Abstract
Proliferin is a recently described, prolactin-related protein whose mRNA appears in several murine cell lines during active growth. We have surveyed a number of mouse organs or tissues for the presence of mRNAs that hybridize to cloned proliferin cDNA. Of the tissues tested, only the placenta yielded proliferin-related mRNA. This placental RNA is about 1 kilobase in length, increases sharply between days 8 and 10 of pregnancy, and then gradually declines through day 18. It is more abundant in RNA extracted from the fetal, compared to the maternal, part of the placenta. From a cDNA plasmid library prepared from poly(A)+ placental RNA, two types of proliferin-related clones were isolated, differing in intensity of hybridization to proliferin cDNA. By nucleotide sequence analysis, a strongly hybridizing clone was found to be nearly identical to the proliferin cDNA clone isolated from a library prepared from mRNA of a growing mouse fibroblastic cell line. Using an antiserum prepared against a synthetic proliferin fusion protein, we show that proliferin is secreted as a glycoprotein by minced placental tissue and that it differs from mouse placental lactogen. We conclude that proliferin is a placental hormone that is synthesized in certain mouse cell lines during active growth. Its function during pregnancy and during the growth of cultured cells is presently unknown.
Full text
PDF
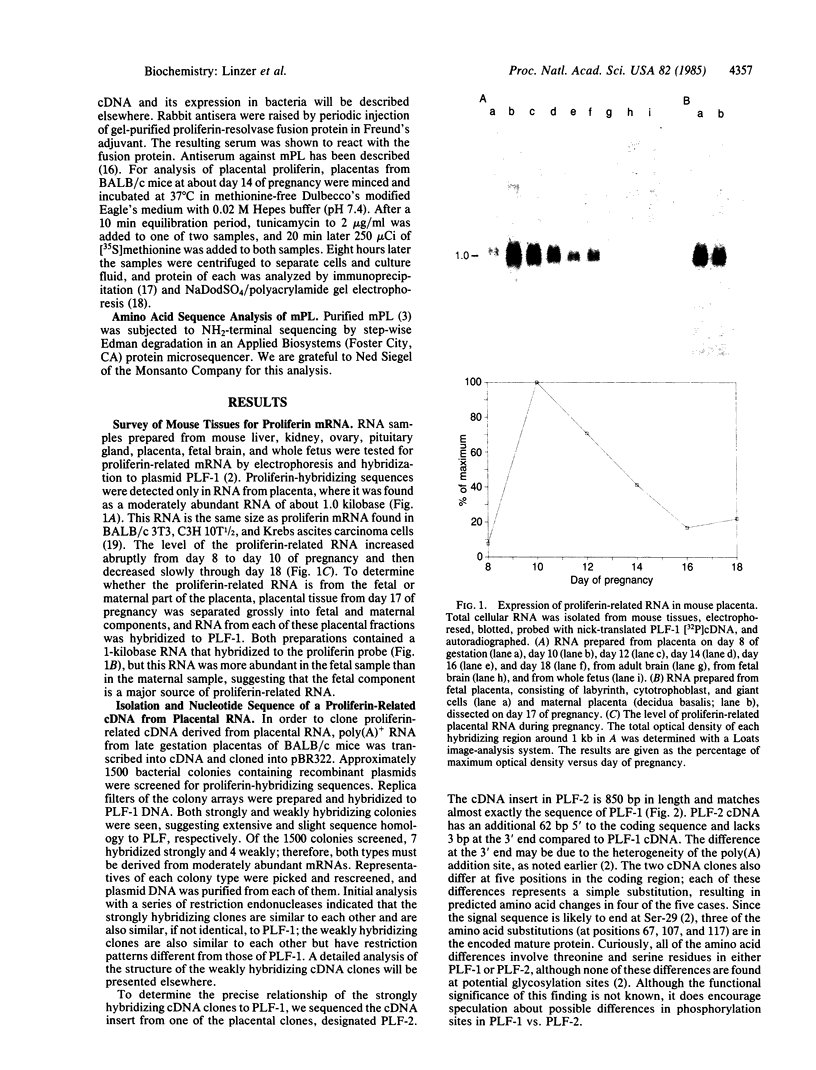

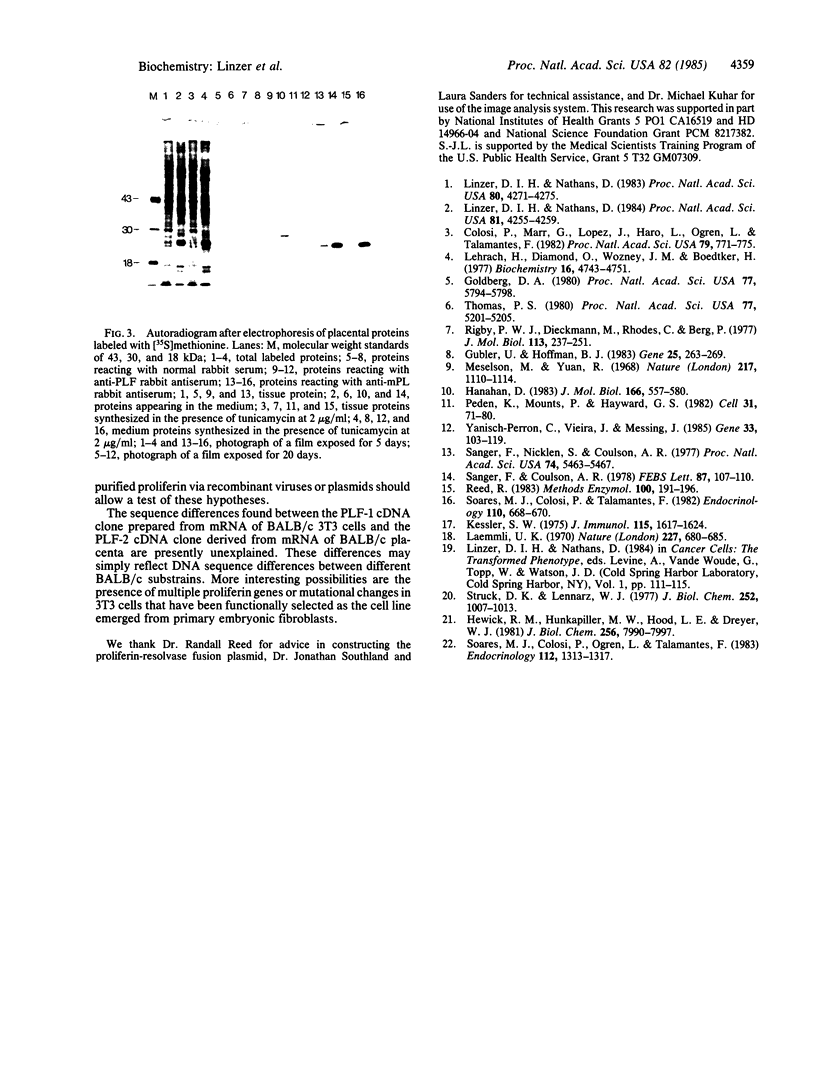
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Colosi P., Marr G., Lopez J., Haro L., Ogren L., Talamantes F. Isolation, purification, and characterization of mouse placental lactogen. Proc Natl Acad Sci U S A. 1982 Feb;79(3):771–775. doi: 10.1073/pnas.79.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg D. A. Isolation and partial characterization of the Drosophila alcohol dehydrogenase gene. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5794–5798. doi: 10.1073/pnas.77.10.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Linzer D. I., Nathans D. Growth-related changes in specific mRNAs of cultured mouse cells. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4271–4275. doi: 10.1073/pnas.80.14.4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzer D. I., Nathans D. Nucleotide sequence of a growth-related mRNA encoding a member of the prolactin-growth hormone family. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4255–4259. doi: 10.1073/pnas.81.14.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meselson M., Yuan R. DNA restriction enzyme from E. coli. Nature. 1968 Mar 23;217(5134):1110–1114. doi: 10.1038/2171110a0. [DOI] [PubMed] [Google Scholar]
- Peden K., Mounts P., Hayward G. S. Homology between mammalian cell DNA sequences and human herpesvirus genomes detected by a hybridization procedure with high-complexity probe. Cell. 1982 Nov;31(1):71–80. doi: 10.1016/0092-8674(82)90406-8. [DOI] [PubMed] [Google Scholar]
- Reed R. R. The resolvase protein of the transposon gamma delta. Methods Enzymol. 1983;100:191–196. doi: 10.1016/0076-6879(83)00055-5. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares M. J., Colosi P., Ogren L., Talamantes F. Identification and partial characterization of a lactogen from the midpregnant mouse conceptus. Endocrinology. 1983 Apr;112(4):1313–1317. doi: 10.1210/endo-112-4-1313. [DOI] [PubMed] [Google Scholar]
- Soares M. J., Colosi P., Talamantes F. The development and characterization of a homologous radioimmunoassay for mouse placental lactogen. Endocrinology. 1982 Feb;110(2):668–670. doi: 10.1210/endo-110-2-668. [DOI] [PubMed] [Google Scholar]
- Struck D. K., Lennarz W. J. Evidence for the participation of saccharide-lipids in the synthesis of the oligosaccharide chain of ovalbumin. J Biol Chem. 1977 Feb 10;252(3):1007–1013. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]