamp-connections present in all tissues.
Habitat & Distribution — In groups in grassy spot near margin of Picea forest, and in mixed woods of Abies, Picea and Betula. Eastern and North-eastern North America, Canada and USA.
Collections examined. Canada, Newfoundland, Gros Morne National Park, Killdevil Camp, 1 Sept. 2005, M.E. Noordeloos 2005108 (L); Québec, City of Bonsecours, 21 Sept. 2005, Y. Lamoureux 3952 (CMMF). – USA, Tennessee, Great Smokey Mountains National Park, Cades Cove, 25 Aug. 1966, L.R. Hesler 29229 (holotype TENN).
Notes — Entoloma luridum is fairly well characterized by the bright yellow gills and otherwise very pale, almost white, rather robust basidioma, and the small, thin-walled, many-angled spores. Entoloma mathinnae G.M. Gates, B.M. Horton & Noordel. and E. manganaense G.M. Gates & Noordel. from Tasmania (Gates & Noordeloos 2007, Gates et al. 2009, Noordeloos & Gates 2012) have similar persistent yellow lamellae, and small, poorly angled spores, but differ by the presence of blue pigments in the pileus and/or stipe. Entoloma subsinuatum, which may occur in similar habitats, has less pronounced yellow lamellae (Fig. 10), a hardly hygrophanous pileus, and larger, more pronouncedly angled spores. Entoloma flavifolium and E. pseudolividum differ by having a brownish pileus, and distinctly angled, slightly larger, thick-walled spores, which place them in the rhodopolioid clade.
II. Entoloma prunuloides group, characterized by small to medium sized tricholomatoid habit and pale to dark brown colours
5. Entoloma pseudoprunuloides Morgado & Noordel., sp. nov. — MycoBank MB802144; Fig. 6f, 11
Fig. 11.
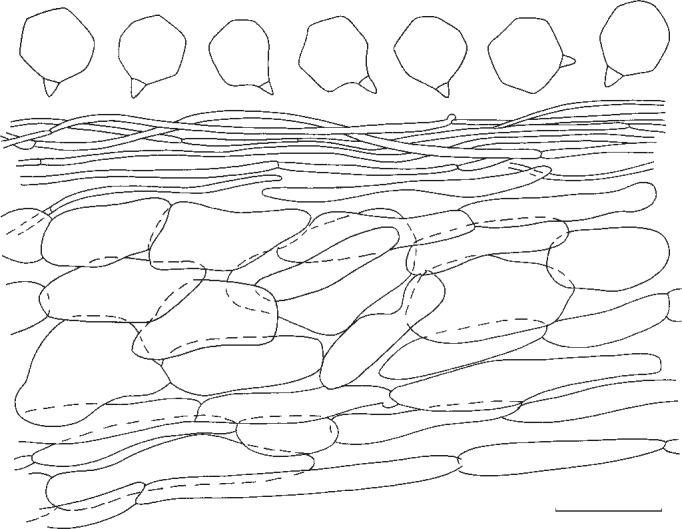
Entoloma pseudoprunuloides. Spores and pileipellis (all: holotype). — Scale bar = 10 μm.
Etymology. pseudo (L) = pseudo, referring to the likeness with E. prunuloides.
Pileus 40–60 mm, conico-convex with involute margin, expanding to convex or plano-convex with umbo and deflexed margin, not hygrophanous, not translucently striate, pale to moderately dark yellow-brown (10 YR 8/4, 7/3–4) glabrous, with micaceous patches at centre. Lamellae rather crowded, L = 80–90, l = 3–5, adnate-emarginate, segmentiform to subventricose, white then sordid pink with serrulate, concolorous edge. Stipe 20–50 × 8–13 mm, cylindrical, attenuated at base, white with pale brown tinges, base white with slight yellow tinge, innately fibrillose, smooth. Context white. Smell and taste strong, farinaceous-rancid. Spores 6.5–8.1 × 6.5–7.8 μm, average 7.5 × 7.1 μm, Q = 1.0–1.17, Qav = 1.05, isodiametric, 5–7-angled in side view. Basidia 34–47 × 8–11 μm, 4-spored, clavate. Cystidia absent. Lamellae edge fertile. Hymenophoral trama regular, 47–126 × 8–20 μm. Pileipellis in the center an ixocutis with some transitions to trichoderm made of cylindrical hyphae of 22–97 × 1.5–6 μm, with intracellular diffuse brown to pale yellow pigment; in the middle of the radius a simple ixocutis made of cylindrical hyphae of 25–63 × 2.5–8 μm, with intracellular diffuse pale yellow pigment; subpellis made of elongated hyphae of 29–64 × 13–26 μm, with intracellular brownish diffuse pigment. Pileitrama made of cylindrical hyphae of 56–160 × 16–42 μm, with intracellular hyaline pigment. Stipitipellis without caulocystidia, although some sterile basidia are present. Stipititrama made of hyphae of 55–200 × 20–30 μm, with intracellular diffuse hyaline pigment. Vascular hyphae present. Clamp-connections abundant in all tissues.
Habitat & Distribution — In groups in grassland with scattered Abies balsamifera. Newfoundland, Canada, known only from the type locality.
Collection examined. Canada, Newfoundland, Gros Morne National Park, Killdevil Campground, 2 Sept. 2005, M.E. Noordeloos 2005113 (holotype L).
Notes — Entoloma pseudoprunuloides resembles the European E. prunuloides in habit and also in microscopic characters very much. The main distinguishing characters are the darker pileus and slightly different spores.
6. Entoloma ochreoprunuloides Morgado & Noordel., nom. nov. — MycoBank MB802176; Fig. 6g, h, 13
Fig. 13.
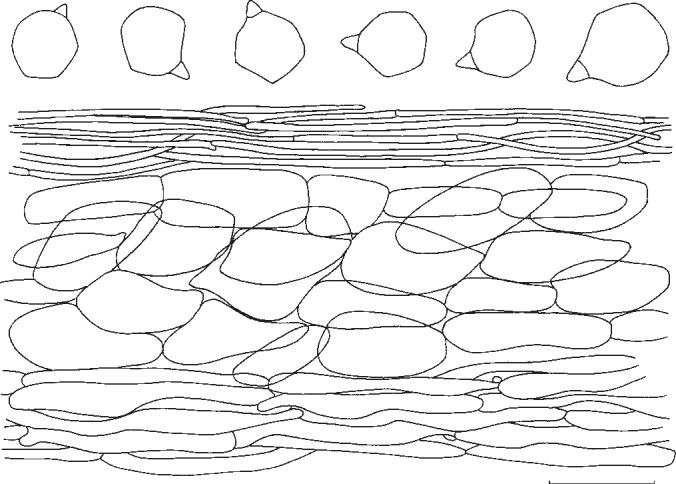
Entoloma ochreoprunuloides. Spores and pileipellis (all: holotype). — Scale bar = 10 μm.
Syn.: Entoloma prunuloides var. obscurum Arnolds & Noordel., Fungi Europei vol. 5a: 838. 2005, non Entoloma obscurum Hesler 1967.
6.1 Typical form
Holotype. Germany, Nordrhein-Westfalen, Teutoburgerwald, Brochterheck, S. of Ibbenbüren, ‘Stallfort’, 5 Oct. 2001, E. Arnolds 01-142 (holotype L).
Pileus 20–50 mm broad, conical soon expanding to plano-convex with low umbo, with straight margin, weakly hygrophanous, moderately dark brown with greyish brown centre (K&W 6D4–6E5), finely radially wrinkled. Lamellae, L = ± 40, l = 3–5, moderately distant, adnate-emarginate, sordid pink with entire, concolorous edge. Stipe 30–50 × 5–8 mm, cylindrical, straight, off-white to pale grey-brown, much paler than pileus, innately fibrillose lengthwise. Context concolorous, rather firm. Smell and taste farinaceous. Spores 5.9–7.1 × 5.7–7.2 μm, average 6.6 × 6.4 μm, Q = 1.0–1.16, Qav = 1.04, isodiametric, 5–6-angled. Basidia (27−)30–35(−43) × 8–11.4 μm, 4-spored clavated, clamped. Lamella edge fertile. Hymenophoral trama regular, elements cylindrical to inflated, 62–148 × 14–38 μm. Pileipellis in the centre a cutis with narrow cylindrical elements of 23–48 × 1.5–7 μm; in the middle of the radius a cutis of cylindrical elements of 29–60 × 2–8 μm and with brown pigment, intracellular and diffuse; subpellis very well differentiated, made of inflated to rounded elements, 35–69 × 22–34 μm with diffuse, intracellular, brown pigment and also parietal pigment. Pileitrama made up of cylindrical to somewhat ‘sausage’ shaped elements, ranging 33–105 × 7–23 μm, with intracellular, diffuse pale yellow pigment. Vascular hyphae present. Stipititrama regular, made up of cylindrical elements, 56–140 × 15–28 μm with pale, diffuse, intracellular pigment. Caulocystidia absent. Clamp-connections abundant.
Habitat & Distribution — In poor, recently mown grassland (Mesobromion), S-facing, on calcareous load above limestone. Probably spread all over Europe, confirmed from Germany, England, Corsica (France).
Additional collections examined. France, Corsica, Galeria, Valley of River Fango, 3 Nov. 1982, R.A. Maas-Geesteranus 15721 (L). – United Kingdom, Pembrokeshire, Somerton Farm, 3 Oct. 2010, David Harries (L).
6.2. Entoloma ochreoprunuloides forma hyacinthinum Noordel. & Morgado, forma nov. — MycoBank MB802148; Fig. 6h
Holotype. United Kingdom, Pembrokeshire, Somerton Farm, 25 Sept. 2010, David Harries (L).
Differs from the typical form of E. ochreoprunuloides by having brown-violet tinges in pileus and stipe.
7. Entoloma fumosobrunneum Noordel. & Morgado, sp. nov. — MycoBank MB802145; Fig. 12a, 14
Fig. 12.
Habit of the following species: a. Entoloma fumosobrunneum (holotype, photos M.E. Noordeloos); b. E. bloxamii (photo M.E. Noordeloos), from epitype; c. E. bloxamii var. rubellum (G.Wolfel E20/04, photo Winterstein (top); Schmitz (bottom)); d. E. madidum (neotype, photo M.E. Noordeloos); e. E. caesiolamellatum (holotype, photo G. Wolfel); f. E. corneri (photo D. Stubbe, 06241); g. E. callidermum (photo Stubbe 06179); h. E. coeruleoviride (photo Stubbe 06236).
Fig. 14.
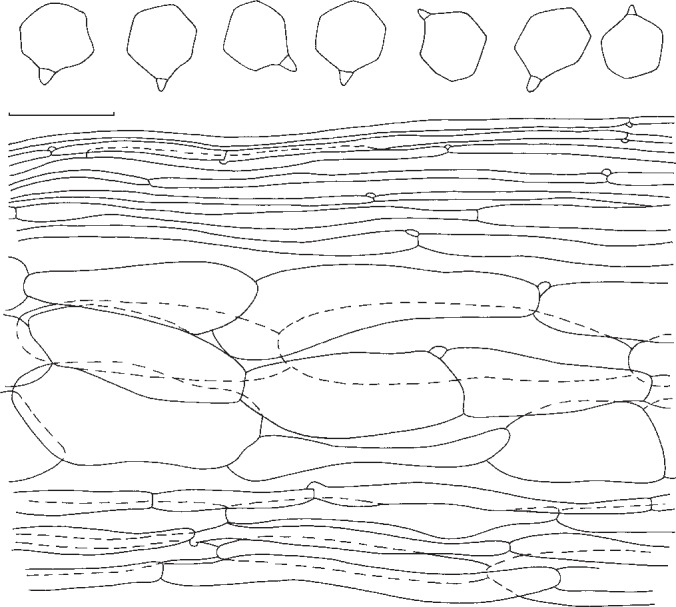
Entoloma fumosobrunneum. Spores and pileipellis (all: holotype). — Scale bar = 10 μm.
Pileus 30–70 mm, convex then plano-convex with low broad umbo, with first involute then deflexed, often undulating margin, not hygrophanous, not translucently striate, very dark grey-brown to grey at centre, slightly paler, variegated with lighter streaks towards margin, innately fibrillose on margin, slightly to distinctly rugulose or veined at centre, and often covered with a fine bloom or micaceous patches. Lamellae crowded to very crowded, L = 80–90, l = 3–7, segmentiform to subventricose, pale greyish cream then sordid pink with uneven, concolorous edge. Stipe 40–65 × 12–15 mm, cylindrical with broadened base (−18 mm), whitish with grey-brown fibrillose covering in upper half or three quarter, white or tinged yellow at base. Context thick, white. Smell and taste strongly farinaceous. Spores 5.8–7.9 × 5.7–8.4 μm, average 6.9 × 6.6 μm, Q = 1.0–1.16, Qav = 1.04, subisodiametrical, 5–6-angled, with relatively thick walls. Basidia (32−)35–42(−47) × 9–12 μm, 2- and 4-spored, clavate, often with pale intracellular pigment, clamped. Lamella edge fertile. Cystidia absent. Hymenophoral trama regular, made up of cylindrical hyphae, ranging 30–140 × 8–35 μm, with vascular hyphae. Pileipellis 2-layered, suprapellis an ixocutis of narrow cylindrical, 2.5–9.5 μm wide hyphae, at centre sometimes with transition to an ixotrichoderm of 2–7 μm wide; subpellis a well differentiated layer of inflated hyphal elements, 35–75 × 7–38 μm. Pileitrama regular, made up of elongated hyphae ranging 34–74 × 7–38 μm, with vascular hyphae present. Pigment intracellular, yellowish to brownish, diffuse and agglutinated in pileipellis and upper pileitrama. Stipitipellis a cutis of narrow, cylindrical hyphae, 2–7 μm wide. Stipititrama regular, made up of inflated elements, 51–197 × 9–23 μm with diffuse yellowish intracellular pigment and sometimes refringent walls. Clamp-connections abundant in all tissues.
Habitat & Distribution — In groups in grassland with scattered Abies balsamifera. Newfoundland, Canada, only known from the type locality.
Collections examined. Canada, Newfoundland, Gros Morne National Park, Killdevil Campground, 4 Sept. 2005, M.E. Noordeloos 2005120 (holotype, L); same locality, 2 Sept. 2005, M.E. Noordeloos 2005113 (L).
Notes — Entoloma fumosobrunneum is characterized by the smoky brown pileus and stipe, finely rugulose to distinctly veined surface of pileus, and relatively thick-walled, 5–6-angled spores. Entoloma ochreoprunuloides lacks grey tinges and has slightly smaller and more thin-walled spores. Entoloma tephreum Hesler from the Smoky Mountains resembles E. fumosobrunneum in having grey tinges in the basidiocarps, but is more fragile with a translucently striate, hygrophanous pileus, and has many-angled, very thin-walled spores, similar to those found in E. sect. Turfosa (Noordeloos 1984).
III. Entoloma bloxamii and look-alikes; characterized by blue pigmented, tricholomatoid basidiomes
In the older literature, E. bloxamii and E. madidum are sometimes treated as two different species, or as one, more variable species. Entoloma bloxamii originally was described as a relatively robust species with a dark blue-purple pileus and cylindrical stipe, attenuated towards the base. Agaricus madidus originally is a species with equally dark blue pileus but with a clavate stipe with a broad base and having a fetid odour (like Russua foetens). However, because of similarity in colour and the variability of the shape of the stipe, Quelet (1886) considered them synonymous and placed bloxamii as only a variant of madidus. Konrad & Maublanc (1924–1932) distinguish two species: Rhodophyllus bloxamii with a robust basidioma, relatively dark colours, a stipe with pointed yellowish base, and spores ranging 8–10 μm in length against R. madidus with a more slender habit, a pileus that fades with age and smaller spores (6–8 μm in length). Kühner & Romagnesi (1953), Orton (1960) and Moser (1978) adopted the same concept. The results of the present study clearly indicate that the bloxamii-complex in Europe consists of more than one species. Therefore we decided to fix the concept of E. bloxamii and E. madidum with an epitype, respectively neotype. Both species can indeed be distinguished on the spore size and shape, as indicated by Konrad & Maublanc (1924–1932).
8. Entoloma bloxamii (Berk. & Broome) Sacc. — Fig. 12b, 15
Fig. 15.
Entoloma bloxamii Spores and pileipellis (all: epitype).
Agaricus bloxamii Berk. & Broome, Ann. Mag. Nat. Hist., ser. II, 13: 399. 1854. — Entoloma bloxamii (Berk. & Broome) Sacc. (‘bloxami’), Syll. Fung. 5: 684. 1887. — Entoloma madidum var. bloxamii (Berk. & Broome) Largent, Madroño 22, 7: 368. 1974.
Type-study. Holotype: Herb. Berk. & Broom 815. Agaricus bloxamii, Twycross, Nov. 1851 (K).
The holotype consists of 4 fragments of sliced basidiocarps glued on paper with habit drawings, indicating the centre of the pileus being fuscus, the marginal zone atromentario-coeruleus; stipe concolorous. The type being in a rather bad state, only a few spores could be recovered and measured: 8.5–10.5 × 7.5–9 μm.
In view of the current insight, that E. bloxamii represents a polyphyletic complex of very similar species, an epitype is designated here to fix the specific epithet bloxamii for the current phylogenetic taxon.
Entoloma bloxamii — Description of the epitype (designated here): Austria, Vorarlberg, Bludenz, Alaas, Zug, Tannlageralpe, 29 Aug. 2004, M.E. Noordeloos 200442 (L).
Pileus 30–50 mm, conical then conico-convex, hardly expanding with age, with deflexed margin, not hygrophanous, not translucently striate, rather pale greyish blue sometimes with slight brown tinge, losing its blue colour almost entirely with age, then pale greyish brown, glabrous or very slightly innately fibrillose. Lamellae crowded, adnate-emarginate, white then pink with serrulate, concolorous edge. Stipe 20–45 × 10–18 mm, clavate with broadest part in lower half, almost white, with faint blue or bluish grey tinge, innately fibrillose, base white, sometimes with yellow tinges. Context thick, white. Smell and taste subfarinaceous, but rather weak. Spores 7.4–9.4(−10.8) × 6.7–9.7 μm, average 8.2 × 8.3 μm, Q = 1.0–1.2, Qav = 1.01, isodiametric to subisodiametric, thin-walled, 7–10-angled in side-view. Basidia (37−)48–70(−85) × 11–14.5 μm, clavate to oblong, clamped, 2- and 4-spored. Lamellae edge fertile, cystidia absent. Hymenophoral trama made up of cylindrical hyphae ranging 90–350 × 15–60 μm. Pileipellis in the centre is an ixocutis (very gelatinous) made of narrow cylindrical hyphae ranging 20–74 × 2–5 μm; in the middle of the radius is an ixocutis but with transitions to an ixotrichoderm, made of narrow cylindrical hyphae ranging 25–113 × 3–9 μm; subpellis not differentiated, pileipellis gradually passing into underlying pileitrama. Pigment diffusely intracellular and also in form of agglutinated yellow and light bluish pigment-clots. Pileitrama regular, made up of elongated cylindrical hyphae ranging 53–343 × 17–43 μm, with intracellular diffuse hyaline and parietal pigment. Stipitipellis is a cutis made of cylindrical narrow 3–9 μm wide hyphae. Stipititrama made up of cylindrical to fusiform hyphae ranging 45–130 × 10–20 μm, with pale to yellow intracellular diffuse pigment and also parietal pigment present. Vascular hyphae present in trama. Clamp-connections present and abundant.
Type specimen habitat and general distribution — In short grazed subalpine meadow on calcareous soil at about 1800–2000 m altitude, probably spread all over Europe, confirmed from Portugal, Italy, Germany and Austria.
Additional collections examined. Germany, Sternberg, Hohenstein, 1 Oct. 2010, S. Baireuther 011010 (L). – Italy, Trento, Val di Sella (Valsugana), 4 Oct. 1982, C. Bas 8003 (L). – Portugal, Lisbon, Parque de Monsanto, 17 Dec. 2011, A. Lebre, LNM 171211 (L).
8.1. Entoloma bloxamii var. rubellum (Scop.: Gillet) Morgado & Noordel., comb.nov. — MycoBank MB802146; Fig. 12c
Basionym. Agaricus rubellus Scop., Fl. Carniol., ed. 2 (Wien) 2: 445. 1772.
Syn. Entoloma rubellum (Scop.) Gillet, Hyménomycètes (Alençon) 1: 1–176. 1874; Rhodophyllus rubellus (Scop.) Quel., Enchir. Fung. (Paris): 58. 1886.
Differs from the type variety by the light pink colours in the pileus and stipe. All the other characters are similar. It can be confused with pale pinkish ochre specimens of E. ochreoprunuloides, which, however, can easily be distinguished by the larger spores (see below).
Habitat & Distribution — Similar to the type variety. Probably spread all over Europe, confirmed from Germany, Italy and France.
Collections examined. France, Dijon, 1 Oct. 2000, J.C. Vegeau, JCV 011000 (L). – Germany, Nordrhein Westfalen, Eierberg, 25 Oct. 2004, G. Wöllfel, E20/04 (L); Sternberg, Hohenstein, 1 Oct. 2010, S. Baireuther 011010 (L). – Italy, Fossombrone, S. Cristrophoro del Valli, 28 Oct. 1998, M. Malleti, MM 281098 (L).
9. Entoloma madidum (Fr. ex) Gillet, Hymenomycetes: 399. 1876. — Fig. 12d, 16
Fig. 16.
Entoloma madidum. Spores and pileipellis (all: neotype). — Scale bar = 10 μm.
Description of the neotype. The Netherlands, prov. Gelderland, Staverden, Loam-Pits, 30 Sept. 2004, M.E. Noordeloos 2004030 (L).
Pileus 30–80 mm, conical at first, expanding to plano-convex with low, broad umbo, with margin first involute then deflexed, not hygrophanous, not translucently striate, deep blue with violaceous tinge, retaining its colour when old, innately radially fibrillose. Lamellae moderately crowded, adnate-decurrent, white then rather purely pink with irregularly serrulate, concolorous edge. Stipe 40–90 × 10–20 mm, cylindrical, blue-violaceous, at least in upper 3/4, basal part white or with faint yellow tinge, innately fibrillose. Context medium thick, white. Smell and taste farinaceous. Spores 5.9–7.6 × 5.8–7.5 μm, average 6.8 × 6.7 μm, Q = 1.0–1.2, Qav = 1.03, thin-walled, with 6–8-angled in side view, thin wall. Basidia 33–48 × 8.5–10.5 μm, clavate, clamped, 2–4-spored. Lamellae edge fertile. Cystidia absent. Hymenophoral trama regular, elements 43–95 × 6–15 μm, with pale blue, diffuse intracellular pigment. Pileipellis a very gelatinouse ixocutis at centre, with some ascending hyphae forming a transition to an ixotrichoderm, made of cylindrical hyphae with elements ranging 30–116 × 2–8 μm, with hyaline bluish diffuse pigment; in the middle of the radius an ixocutis with transitions to an ixotrichoderm, hyphae ranging 30–65 × 3–6.5 μm, with pale blue, intracellular diffuse pigment; subpellis differentiated, made up of elongated, inflated elements, ranging 20–72 × 11–28 μm, with bluish and brownish diffuse intracellular pigment. Pileitrama regular, made of cylindrical elements, ranging 25–70 × 10–20 μm, with pale blue, intracellular diffuse and agglutinated pigment. Vascular hyphae present. Stipitipellis a thin ixocutis of 3–8 μm wide hyphae, with pale yellow and light bluish, diffuse intracellular pigment. Stipititrama regular, made up of cylindrical elements, ranging 38–160 × 8.5–20 μm, with yellowish intracellular and parietal pigment. Clamp-connections present in all tissues.
Habitat & Distribution — In poorly managed grassland on heavy loamy soil, with scattered deciduous trees (Quercus, Betula). Probably spread all over Europe, confirmed from Norway, The Netherlands and Italy.
Additional collections examined. Italy, Trentino, Baselga del pini, 3 Oct. 2002, M.E. Noordeloos 02165 (L). – Norway, Herøy kom., Tenna, Geithammaren, 19 Sept. 2004, M. Gjestland, T. Solem, E. Håve, E. Johannesen, E.W. Hanssen, B.H. Larsen 67195 (O). – The Netherlands, prov. Gelderland, Staverden, 9 Oct. 1990, E.C. Vellinga & E.J. Weeda 1702 (L).
Notes — The name Agaricus madidus Fr. 1836, is not sanctioned, therefore it is a later homonym of Agaricus madidus Pers. 1828 and illegitimate. When Gillet (1876) combined the epithet madidus of Fries in the genus Entoloma, it became legitimate and available for the present taxon. The neotype fixes now the name E. madidum for the small-spored look-alike of E. bloxamii.
10. Entoloma caesiolamellatum (Noordel. & Wölfel) Noordel. & Morgado, comb. nov. — MycoBank MB802147; Fig. 12e, 17
Fig. 17.
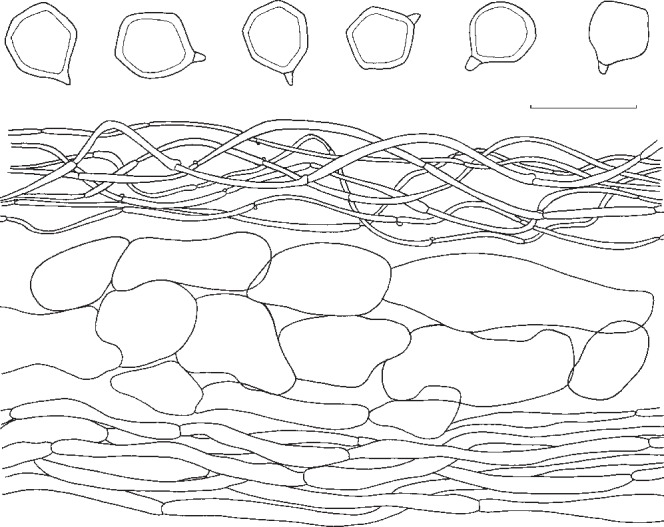
Entoloma caesiolamellatum (all: holotype). — Scale bar = 10 μm.
Basionym. Entoloma bloxamii forma caesiolamellatum Noordel. & Wölfel. Öster. Z. Pilzk. 10: 187. 2001.
Pileus 40–60 mm, conico-convex then convex or plano-convex, with deflexed margin, not hygrophanous, not translucently striate, deep blackish blue all over, innately fibrillose, at centre with micaceous sheen. Lamellae rather crowded, adnate-emarginate, dark bluish grey then with pink tinge, with irregular, concolorous edge. Stipe 30–60 × 10–15 mm, cylindrical, upper part dark blackish blue fibrillose on paler background, base white. Smell and taste unknown. Spores 6–8.1 × 6.3–8 μm, average 7.1 × 7.1 μm, Q = 1.0–1.13, Qav = 1.01, isodiametric, weakly 5–6-angled, with thick wall 0.5–1 μm. Basidia 33–41 × 8.5–10.6 μm, clavate, 2- and 4-spored, with hyaline intracellular pigment. Lamella edge fertile. Cystidia absent. Hymenophoral trama regular, made up of cylindrical elements ranging 45–135 × 11–27 μm, with hyaline to pale diffuse intracellular pigment, vascular hyphae present. Pileipellis an ixocutis with narrow cylindrical hyphae of short length 17–51 × 2–9 μm, with very pale to hyaline intracellular diffuse pigment; in the centre of the pileus the hyphae of the suprapellis are more undulated than in the middle of the radius; subpellis is made of inflated and rounded hyphae ranging 30–72 × 14–45 μm, with dark blue intracellular vaccuolar pigment (absent in the suprapellis). Pileitrama regular, made up of cylindrical hyphae elements ranging 38–100 × 11–35 μm, with pale yellowish intracellular diffuse and dark blue vaccuolar pigment; vascular hyphae present. Stipitipellis a narrow cutis, reaching up to 5 μm wide hyphae, with yellowish to hyaline intracellular diffuse pigment; without caulocystidia. Stipititrama regular, made up of cylindrical elements, 95–207 × 19–61 μm, with thick walls. Pigment blue and yellowish to hyaline intracellular agglutinated and diffuse, respectively; vascular hyphae present. Clamp-connections present in all tissues and abundant in the suprapellis.
Collections examined. Spain, Canary Islands, Isla La Palma, Pared Viega, 20 Jan. 2000, G. Wölfel, E20/04 (holotype of E. bloxamii forma caesiolamellatum, L). – USA, CA, Mendocino Co., Tom Bell Flat, 12 Jan. 1989, Timothy J. Baroni, TB6117 (State University of New York – College at Cortland).
Notes — This species is characterized by rather dull greyish blue tinges in the whole basidioma, including the lamellae. It differs from E. bloxamii by the short length of the pileipellis elements. Similar taxa occur in the USA (TB6117)
11. Notes on Entoloma bloxamii-NA from California
Largent (1974) studied E. bloxamii look-alikes in the Pacific Northwest of the USA, and made a taxonomical distinction between E. bloxamii and E. madidum based on spore-size. However, in a later work Largent (1994) changed his opinion, and proposed them as a single taxon. As our study shows neither E. bloxamii or E. madidum occur in North America and therefore, the North American collections of E. bloxamii look-alikes need to be re-evaluated.
Entoloma cf. bloxamii from California
Microscopic characters.
Spores (6−)7–9 × 6–8.5(−9.3) μm, average 7.7 × 7.0 μm, Q = 1.0–1.17, Qav = 1.1, subisodiametric, 5–6-angled in side view. Basidia 40–47 × 10–12 μm, clavate, 2- and 4-spored, with intracellular agglutinated hyaline to greyish pigment. Cystidia absent. Lamellae edge fertile. Hymenophoral trama ranging 22–165 × 5–25 μm, with intracellular diffuse and agglutinated greyish pigment. Pileipellis at centre is an ixocutis made of cylindrical hyphae ranging 21–96 × 2–6 μm, with intracellular yellow diffuse and blue agglutinated pigment, in addition there is also parietal pigment; in the middle of the radius is an ixocutis made of narrow cylindrical hyphae ranging 20–85 × 3–8 μm, and intracellular diffuse and agglutinated yellow and blue pigment, in addition there is also parietal pigment; subpellis made up of elongated and inflated hyphae in the middle of the radius ranging 35–104 × 15–30 μm, and more or less rounded hyphae ranging 9–35 × 7.5–15 μm, with intracellular diffuse pale yellowish to brownish pigment, and also parietal pigment in the middle of the radius; the pigment is more evident between the supra and subpellis region. Pileitrama made up of cylindrical hyphae ranging 38–134 × 12–18 μm, with intracellular diffuse yellowish pigment. Vascular hyphae absent. Stipititrama ranging 48–160 × 9–28 μm, with intracellular diffuse and agglutinated brown to yellowish pigment. Clamp-connections abundant in all tissues.
Collections examined. USA, CA, Mendocino Co. West Willits, 6 Jan. 1996, Timothy Baroni 7876 (State University of New York – College at Cortland); CA, Mendocino Co., 30 Nov. 1991, H.D. Thiers 53901 (The New York Botanical Garden); CA, Mendocino Co., E.C. Vellinga (collection missing).
Notes — Macroscopic features of the collections studied here are unfortunately missing and since this species possibly occurs in sympatry (in USA CA) with E. caesiolamellatum we prefer to wait for further collections to give a full description and propose this taxon as a new species. Based on the microscopy however, we can already state that the spore size will be a useful character to separate this species from E. caesiolamellatum. Entoloma cf. bloxamii from California (= E. bloxamii-NA in Fig. 3 clade III) has a broader range and on average slightly bigger spores than E. caesiolamellaum.
IV. Notes on some other species used in the analysis
12. Entoloma corneri E. Horak, Beih. Nova Hedwigia 65: 137. 1980. — Fig. 12f, 18
Fig. 18.
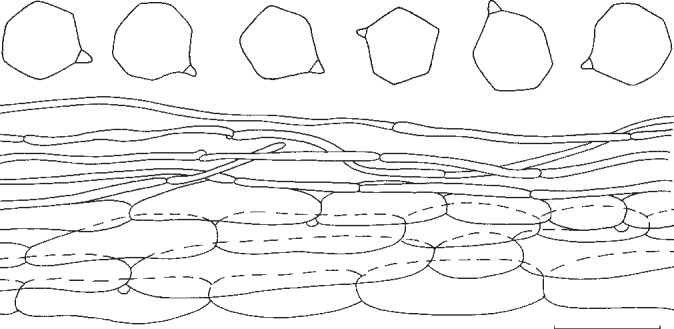
Entoloma corneri. Spores and pileipellis (all: Stubbe 06241). — Scale bar = 10 μm.
Pileus 37–92 mm, convex-umbonate with inflexed then straight margin, not hygrophanous, not translucently striate, dark olivaceous brown at centre, olive-green towards margin, with white outermost margin, smooth, glabrous, silky shining. Lamellae deeply emarginate, moderately crowded, subventricose, 5–7 mm broad, pale pinkish white with entire and concolorous edge. Stipe 23–73 × 6–15 mm, cylindrical, equal or slightly tapering downwards, pale yellow with slightly greyish to olivaceous tinge, whitish at apex and at base, smooth or innately fibrillose. Context white, rather thin in pileus, solid in stipe. Smell sweetish, more or less anise-like. Taste very bitter. Spores 7.0–7.5 × 6.0–7.5 μm, Q = 1.0–1.15, Qav = 1.05, subisodiametrical, 5–8-angled in side-view, with thin to slightly thickened walls. Basidia 18–40 × 6–11 μm, 4-spored, clamped. Lamella edge fertile. Cystidia absent. Hymenophoral trama regular, made up of long, cylindrical elements, 5–20 μm wide. Pileipellis 2-layered, suprapellis a cutis to ixocutis of very narrow, 2–4 μm wide, cylindrical hyphae; subpellis regular, made up of short, inflated elements, 20–60 × 5–20 μm, rather well delimited from underlying pileitrama of cylindrical elements. Clamp-connections abundant in all tissues.
Collections examined. Malaysia, Negeri Sembilan, Pasoh National Forest Reserve, along main trail, between stream and Old Tree Tower, on soil near Shorea, 17 Sept. 2009, D. Stubbe 06241 (GENT).
Notes — Large Entoloma species from E. sect. Entoloma sensu Noordeloos (1992) are rarely found in tropical areas (Horak 1980, Largent et al. 2008). This collection fits fairly well with E. corneri, described from the Singapore Botanic Gardens, and also recorded from New Guinea, differing mainly by the lack of olivaceous tinges in the pileus and pale brown stipe (Horak 1980). Further studies are needed to determine if this is due to infra- or intraspecific variation. For the time being it seems sensible to use this name for this material. Entoloma olivaceocoloratum Largent & T.W. Henkel from Guyana fits also well with its olivaceous-brown pileus, and yellowish stipe, and small spores (Largent et al. 2008).
13. Entoloma callidermum (Romagn.) Noordel. & Co-David, in Co-David et al., Persoonia 23: 165. 2009. — Fig. 12g, 19
Fig. 19.
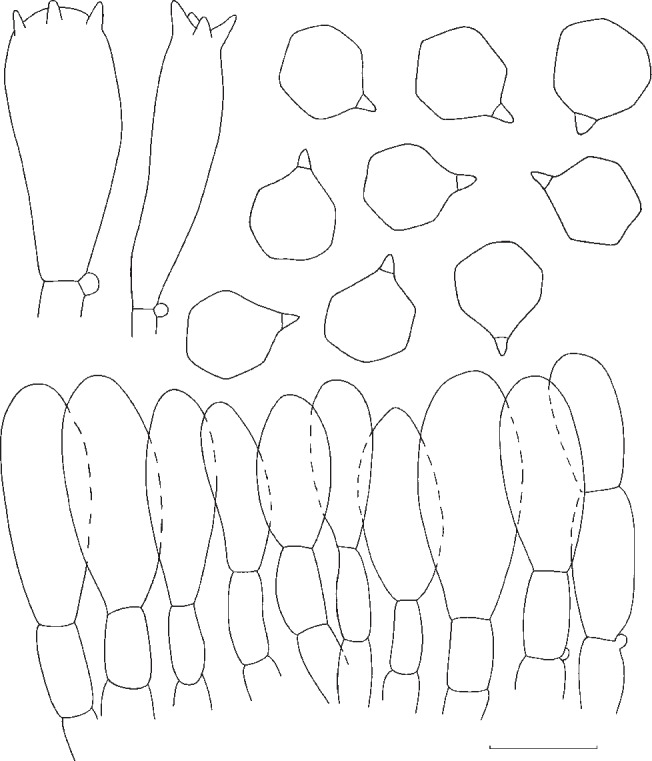
Entoloma callidermum Spores and pileipellis (all: Stubbe 06179). — Scale bar = 10 μm.
Rhodophyllus callidermus Romagn., Bull. Jard. Bot. État Bruxelles 26: 171. 163. 1956.
Pileus 35–73 mm, convex then plano-convex with slightly depressed centre, with involute to deflexed, wavy margin, surface dry, completely and minutely velutinous and radially wrinkled, dark blue, like blue velvet (19–20F4–8) with more violet tinges in centre and locally towards the margin (18–19E5–8). Lamellae emarginate-adnate, crowded, rather broadly ventricose, pale cream coloured (3A2), locally with a faint pinkish tinge; with subentire, concolorous edge. Stipe 50–68 × 9–10 mm, cylindrical, slightly broadening towards the apex, whitish violet (18D4–6), whitish towards base, surface dry, adpressed fibrillose, shiny (silky gloss). Context moderately thin in pileus, solid in stipe, whitish. Smell unremarkable. Taste slightly mealy. Spores 7.5–9.5 × 6.5–8.0 μm, Q = 1.0–1.2, (sub)isodiametrical, 5–6(−7)-angled in side-view, rather thin-walled. Basidia 27–35 × 6–11 μm, 4-spored, clamped. Lamella edge fertile. Cystidia absent. Hymenophoral trama made up of inflated elements, 40–120 × 6–20 μm. Brilliant granules sparse. Pileipellis a hymeniderm, of clavate elements, 22–40 × 9–20 μm. Pigment bright blue, intracellular in pileipellis and in upper pileitrama. Pileitrama regular, made up of cylindrical to inflated elements, 50–155 × 4–22 μm. Clamp-connections abundant in hymenium, scattered in other tissues.
Collections examined. Malaysia, Negeri Sembilan, Pasoh National Forest Reserve, along main trail, between beginning of Nature Trail and stream, near Shorea sp., 19 Sept. 2006, D. Stubbe 06252 (GENT); Negeri Sembilan, Pasoh National Forest Reserve, in experimental plots A/D, dominated by Shorea (regenerating rainforest, formerly completely logged over), 12 Sept. 2006, D. Stubbe 06179 (GENT).
Notes — The collections studied match well with the original description of E. callidermum from Africa, which also has been recorded from various other tropical areas (Horak 1980, Karstedt & Capelari 2010). It is a striking species with a deep blue and velvety pileus, which shows in radial section a perfect hymeniderm of clavate elements, also called a calliderm. This type of pileipellis was the main criterion for Romagnesi to create E. sect. Calliderma within E. subg. Inopilus (Romagnesi 1978), and later, for Largent (1994) to consider it a genus in its own right. Entoloma rugosopruinatum Corner & E. Horak from Sabah (Malaysia) is very similar, differing mainly by the supposedly clampless hyphae (Horak 1980). The inclusion of E. callidermum within the basal grade of Entoloma phylogeny indicated that the value of pileipellis structure as criterion for higher taxonomic differentiation within the entolomatoid fungi needs to be revaluated.
14. Entoloma coeruleoviride Corner & E. Horak, Beih. Nova Hedwigia 65: 137. 1980. — Fig. 12h, 20
Fig. 20.
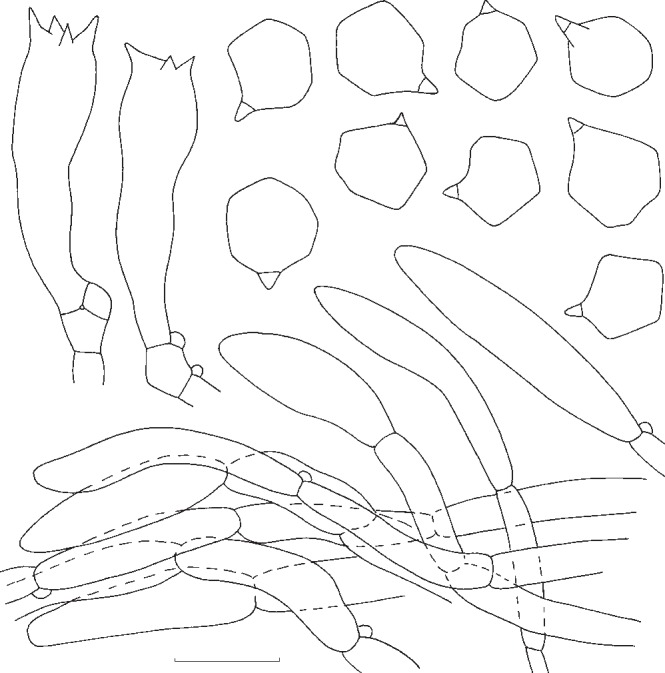
Entoloma coeruleoviride. Spores and pileipellis (all: Stubbe 06236). — Scale bar = 10 μm.
Pileus 62 mm, convex-subumbonate with straight, smooth margin, not hygrophanous, not translucently striate, deep dark violet to blackish violet (17–18F4–5), dry, adpressed tomentose and radially rugulose. Lamellae crowded, emarginate, ventricose, up to 8 mm broad, pale pink with slightly eroded and concolorous edge. Stipe 125 × 8 mm, cylindrical, slightly broadening at apex, blue (19D3–5), whitish towards apex and base, dry, longitudinally striate, shiny. Context white. Smell sweetish. Taste not recorded. Spores 7.5–9.0 × 7.0–8.0 μm, Q = 1.0–1.2, Qav = 1.1, 5–7-angled with rather pronounced angles, (sub-)isodiametrical. Basidia 20–36 × 6–10 μm, 4-spored, clamped. Lamella edge appearing fertile. Cystidia not observed (see comment). Hymenophoral trama regular, made up of cylindrical or slightly inflated hyphae, elements 50–120 × 4–12 μm. Brilliant granules absent. Pileipellis a trichoderm of clavate to fusiform terminal elements, 40–65 × 11–25 μm, arising from a regular subpellis made up of cylindrical hyphae, 5–12 μm wide. Pigment intracellular, deep blue, abundant in pileipellis and upper pileitrama. Pileitrama regular, made up of cylindrical to inflated hyphae, 4–14 μm wide. Clamp-connections abundant in all tissues.
Collections examined. Malaysia, Negeri Sembilan, Pasoh National Forest Reserve, along main trail, near Old Tree Tower, on soil, 17 Sept. 2006, D. Stubbe 06236 (GENT).
Notes — The present collection consists of only one, mature basidioma. In many respects it can be identified as E. coeruleoviride. This striking species, only known so far from Singapore Botanic Gardens, has a sterile lamella edge with clavate cheilocystidia (Horak 1980, pl. 6c). More material is needed to evaluate the differences between our material and the holotype. Entoloma rugosopruinatum from Sabah (Malaysia) is macroscopically similar, but differs by having a true hymeniderm of clavate, much wider elements, and clampless hyphae. Entoloma kermandii G.M. Gates & Noordel. is also similar, differing mainly by the different colour of the basidioma, and fusiform stipe, frequently with yellow tinge at base.
Acknowledgments
Delia Co-David is gratefully acknowledged for her help and guidance in getting a large number of sequences for this study during the MSc project of LNM in 2007. Many people contributed to this study with collections, notes and advice, in particular Timothy J. Baroni, Dirk Stubbe, Sieglinde Baireuther, David Harries, Ken Preston Maffin, A. Lebre and Gerhard Wölfel. Anita Walsmit Sachs prepared the line drawings for print. Andrus Voitk kindly invited MEN to visit Newfoundland and Labrador (Canada) in 2005 where several interesting collections initiated this study. Cilia Grebenstein, Nicolas Davin and Luisa Carvalheiro are thanked for help in the statistical analyses and Dorien Langeveld for providing scan electronic microscopic pictures for the preliminary analyses in this study. We are also grateful for the constructive critics of two anonymous reviewers.
REFERENCES
- Agerer R. 1997. Entoloma sinuatum (Bull.: Fr.) Kummer+Salix spec. Descriptions of Ectomycorrhizae 2: 13–18 [Google Scholar]
- Avise JC, Ball RM. 1990. Principles of genealogical concordance in species concepts and biological taxonomy. Oxford Surveys in Evolutionary Biology 7: 45–67 [Google Scholar]
- Baroni TJ, Hofstetter V, Largent D, Vilgalys R. 2011. Entocybe is proposed as a new genus in the Entolomataceae (Agaricomycetes, Basidiomycota) based on morphological and molecular evidence. North American Fungi Vol. 6, 12: 1–19 [Google Scholar]
- Baroni TJ, Matheny B. 2011. A re-evaluation of gasteroid and cyphelloid species of Entolomataceae from eastern North America. Harvard Papers in Botany 16: 293–310 [Google Scholar]
- Co-David D, Langeveld D, Noordeloos ME. 2009. Molecular phylogeny and spore evolution of Entolomataceae. Persoonia 23: 147–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee MPA, Wingfield BD, Harrington TC, Dalevi D, Coutinho TA, Wingfield MJ. 2000. Geographic diversity of Armillaria mellea s.s. based on phylogenetic analysis. Mycologia 92: 105–113 [Google Scholar]
- Dahlberg A, Croneborg H. (eds). 2006. The 33 threatened fungi in Europe. Nature and Environment No. 136, Council of Europe Publishing, Strasbourg [Google Scholar]
- Dettman JR, Jacobson DJ, Taylor JW. 2003. A multilocus genealogical approach to phylogenetic species recognition in the model eukaryote Neurospora. Evolution 57, 12: 2703–2720 [DOI] [PubMed] [Google Scholar]
- Drummond AJ, Ashton B, Buxton S, Cheung M, Cooper A, et al. 2011. Geneious v5.4. Available from http://www.geneious.com
- Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high 25 throughput. Nucleic Acids Research 32: 1792–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayod V. 1889. Prodrome d’une histoire naturelle de Agaricinées. Annales des Sciences Naturelles. Botanique VII 9: 181–411 [Google Scholar]
- Felsenstein J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39: 783–791 [DOI] [PubMed] [Google Scholar]
- Finlay B. 2002. Global dispersal of free-living microbial eukaryote species. Science 296: 1061–1063 [DOI] [PubMed] [Google Scholar]
- Gates G, Horton B, Noordeloos ME. 2009. A new Entoloma (Basidiomycetes, Agaricales) from Tasmania. Mycotaxon 102: 175–179 [Google Scholar]
- Gates G, Noordeloos ME. 2007. Preliminary studies in the genus Entoloma in Tasmania – I. Persoonia 19: 157–226 [Google Scholar]
- Geml J, Davis DD, Geiser DM. 2005a. Systematics of the genus Sphaerobolus based on molecular and morphological data, with the description of Sphaerobolus ingoldii sp. nov. Mycologia 97: 680–694 [DOI] [PubMed] [Google Scholar]
- Geml J, Davis DD, Geiser DM. 2005b. Phylogenetic analyses reveal deeply divergent species lineages in the genus Sphaerobolus (Phallales: Basidiomycota). Molecular Phylogenetics and Evolution 35: 313–322 [DOI] [PubMed] [Google Scholar]
- Geml J, Kauff F, Brochmann C, Lutzoni F, Laursen GA, Redhead SA, Taylor L. 2012. Frequent circumarctic and rare transequatorial dispersals in the lichenised agaric genus Lichenomphalia (Hygrophoraceae, Basidiomycota). Fungal Biology 116, 3: 388–400 [DOI] [PubMed] [Google Scholar]
- Geml J, Tulloss RE, Laursen GA, Sazanova NA, Taylor DL. 2008. Evidence for strong inter- and intracontinental phylogeographic structure in Amanita muscaria, a wind-dispersed ectomycorrhizal basidiomycete. Molecular Phylogenetics and Evolution 48: 694–701 [DOI] [PubMed] [Google Scholar]
- Gillet CC. 1876. Hyménomycètes de France. Paris [Google Scholar]
- Giraud T, Refrégier G, Le Gac M, Vienne DM, Hood ME. 2008. Speciation in fungi. Fungal Genetics and Biology 45: 791–802 [DOI] [PubMed] [Google Scholar]
- Graham V. 1944. Mushrooms of the Great Lakes region. Dover Publications, Inc. New York, USA [Google Scholar]
- Hamady M, Lozupone C, Knight R. 2010. Fast UniFrac: facilitating high-throughput phylogenetic analyses of microbial communities including analysis of pyrosequencing and PhyloChip data. The ISME Journal 4: 17–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesler RL. 1967. Entoloma in southeastern North America. Beihefte zur Nova Hedwigia [Google Scholar]
- Hibbett DS. 2001. Shiitake mushrooms and molecular clocks: historical biogeography of Lentinula. Journal of Biogeography 28: 231–241 [Google Scholar]
- Horak E. 1980. Entoloma (Agaricales) in Indomalaya and Australasia. Beih. Nova Hedwigia 65: 1–352 [Google Scholar]
- Horak E. 2008. Agaricales of New Zealand. 1: Pluteaceae (Pluteus, Volvariella) Entolomataceae (Caludopus, Clitopilus, Entoloma, Pouzarella, Rhodocybe, Richoniella). Fungi of New Zealand Volume 5. Fungal Diversity Research Series 19: 1–305 [Google Scholar]
- Karstedt F, Capelari M. 2010. New species and new combinations of Calliderma (Entolomataceae, Agaricales). Mycologia 102: 163–173 [DOI] [PubMed] [Google Scholar]
- Kasuya T, Takehashi S, Hoshino T, Noordeloos ME. 2010. Entoloma aprile (Agaricales, Entolomataceae) new to Japan, with notes on its mycorrhiza associated with Populus maximowiczii in cool-temperate deciduous forests of Hokkaido. Sydowia 62, 2: 205–223 [Google Scholar]
- Katoh K, Toh H. 2008. Improved accuracy of multiple ncRNA alignment by incorporating 16 structural 11 information into a MAFFT-based framework. BMC Bioinformatics 9: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk PM, Cannon PF, Minter DW, Stalpers JA. 2008. Ainsworth and Bisby’s Dictionary of the Fungi, 10th edn.CAB International, Wallingford, UK [Google Scholar]
- Konrad P, Maublanc A. 1924–1932. Icones Selectae Fungorum. Vol. 2 Ed. Lechevalier Paul, Paris [Google Scholar]
- Kornerup A, Wanscher JH. 1978. Methuen handbook of colour, ed. 3 London [Google Scholar]
- Kroken S, Taylor JW. 2001. A gene genealogical approach to recognize phylogenetic species boundaries in the lichenized fungus Letharia. Mycologia 93: 38–53 [Google Scholar]
- Kühner R, Romagnesi H. 1953. Flore analytique des champignons supérieurs (Agarics, Bolets, Chanterelles). Masson, Paris [Google Scholar]
- Largent D. 1974. New or interesting species of Claudopus and Entoloma from the Pacific Coast. Madroño 22: 363–373 [Google Scholar]
- Largent D. 1994. Entolomatoid fungi of the Pacific Northwest and Alaska. Mad River Press, USA [Google Scholar]
- Largent D, Baroni TJ. 1988. How to identify mushrooms to genus VI: Modern genera. Mad River Press, Eureka, CA [Google Scholar]
- Largent D, Henkel TW, Aime MC, Baroni TJ. 2008. The Entolomataceae of the Pakaraima Mountains of Guyana I: four new species of Entoloma s.str. Mycologia 100, 1: 132–140 [DOI] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, et al. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23, 21: 2947–2948 [DOI] [PubMed] [Google Scholar]
- Lassmann T, Sonnhammer ELL. 2005. Kalign – an accurate and fast multiple sequence alignment algorithm. BMC Bioinformatics 6: 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassmann T, Sonnhammer ELL. 2006. Automatic assessment of alignment quality. Nucleic Acids Research 33: 7120–7128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YC, Yang ZL, Tolgor B. 2009. Phylogenetic and biogeographic relationships of Chroogomphus species as inferred from molecular and morphological data. Fungal Diversity 38: 85–104 [Google Scholar]
- Manimohan P, Noordeloos ME, Dhanya AM. 2006. Studies on the genus Entoloma (Basidiomycetes, Agaricales) in Kerala State, India. Persoonia 19: 45–94 [Google Scholar]
- Martin AP. 2002. Phylogenetic approaches for describing and comparing the diversity of microbial communities. Applied and Environmental Microbiology 68, 8: 3673–3682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheny PB, Curtis JM, Hofstetter V, Aime MC, Moncalvo J-M, et al. 2006. Major clades of Agaricales: a multilocus phylogenetic overview. Mycologia 98: 982–995 [DOI] [PubMed] [Google Scholar]
- Mayr E. 1963. Animal species and evolution. Cambridge, Belknap Press of Harvard University Press [Google Scholar]
- Miller OK, Jr, Farr DE. 1975. Index of the common fungi of North America, (synonymy and common names). Bibliotheca Mycologica 44. Cramer [Google Scholar]
- Moncalvo J-M, Buchanan PK. 2008. Molecular evidence for long distance dispersal across the Southern Hemisphere in the Ganoderma applanatum-australe species complex (Basidiomycota). Mycological Research 112, 4: 425–436 [DOI] [PubMed] [Google Scholar]
- Moncalvo J-M, Vilgalys R, Redhead S, Johnson J, James T, et al. 2002. One hundred and seventeen clades of euagarics. Molecular Phylogenetics and Evolution 23: 357–400 [DOI] [PubMed] [Google Scholar]
- Montecchio L, Rossi S, Courty PE, Garbaye J. 2006. Entoloma nitidum Quél. plus Carpinus betulus L. Descriptions of Ectomycorrhizae 9/10: 33–38 [Google Scholar]
- Moser MM. 1978. Key to Agarics and Boleti (Polyporales, Boletales, Agaricales, Russulales). Fischer Verlag, Stuttgart [Google Scholar]
- Mueller GM, Wu QX, Huang YQ, Guo SY, Aldana-Gomez R, Vilgalys R. 2001. Assessing biogeographic relationships between North American and Chinese macrofungi. Journal of Biogeography 28: 271–281 [Google Scholar]
- Murrill WA. 1917. North American Flora Vol. 10 Part 2. The New York Botanical Garden [Google Scholar]
- Noordeloos ME. 1984. Studies in Entoloma 10–13. Persoonia 12: 193–223 [Google Scholar]
- Noordeloos ME. 1992. Entoloma s.l. in Fungi Europaei, vol. 5 Ed. Candusso, Alassio. [Google Scholar]
- Noordeloos ME. 2004. Entoloma s.l. in Fungi Europaei, vol. 5a Ed. Candusso, Alassio. [Google Scholar]
- Noordeloos ME. 2008. Entoloma in North America 2: the species described by C.H. Peck – type studies and comments. Österreichische Zeitschrift für Pilzkunde 17: 87–152 [Google Scholar]
- Noordeloos ME, Gates GM. 2012. The Entolomataceae of Tasmaia. Fungal Diversity Research Series, vol. 22 Springer [Google Scholar]
- Noordeloos ME, Gulden G. 1989. Entoloma (Basidiomycetes, Agaricales) of alpine habitats on the Hardangervidda near Finse, Norway, with a key including species from Northern Europe and Greenland. Canadian Journal of Botany 67: 1727–1738 [Google Scholar]
- Orton PD. 1960. New checklist of British Agarics and Boleti. III. Notes on genera and species in the list. Transactions of the British Mycological Society 43: 159–439 [Google Scholar]
- Peck C. 1906. New species of fungi. Bulletin of the Torrey Botanical Club 33: 213–221 [Google Scholar]
- Putte K van de, Nuytinck J, Das K, Verbeken A. 2012. Exposing hidden diversity by concordant genealogies and morphology – a study of the Lactifluus volemus (Russulales) species complex in Sikkim Himalaya (India). Fungal Diversity 55: 171–194 [Google Scholar]
- Quélet N. 1886. Enchiridion Fungorum in Europa media et prasesertim in Gallia vigentum. Paris [Google Scholar]
- R development core team 2012.
- Rambaut A. 2002. Se-Al v2.0a11 carbon. Oxford University, Oxford [Google Scholar]
- Romagnesi H. 1974. Essai d’une classification des Rhodophylles. Bulletin Mensuel de la Société Linnéenne de Lyon 43: 325–332 [Google Scholar]
- Romagnesi H. 1978. Les fondéments de la taxonomie des Rhodophylles et leur classification. Beihefte zur Nova Hedwigia 59: 1–80 Cramer, Germany. [Google Scholar]
- Ronquist F, Teslenko M, Mark P, Ayres D, Darling A, et al. 2012. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roody W. 2003. Mushrooms of West Virginia and the Central Appalachians, Louisville, University Press of Kentucky [Google Scholar]
- Taylor JW, Jacobson DJ, Kroken S, Kasuga T, Geiser DM, et al. 2000. Phylogenetic species recognition and species concepts in fungi. Fungal Genetics and Biology 31, 1: 21–32 [DOI] [PubMed] [Google Scholar]
- Taylor JW, Turner E, Townsend JP, Dettman JR, Jacobson D. 2006. Eukaryotic microbes, species recognition and the geographic limits of species: examples from the kingdom fungi. Philosophical Transactions of the Royal Society of London, ser. B, 361: 1947–1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor JW. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ. (eds), PCR protocols: a guide to methods and applications: 315–322 Academic Press, New York [Google Scholar]
- Xiao-Lan H, Tai-Hui L, Zi-De J, Ya-Heng S. 2012. Four new species of Entoloma s.l. (Agaricales) from southern China. Mycological Progress 11, 4: 915–925 [Google Scholar]
- Zwickl D. 2006. Genetic algorithm approaches for the phylogenetic analysis of large biological sequence datasets under the maximum likelihood criterion. PhD thesis, The University of Texas at Austin [Google Scholar]