Abstract
Intravenous immunoglobulin (IVIg) is successfully used in the treatment of autoimmune diseases involving self-reactive CD8+ T cells. However, its direct influence on the cytotoxic response remains unknown. Using an antigen cross-presentation assay and a mouse model of ovalbumin (OVA) immunization, we showed that IVIg decreases the in vitro activation, proliferation and cytokine secretion of OVA-specific CD8+ T cells (OT-I), as well as the in vivo generation of OVA-specific CD8+ T cells. In addition, IVIg significantly decreases the proportion of perforin- and CD107a-expressing CD8+ T cells, and inhibits the cytotoxic activity of OVA-activated OT-I cells. The interference of IVIg with the CD8+ T-cell response is associated with T-cell receptor blockade, therefore reducing the interaction between effector and target cells. A similar blockade is observed on human CD8+ T cells, suggesting that the observations reported here could apply to the IVIg-mediated improvement of CD8+ T-cell-mediated autoimmune conditions in human patients.
Keywords: CD8+ T cells, cross-presentation, cytotoxicity, immunization, intravenous immunoglobulin
Introduction
Autoimmune diseases occur when self-reactive T cells become activated by deregulated presentation of self-peptides in the presence of inflammatory co-stimulatory molecules.1 The involvement of CD4+ (helper) and CD8+ (cytotoxic) T cells in autoimmune disorders leads to autoantibody production and self-reactive cytotoxicity, respectively.2,3 Cytotoxic CD8+ T cells induce immune-mediated damage by secreting perforin and granzyme B within the immunological synapse and expressing Fas ligand (FasL) on their surface.5 These cells are capable of organ destruction and contribute to the persistence and severity of many autoimmune diseases,6 for example by damaging β-cells in type 1 diabetes7–10 and myelin in chronic inflammatory demyelinating polyneuropathy (CIDP) or its experimental equivalent, experimental autoimmune encephalomyelitis.11,12
Intravenous immunoglobulin (IVIg) is a therapeutic preparation of human polyclonal IgG used as replacement therapy in patients with primary or secondary immunodeficiency, but also to treat more than one hundred inflammatory or autoimmune disorders.14 Considering that IVIg therapy was shown to improve immune conditions such as Crohn’s disease15 and CIDP,16–17 in which cytotoxic T cells play a dominant role, we hypothesized that IVIg could modulate the CD8+ T-cell response and influence their cytotoxic activity. Indeed, we recently reported that IVIg inhibited the in vitro activation of CD8+ T cells during cross-presentation of immune complexes of ovalbumin (OVA) by antigen-presenting cells (APC).18 The inhibition was largely explained by a reduction in immune complex internalization as the result of competition between IVIg and immune complexes for binding to activating FcγR.19 However, we could not rule out the possibility that IVIg also directly affects the ability of antigen-loaded APC to activate CD8+ T cells by cross-presentation. In the present work, we evaluated whether IVIg can directly interfere with the in vitro priming and expansion of CD8+ T cells by antigen-loaded APC and with the in vivo generation of antigen-specific CD8+ T cells, using a mouse model of OVA immunization. We also measured the cytotoxic activity of antigen-activated CD8+ T cells in the presence or absence of IVIg and explored the possible mechanisms of IVIg interference with the antigen-specific CD8+ T-cell response.
Materials and methods
Animals
Wild-type female C57BL/6 mice (18–22 g) were obtained from Charles River (Montreal, QC, Canada) and C57BL/6-Tg(TcraTcrb)1100Mjb/J (OT-I) mice were obtained from the Jackson Laboratory (Bar Harbor, ME). Mice were kept at the animal facility at Laval University (Quebec City, QC, Canada) and all procedures were approved by the Animal Ethics Committee of Laval University.
Cells and reagents
Bone marrow-derived dendritic cells (BMDC) from C57BL/6 mice were generated using 20 ng/ml of granulocyte–macrophage colony-stimulating factor (Peprotech, Rocky Hill, NJ) and cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (Invitrogen Canada Inc, Burlington, ON, Canada), as previously described.19–20 The OVA-specific CD8+ T cells (OT-I) were prepared from lymph nodes and spleens of OT-I mice by negative selection using the EasySep separation system (STEMCELL Technologies, Vancouver, BC, Canada). Purity was at least 98%, as determined by flow cytometry using a mouse CD8-specific fluorescent antibody. For in vitro experiments, IVIg (Gamunex, Grifols Canada Ltd, Mississauga, ON, Canada) was dialysed at 4° against endotoxin-free PBS to remove stabilizing agents and was kept frozen until use. Dialysed IVIg was analysed by size-exclusion chromatography on a Superdex 200 10/300 GL column (GE Healthcare Canada, Mississauga, ON, Canada) to confirm that the proportion of monomers and dimers remains unchanged after dialysis and thawing.
Cross-presentation assay
The BMDC (2·5 × 105/ml) were incubated for 4 hr with 1 mg/ml OVA (MP Biomedicals, Solon, OH), then washed five times with warm medium. Purified OT-I cells (2·5 × 105/ml) were fluorescently labelled with CellVue Maroon (Molecular Targeting Technologies, Inc. West Chester, PA) following the manufacturer’s instructions and added to the OVA-pulsed BMDC, in the presence or absence of the indicated doses of dialysed IVIg. OT-1 cell activation was measured by flow cytometry after 24 hr, using a fluorescently labelled CD69-specific antibody (eBioscience, San Diego, CA). Proliferation was evaluated after 72 hr by measuring the fluorescence intensity of CellVue Maroon-stained OT-I cells and expressed as proliferation index calculated using Modfit LT (Verity Software House Inc., Topsham, ME).
Analysis of T-cell response following OVA immunization
Groups of C57BL/6 mice received two subcutaneous injections (day 1 and day 14) of 100 μg OVA emulsified in complete Freund’s adjuvant (Sigma-Aldrich Canada, Oakville, ON, Canada) on day 1 and incomplete Freund’s adjuvant on day 14. The IVIg was injected every day at the indicated doses, starting 2 days before and ending 2 days after OVA injections. Mice were killed 28 days later. Spleens were recovered and homogenized with an organ grinder to obtain a single-cell suspension. Cells were then labelled with phycoerythrin-conjugated SIINFEKL-specific MHC-I tetramers (BD Biosciences, Mississauga, ON, Canada) according to the manufacturer’s protocol and analysed by flow cytometry to evaluate the amount of OVA-specific T cells. The OVA-specific antibody titres in mouse plasma were determined by ELISA using OVA as capture antigen. In parallel, a standard curve was established using anti-mouse IgG (Fab-specific) antibodies (Jackson Immunoresearch Laboratories, Inc., West Grove, PA) to capture mouse IgG from serial dilutions of a standardized murine serum (Bethyl Laboratories Inc., Montgomery, TX). A goat anti-mouse IgG (Fc-specific) horseradish peroxidase conjugate (Jackson Immunoresearch Laboratories Inc.) was used for detection.
Flow cytometry
The expression of perforin, granzyme B, FasL and the cytotoxicity-associated marker CD107a (LAMP-1) was measured on OT-I cells activated by OVA-pulsed BMDC from C57BL/6 mice during 24 hr in the presence or absence of 10 mg/ml IVIg, using specific fluorescent antibodies (all from eBiosciences). The expression of the same markers was also evaluated on splenic CD8+ T cells recovered from OVA-immunized mice. The effect of IVIg on the detection of MHC-I on BMDC, of CD8 on OT-I T cells and of T-cell receptor (TCR) αβ on human peripheral blood mononuclear cells (PBMC) was evaluated using specific antibodies (eBiosciences) by incubating the cells on ice for 1 hr in the presence or absence of IVIg (20 mg/ml) followed by extensive washes before flow cytometry analysis. The use of PBMC from healthy human volunteers was approved by the Héma-Québec Ethics Committee. PBMC were isolated by density centrifugation over Ficoll-Paque (GE Healthcare Bio-Sciences, Inc., Baie d’Urfé, QC, Canada), starting with whole blood obtained from the participants after informed consent. Similarly, the detection of the OVA-specific TCR on OT-I T cells in the presence or absence of IVIg was performed using fluorescent SIINFEKL/MHC-I tetramers (BD Biosciences). All flow cytometry analyses were carried out on a Partec Cyflow ML flow cytometer (Partec North American, Inc., Swedesboro, NJ).
Cytotoxicity assay
Splenocytes from C57BL/6 mice (2·0 × 107/ml) were pulsed with 1 μm OVA257–264 peptide (SIINFEKL) (Anaspec, Fremont, CA) for 4 hr in RPMI-1640 supplemented with 10% fetal bovine serum. Purified OT-I cells were added (1·0 × 106/ml) to the OVA-pulsed splenocytes and incubated for the following 3 days, for activation. EL4 target cells (ATCC, Manassas, VA) were fluorescently labelled with the Cell Proliferation Dye eFluor® 670 (eBiosciences) and pulsed with 5 μm SIINFEKL peptide for 1 hr, washed once and added at a 10 : 1 effector-to-target cell ratio in the presence or absence of 10 mg/ml IVIg. The specific killing of EL4 cells by cytotoxic T cells was determined by flow cytometry using a viability dye (Sytox Blue, eBiosciences).
Statistical analysis
All statistical analyses were performed using GraphPad InStat (GraphPad Software, La Jolla, CA) using appropriate tests. Values of P < 0·05 were considered to indicate statistical significance.
Results
IVIg inhibits the in vitro CD8+ T-cell response to native OVA
To study the direct effect of IVIg on CD8+ T-cell activation by antigen-loaded BMDC, we used OVA-specific CD8+ T cells (OT-I cells) obtained from OT-I mice, which express a rearranged TCR transgene specific for the H2-Kb-OVA257–264 complex.21 OT-I cells were incubated with OVA-pulsed BMDC and the extent of activation was measured 24 hr later by evaluation of the expression of the CD69 early activation marker. The results (Fig. 1a) first showed that OT-I cells were not activated in the absence of OVA. In the presence of OVA-pulsed BMDC, > 90% of the cells expressed CD69, indicating the efficient OT-I cell activation by cross-presentation of native OVA. Addition of IVIg during OVA cross-presentation resulted in a 30% reduction in CD69-positive OT-I cells, indicating that IVIg interferes with activation of OT-I cells by cross-presentation of native antigens.
Figure 1.
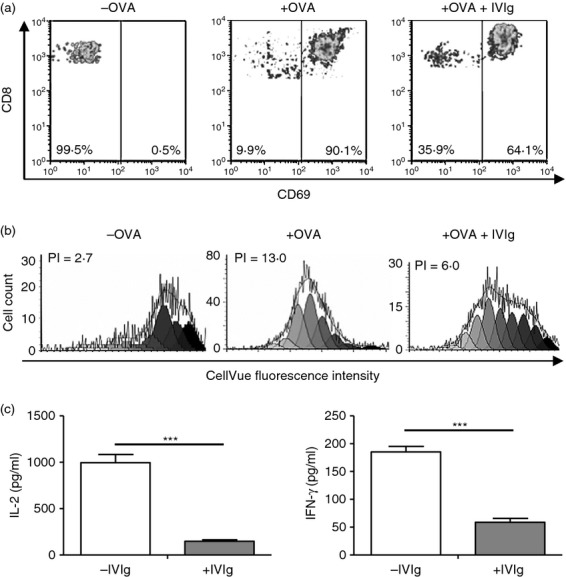
Effect of intravenous immunoglobulin (IVIg) on the MHC-I-specific CD8+ T-cell activation, proliferation and cytokine secretion. (a) Ovalbumin (OVA)-specific purified OT-I cells were activated with OVA-pulsed bone marrow-derived cells (BMDC) for 24 hr in the presence or absence of IVIg (10 mg/ml). The background OT-I cell activation was established using unpulsed BMDC (left panel). Cell activation was evaluated by measuring the expression of CD69 using flow cytometry. The data are representative of six independent experiments. (b) The same assay was conducted for 72 hr to evaluate proliferation using CellVue Maroon-stained OT-I cells. The OT-I cell fluorescence was measured by flow cytometry to allow calculation of the proliferation index (PI) using ModFit LT. The data are representative of four separate experiments. (c) The concentration of interleukin-2 (IL-2) and interferon-γ (IFN-γ) in the supernatants of OT-I cells activated with OVA-pulsed BMDC with or without IVIg (10 mg/ml) for 72 hr was evaluated by ELISA. The data are combined from three different experiments. Statistical analysis was used a two-tailed unpaired t-test, ***P < 0·001.
To confirm this result, we measured the proliferation of CellVue Maroon-stained OT-I cells, 72 hr after OVA cross-presentation in the presence or absence of IVIg (Fig. 1b). The results are expressed as proliferation index (PI) calculated using Modfit LT, which is a measure of the increase in cell number over the course of the assay.22 The background PI of OT-I cells activated in the absence of OVA was 2·7. In comparison, activation of OT-I cells in the presence of native OVA resulted in a PI of 13·0. A > 50% reduction in OT-I cell proliferation was observed in the presence of IVIg (PI 6·0), in agreement with the observation made using the early activation marker CD69, confirming that IVIg interferes with the activation of CD8+ T cells by cross-presentation of native antigens. Finally, the concentration of the proliferation-promoting cytokine interleukin-2 (IL-2) as well as the pro-inflammatory cytokine interferon-γ was measured in the culture supernatants, at the end of the proliferation assay. As expected, the presence of IVIg resulted in a ninefold and threefold decrease in the secretion of IL-2 and interferon-γ, respectively (Fig. 1c). In contrast, the presence of an equal concentration of human serum albumin (10 mg/ml) did not affect secretion of IL-2 by CD8+ T cells (data not shown).
IVIg inhibits the CD4 and CD8 responses in immunized C57BL/6 mice
To address the physiological relevance of the above observations, we used a mouse model of OVA immunization to measure the helper and cytotoxic T-cell responses in the presence of IVIg. Mice were immunized with native OVA using complete Freund’s adjuvant to stimulate both humoral and cellular immune responses.23 The experimental group of mice also received IVIg injections (either 1·0 or 2·5 g/kg) during the immunization period, whereas control mice received a corresponding volume of vehicle (glycine). The efficacy of immunization was confirmed by the presence of OVA-specific antibodies, 28 days after the first immunization. The OVA-specific antibodies were quantified in plasma samples using a standard curve prepared with immobilized mouse IgG using Fab-specific antibodies to mimic the binding of IgG to an antigen. The data presented are normalized for the total mouse IgG concentration in each animal (ng anti-OVA/mg mouse IgG). Control immunized mice had an average of 107 ng anti-OVA/mg mouse IgG (Fig. 2a). The amount of OVA-specific IgG in the non-immunized control group was below the detection limit of the ELISA (data not shown). Treatment with IVIg during the immunization resulted in a 2·7-fold (not statistically significant) and 4·6-fold (P < 0·05) decrease in OVA-specific IgG production (40 and 23 ng anti-OVA/mg mouse IgG) for the 1·0 g/kg and 2·5 g/kg IVIg dose, respectively. These results indicate that IVIg interferes with the MHC-II-dependent CD4 T-cell generation and activation in C57BL/6 mice, resulting in a decreased production of OVA-specific B cells and the subsequent OVA-specific antibody secretion, in agreement with our previous demonstration using BALB/c mice.19
Figure 2.
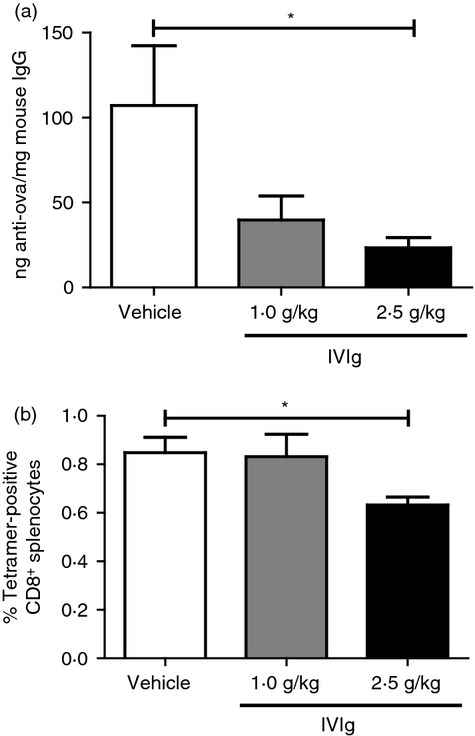
Effect of intravenous immunoglobulin (IVIg) on ovalbumin (OVA) -specific antibody and CD8+ T-cell generation in vivo. (a) The proportion of OVA-specific antibodies among total IgG was measured in the serum of OVA-immunized mice treated (n = 6) or not (n = 6) with IVIg at the indicated doses, 28 days after immunization. (b) The relative abundance of splenic OVA-specific CD8+ T cells in the groups of mice described in (a) was determined by flow cytometry using SIINFEKL-specific MHC-I tetramers. Statistical analysis used a one-way analysis of variance with Bonferroni post-test, *P < 0·05.
The spleens of the animals were recovered at sacrifice and the presence of OVA-specific CD8+ T cells was quantified by flow cytometry using phycoerythrin-conjugated SIINFEKL-specific MHC-I tetramers. Figure 2(b) shows the percentage of tetramer-positive CD8+ T cells among the total CD8+ T cells in the spleens of immunized mice (0·81%). The background percentage of tetramer-positive CD8+ T cells was determined in non-immunized mice and represented < 0·1% of total CD8+ T cells (data not shown). In the presence of IVIg, a significant decrease (24%, P < 0·05) in tetramer-positive CD8+ T cells was observed, but only at the highest dose used (2·5 g/kg). The absence of IVIg effect on CD8+ T cells at the 1·0 g/kg dose contrasts with the decreased humoral response observed at the same dose, suggesting that CD4+ T cells are more responsive to IVIg treatment. Together, these results show that IVIg decreases the antigen-specific CD4+ and CD8+ T-cell responses in vivo, at doses similar to those used for therapy of patients with autoimmune disorders.
IVIg decreases the number of T cells expressing cytotoxicity markers
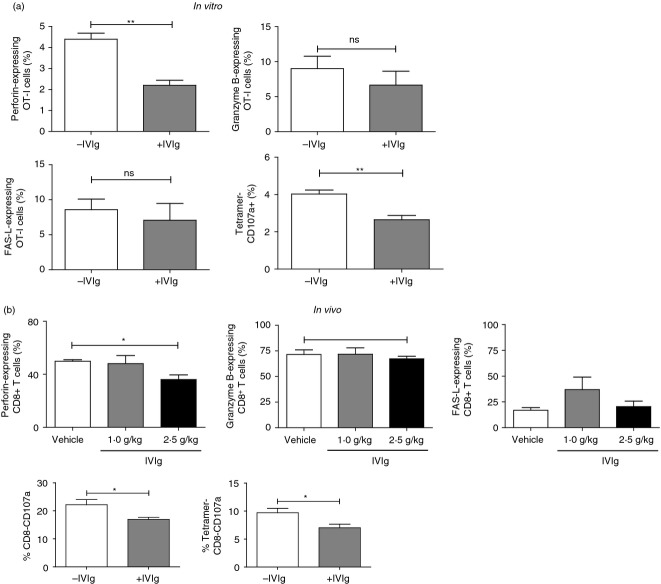
To further explore the effects of IVIg on the CD8+ T-cell population, we measured the expression of intracellular perforin and granzyme B, as well as the surface expression of FasL (CD95) and CD107a on T cells obtained from our in vitro antigen presentation and in vivo immunization assays. The flow cytometry analysis of OVA-specific OT-I cells from the in vitro cross-presentation assay shows a 2·0-fold decrease in the percentage of cells expressing perforin in the presence of 10 mg/ml of IVIg (Fig. 3a). Consistent with this observation, we measured a 1·4-fold decrease in perforin-expressing CD8+ T cells in the spleen of OVA-immunized mice that received 2·5 g/kg of IVIg compared with the control group (Fig. 3b), whereas mice treated with 1·0 g/kg of IVIg did not show a reduction in the number of perforin-expressing CD8+ T cells. The intracellular expression of granzyme B as well as the surface expression of FasL did not vary significantly in the presence or absence of IVIg, in both our in vitro and in vivo experiments (Fig. 3a,b). The expression of CD107a on OT-I cells was used to evaluate the cytotoxic potential of the cells, because its surface expression on CD8+ T cells is in direct correlation with cytolytic degranulation.24–25 The presence of IVIg during OT-I cell activation results in a significant 34% decrease in the number of OT-I cells expressing CD107a (Fig. 3a). The effect of IVIg treatment during mouse immunization on the generation of splenic CD107a+ CD8+ T cells was also examined. The analyses reveal that total and OVA-specific CD8+ T cells recovered from IVIg-treated mice contain 24% and 28% less CD107a-positive CD8 T cells respectively, compared with T cells recovered from untreated mice (Fig. 3b). Altogether, these results show that in addition to decreasing the activation and expansion, the presence of IVIg during the cross-presentation of a native antigen also reduces the percentage of perforin and CD107a-expressing CD8+ T cells.
Figure 3.
Intravenous immunoglobulin (IVIg) decreases the number of perforin- and CD107a-, but not granzyme B- or FasL-expressing CD8+ T cells. (a) The proportion of OT-I cells expressing perforin (upper left panel), granzyme B (upper right panel), FasL (lower left panel) and CD107a (lower right panel) was determined by flow cytometry, 24 hr after in vitro activation with ovalbumin (OVA) -pulsed bone marrow-derived cells (BMDC) in the presence or absence of IVIg (10 mg/ml). The data are representative of three independent experiments. Statistical analysis was carried out using a two-tailed unpaired t-test, **P < 0·01. (b) The proportion of CD8+ T cells expressing perforin, granzyme B, FasL (upper panels) and CD107a (lower panels) in the spleen of OVA-immunized mice (see legend to Fig. 2) was determined by flow cytometry. CD107a expression was analysed on total (left panel) and OVA-specific CD8+ gated T cells (right panel). Statistical analysis of perforin, granzyme B and FasL results was performed using a one-way analysis of variance with a Bonferroni post-test while CD107a results were analysed using a two-tailed unpaired t-test, *P < 0·05.
IVIg modulates the cytotoxic activity of CD8+ T cells
To study whether IVIg can also interfere directly with the cytotoxicity of activated CD8+ T cells, OT-I cells were first activated by SIINFEKL peptide-pulsed wild-type splenocytes in the absence of IVIg during 3 days. In parallel, EL4 cells (derived from a lymphoma induced in C57BL/6 mice)26 were stained with a viability dye, pulsed with the SIINFEKL peptide and used as target cells in a cytotoxicity assay that was performed in the presence or absence of IVIg. The pulsed EL4 cells were added to the OVA-activated OT-I cells. The extent of EL4 cytolysis was evaluated 1 hr later by measuring EL4 cell viability by flow cytometry. Our results first showed that > 95% of unpulsed EL4 cells remained viable (negative for Sytox Blue fluorescence) after incubation with OVA-activated OT-I cells (Fig. 4). Incubation of peptide-pulsed EL4 cells with OVA-activated OT-I cells resulted in a significant reduction in cell viability (> 70%), as expected. The presence of IVIg during the incubation of EL4 and OT-I cells led to a significant decrease in EL4 cytolysis, indicating that IVIg not only affects CD8+ T cells during cross-presentation but also directly interferes with their cytotoxic activity.
Figure 4.
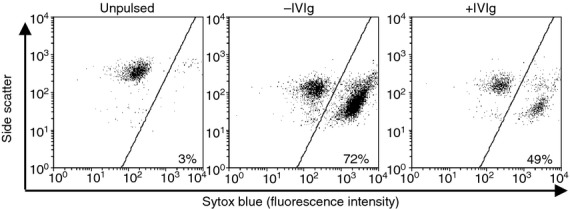
Intravenous immunoglobulin (IVIg) inhibits the class-I restricted T-cell-mediated cytotoxicity. The effect of IVIg on ovalbumin (OVA) -activated OT-I cell cytotoxicity was measured using OVA peptide-pulsed EL4 as target cells. The cytometry dot plots from one of three representative experiments show the percentage of dead and viable EL4 cells following staining with Sytox Blue. Background EL4 cell death was established by incubating unpulsed EL4 cells with activated OT-I cells (left panel). The slight change in side scatter observed in EL4 cells in the middle and right panels is attributed to peptide pulsing. The assay with OVA peptide-pulsed EL4 cells was performed in the presence or absence of IVIg (10 mg/ml). Results are representative of three separate experiments.
IVIg blocks TCR but not MHC-I and CD8 surface molecules
To better understand how IVIg can directly interfere with CD8+ T-cell cytotoxicity, we studied whether IVIg could block cell surface molecules such as MHC-I, CD8 and TCR, which are known to be involved in the interaction between T cells and their targets. To study MHC-I blockade, BMDC from C57BL/6 mice were incubated on ice in the presence or absence of IVIg, followed by MHC-I detection by flow cytometry. Figure 5(a) shows that the mean fluorescence intensity (MFI) was similar with or without IVIg, indicating that IVIg does not prevent the detection of MHC-I molecules on BMDC by MHC-I-specific fluorescent antibodies and therefore, should not interfere with MHC-I binding to its target. We next studied the effect of IVIg on the detection of CD8 and TCR on CD8+ T cells by incubating OT-I cells with IVIg on ice followed by detection of CD8 with a specific fluorescent antibody and of TCR with MHC-I tetramers (Fig. 5b). Although IVIg did not block the detection of the CD8, a 52% decrease of OVA-specific TCR detection was measured in the presence of IVIg (MFI of 41 compared with 86 in the absence of IVIg). To assess the relevance of this latter observation in humans, PBMC obtained from three healthy volunteers after informed consent were incubated with IVIg for 1 hr on ice followed by extensive washing, and the detection of TCR-αβ was evaluated on gated CD8+ T cells by flow cytometry. A statistically significant decrease in MFI was observed on the cells from all three donors when IVIg was present (Fig. 5c), showing the interference of IVIg with TCR on human T cells. In contrast, and similar to the observations carried out with OT-I cells, the detection of CD8 on human cells was not influenced by the presence of IVIg (data not shown).
Figure 5.
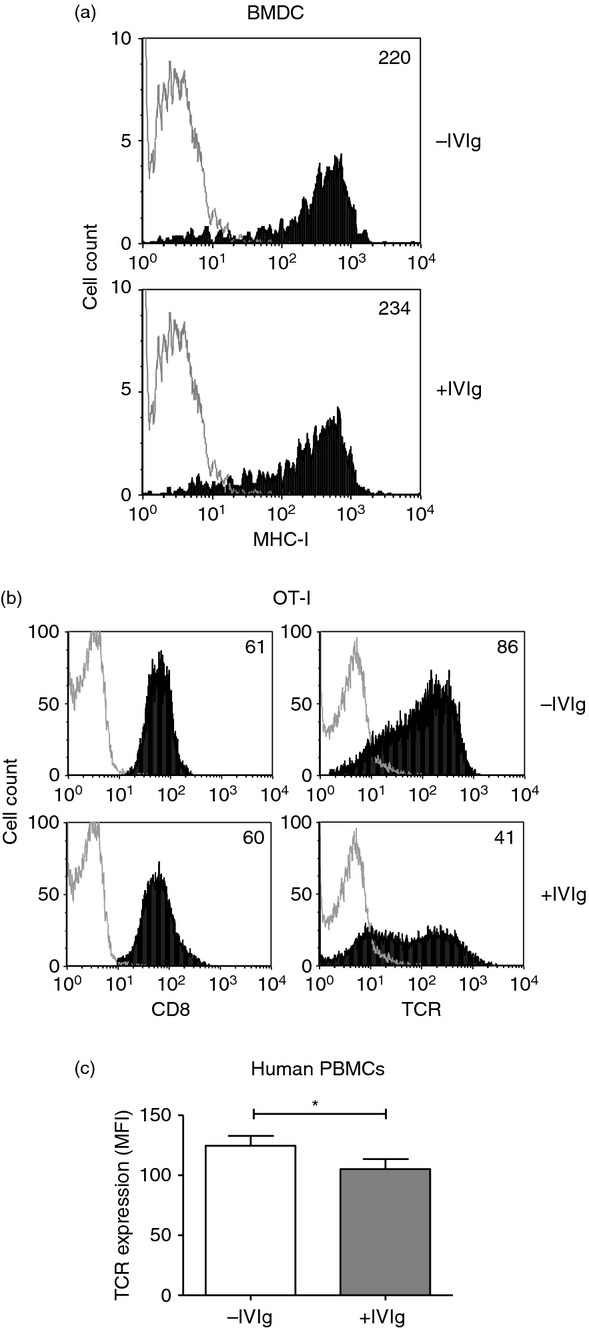
Analysis of intravenous immunoglobulin (IVIg) binding to MHC-I, CD8 and T-cell receptor (TCR). (a) MHC-I molecules expressed on bone marrow-derived cells (BMDC) were detected using a fluorescent specific antibody, after a 1-hr incubation on ice followed by washing, in the presence or absence of IVIg (20 mg/ml). (b) OT-I cells were treated as in (a) followed by detection of CD8 using a fluorescent specific antibody and of TCR using SIINFEKL-specific MHC-I tetramers. The data in (a) and (b) are representative of three independent experiments. The mean fluorescence intensities (MFI) are indicated in the upper right corner (c) Human peripheral blood mononuclear cells (PBMC) from three healthy volunteers were incubated with or without IVIg, as described in (a), followed by detection of human TCR-αβ using a fluorescent specific antibody. Data shown are the mean of the MFI obtained for the three donors. Statistical analysis used a two-tailed paired t-test, *P < 0·05.
Discussion
In this work, we demonstrated that IVIg has the ability to decrease the in vitro OVA-specific CD8+ T-cell response. More precisely, IVIg reduces CD8+ T-cell activation during cross-presentation of native antigens, as measured by the expression of the CD69 activation marker and the release of pro-inflammatory cytokines. Proliferation was also decreased, as evaluated by flow cytometry analysis of CellVue Maroon-stained cells. In addition, fewer perforin- and CD107a-expressing cells were obtained after cross-presentation of a native antigen or after OVA immunization of mice in the presence of IVIg. Finally, we showed that IVIg directly interferes with the cytotoxicity of activated CD8+ T cells, possibly by blocking the TCR and therefore preventing the binding to MHC-I molecules expressed by their targets. This latter observation may also explain the reduction in CD8+ T-cell activation during cross-presentation of native antigens in the presence of IVIg, as discussed above.
The results presented in this work complement our previous work that revealed the effect of IVIg on CD4+ and CD8+ T-cell activation by cross-presentation of immune complexes of antigens.18–19 Our observations are also in agreement with previously reported results obtained in the experimental autoimmune encephalomyelitis mouse model of multiple sclerosis. Indeed, the decreased clinical score in experimental autoimmune encephalomyelitis mice treated with IVIg has been shown to correlate with a decreased secretion of the proliferation-inducing IL-2 and the pro-inflammatory cytokine interferon-γ.27 Interleukin-2 has been reported to regulate perforin and granzyme gene expression in CD8+ T cells.28 The reduction in perforin-expressing CD8+ T cells could therefore be related to the reduced IL-2 secretion, although a similar decrease in granzyme-expressing T cells was not observed in our study. This suggests that perforin and granzyme expression may be regulated by different pathways. The reduction in perforin-expressing CD8+ T cells in the presence of IVIg may be relevant for the treatment of autoimmune disorders, as perforin+ CD8+ T cells have been associated with exacerbation of lichen planus, a T-cell-mediated autoimmune disease,29 with Hashimoto’s thyroiditis30 and with the development of autoimmune diabetes following adoptive transfer of splenocytes from diabetic mice to irradiated NOD mouse recipients.31
Many mechanisms have been proposed to explain the diversity of immunomodulatory effects of IVIg, including the binding of natural antibodies contained in IVIg to cytokines and cell surface receptors.32 Indeed, binding of IVIg to a conserved region of HLA-B7.01 expressed on human T cells has been previously associated with modulation of CD8+ T-cell-mediated functions.33 In the present work, we could not detect binding of IVIg to MHC-I on mouse APC, although the amino acid sequence recognized by IVIg on human HLA-I appears to be conserved in mouse MHC-I molecules (Protein-BLAST using NCBI Protein Database).34 This discrepancy may be explained by the different methods used to detect blocking antibodies: purification by affinity chromatography of MHC I-reactive IgG from IVIg using peptide B07.75-84 followed by acid elution in the Kaveri study, which may have affected the binding specificity of the purified IgG,35 versus direct analysis of binding to cell surface by flow cytometry in the present work. The analysis of surface expression of MHC-II, CD80, CD86 and CD40 on APC also revealed that IVIg did not block any of these receptors (data not shown). We further showed that IVIg does not block the detection of CD8 on mouse T cells, indicating that CD8 can interact with its ligand even in the presence of IVIg. Similarly, we recently reported that IVIg does not block the detection of CD3 and CD28 on the surface of human T cells,36 suggesting that these molecules can normally interact with their ligands. In contrast, we showed that IVIg blocks the TCR detection on OVA-specific OT-I cells, using OVA-specific MHC tetramers and on human PBMC, using TCR-αβ-specific antibodies, suggesting that blocking cell surface molecules contributes to the therapeutic effects of IVIg. This latter hypothesis is supported by our observation that pre-incubation of APCs with IVIg before CD8+ T-cell addition leads to a modest inhibition of T-cell activation whereas pre-incubation of T cells with IVIg before incubation with APC results in much stronger inhibition (data not shown).
The important contribution of CD8+ T cells to the pathogenesis of CIDP was recently uncovered.16 Considered as a first-line treatment for CIDP,37 IVIg was shown to normalize the TCR repertoire and to reduce the proportion of highly activated Vβ elements within the CD8+ T-cell population in CIDP patients.38 However, the exact mechanism by which IVIg exerts its therapeutic effect in this disorder remains unclear. Based on the results presented here, it can be hypothesized that the interference of IVIg with the generation and activation of CD8+ T cells following cross-presentation of antigens and the reduction in cytotoxicity of activated CD8+ T cells observed in the presence of IVIg play important roles in the therapeutic effects of IVIg in CIDP and other autoimmune disorders involving CD8+ T cells.
In summary, we herein demonstrate that IVIg interferes with the generation and activation of CD8+ T cells and decreases their cytotoxic activity, by blocking important cell surface molecules such as antigen-specific TCR. Our results contribute to a better understanding of both the short-term and sustained anti-inflammatory effects of IVIg in CD8-mediated autoimmune disorders.
Acknowledgments
PT designed and performed the experiments, analysed data and wrote the paper. DC performed the experiments, analysed data and wrote the paper. RB designed the research, analysed data and wrote the paper. The authors thank Marie-Ève Rhéaume and Lauriane Padet for excellent technical assistance. PT and DC are recipients of an Industrial Innovation Scholarship from the National Sciences and Engineering Research Council of Canada (NSERC) and Fonds Québécois de Recherche Nature et Technologies (FQRNT).
Glossary
- APC
antigen-presenting cells
- BMDC
bone marrow-derived dendritic cells
- CIDP
chronic inflammatory demyelinating disease
- FasL
Fas ligand
- IL-2
interleukin-2
- IVIg
intravenous immunoglobulin
- MFI
mean fluorescence intensity
- OVA
ovalbumin
- PBMC
peripheral blood mononuclear cells
- PI
proliferation index
- TCR
T-cell receptor
Disclosures
The authors declare no conflict of interests.
References
- Kamradt T, Mitchison NA. Tolerance and autoimmunity. N Engl J Med. 2001;344:655–64. doi: 10.1056/NEJM200103013440907. [DOI] [PubMed] [Google Scholar]
- Stranges PB, Watson J, Cooper CJ, et al. Elimination of antigen-presenting cells and autoreactive T cells by Fas contributes to prevention of autoimmunity. Immunity. 2007;26:629–41. doi: 10.1016/j.immuni.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112:1557–69. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurts C, Heath WR, Carbone FR, Kosaka H, Miller JF. Cross-presentation of self antigens to CD8+ T cells: the balance between tolerance and autoimmunity. Novartis Found Symp. 1998;215:172–81. doi: 10.1002/9780470515525.ch13. discussion 81-90. [DOI] [PubMed] [Google Scholar]
- Lieberman J. The ABCs of granule-mediated cytotoxicity: new weapons in the arsenal. Nat Rev Immunol. 2003;3:361–70. doi: 10.1038/nri1083. [DOI] [PubMed] [Google Scholar]
- Walter U, Santamaria P. CD8+ T cells in autoimmunity. Curr Opin Immunol. 2005;17:624–31. doi: 10.1016/j.coi.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Nagata M, Yoon JW. Studies on autoimmunity for T-cell-mediated β-cell destruction. Distinct difference in β-cell destruction between CD4+ and CD8+ T-cell clones derived from lymphocytes infiltrating the islets of NOD mice. Diabetes. 1992;41:998–1008. doi: 10.2337/diab.41.8.998. [DOI] [PubMed] [Google Scholar]
- Bulek AM, Cole DK, Skowera A, et al. Structural basis for the killing of human β cells by CD8+ T cells in type 1 diabetes. Nat Immunol. 2012;13:283–9. doi: 10.1038/ni.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankathatti Munegowda M, Deng Y, Chibbar R, et al. A distinct role of CD4+ Th17- and Th17-stimulated CD8+ CTL in the pathogenesis of type 1 diabetes and experimental autoimmune encephalomyelitis. J Clin Immunol. 2011;31:811–26. doi: 10.1007/s10875-011-9549-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallone R, Martinuzzi E, Blancou P, et al. CD8+ T-cell responses identify β-cell autoimmunity in human type 1 diabetes. Diabetes. 2007;56:613–21. doi: 10.2337/db06-1419. [DOI] [PubMed] [Google Scholar]
- Jiang H, Braunstein NS, Yu B, Winchester R, Chess L. CD8+ T cells control the TH phenotype of MBP-reactive CD4+ T cells in EAE mice. Proc Natl Acad Sci USA. 2001;98:6301–6. doi: 10.1073/pnas.101123098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Q, Goverman J. Experimental autoimmune encephalomyelitis mediated by CD8+ T cells. Ann N Y Acad Sci. 2007;1103:157–66. doi: 10.1196/annals.1394.017. [DOI] [PubMed] [Google Scholar]
- Steinman L. Myelin-specific CD8 T cells in the pathogenesis of experimental allergic encephalitis and multiple sclerosis. J Exp Med. 2001;194:F27–30. doi: 10.1084/jem.194.5.f27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivity S, Katz U, Daniel N, Nussinovitch U, Papageorgiou N, Shoenfeld Y. Evidence for the use of intravenous immunoglobulins – a review of the literature. Clin Rev Allergy Immunol. 2010;38:201–69. doi: 10.1007/s12016-009-8155-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korber J, Kottgen E, Renz H. A case of Crohn’s disease with increased CD8 T-cell activation and remission during therapy with intravenous immunoglobulins. Scand J Gastroenterol. 1998;33:1113–7. doi: 10.1080/003655298750026840. [DOI] [PubMed] [Google Scholar]
- Schneider-Hohendorf T, Schwab N, Uceyler N, Gobel K, Sommer C, Wiendl H. CD8+ T-cell immunity in chronic inflammatory demyelinating polyradiculoneuropathy. Neurology. 2012;78:402–8. doi: 10.1212/WNL.0b013e318245d250. [DOI] [PubMed] [Google Scholar]
- Deckert M, Sanchez-Ruiz M, Brunn A, Schluter D. Role of CD8 T-cell-mediated autoimmune diseases of the central nervous system. Crit Rev Immunol. 2010;30:311–26. doi: 10.1615/critrevimmunol.v30.i4.10. [DOI] [PubMed] [Google Scholar]
- Trepanier P, Bazin R. Intravenous immunoglobulin (IVIg) inhibits CD8 cytotoxic T-cell activation. Blood. 2012;120:2769–70. doi: 10.1182/blood-2012-07-445007. [DOI] [PubMed] [Google Scholar]
- Aubin E, Lemieux R, Bazin R. Indirect inhibition of in vivo and in vitro T-cell responses by intravenous immunoglobulins due to impaired antigen presentation. Blood. 2010;115:1727–34. doi: 10.1182/blood-2009-06-225417. [DOI] [PubMed] [Google Scholar]
- Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Tario JD, Jr, Humphrey K, Bantly AD, Muirhead KA, Moore JS, Wallace PK. Optimized staining and proliferation modeling methods for cell division monitoring using cell tracking dyes. J Vis Exp. 2012:e4287. doi: 10.3791/4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke Y, Li Y, Kapp JA. Ovalbumin injected with complete Freund’s adjuvant stimulates cytolytic responses. Eur J Immunol. 1995;25:549–53. doi: 10.1002/eji.1830250237. [DOI] [PubMed] [Google Scholar]
- Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- Aktas E, Kucuksezer UC, Bilgic S, Erten G, Deniz G. Relationship between CD107a expression and cytotoxic activity. Cell Immunol. 2009;254:149–54. doi: 10.1016/j.cellimm.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Gorer PA. Studies in antibody response of mice to tumour inoculation. Br J Cancer. 1950;4:372–9. doi: 10.1038/bjc.1950.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashov A, Bellon B, Kaveri SV, Kazatchkine MD. A shift in encephalitogenic T cell cytokine pattern is associated with suppression of EAE by intravenous immunoglobulins (IVIg) Mult Scler. 1997;3:153–6. doi: 10.1177/135245859700300218. [DOI] [PubMed] [Google Scholar]
- Janas ML, Groves P, Kienzle N, Kelso A. IL-2 regulates perforin and granzyme gene expression in CD8+ T cells independently of its effects on survival and proliferation. J Immunol. 2005;175:8003–10. doi: 10.4049/jimmunol.175.12.8003. [DOI] [PubMed] [Google Scholar]
- Prpic Massari L, Kastelan M, Gruber F, et al. Perforin expression in peripheral blood lymphocytes and skin-infiltrating cells in patients with lichen planus. Br J Dermatol. 2004;151:433–9. doi: 10.1111/j.1365-2133.2004.06086.x. [DOI] [PubMed] [Google Scholar]
- Wu Z, Podack ER, McKenzie JM, Olsen KJ, Zakarija M. Perforin expression by thyroid-infiltrating T cells in autoimmune thyroid disease. Clin Exp Immunol. 1994;98:470–7. doi: 10.1111/j.1365-2249.1994.tb05515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LH, Peterson LB, Wicker LS, Persechini PM, Young JD. In vivo expression of perforin by CD8+ lymphocytes in autoimmune disease. Studies on spontaneous and adoptively transferred diabetes in nonobese diabetic mice. J Immunol. 1989;143:3994–9. [PubMed] [Google Scholar]
- Kazatchkine MD, Kaveri SV. Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. N Engl J Med. 2001;345:747–55. doi: 10.1056/NEJMra993360. [DOI] [PubMed] [Google Scholar]
- Kaveri S, Vassilev T, Hurez V, et al. Antibodies to a conserved region of HLA class I molecules, capable of modulating CD8 T cell-mediated function, are present in pooled normal immunoglobulin for therapeutic use. J Clin Invest. 1996;97:865–9. doi: 10.1172/JCI118488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–10. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- St-Amour I, Laroche A, Bazin R, Lemieux R. Activation of cryptic IgG reactive with BAFF, amyloid β peptide and GM-CSF during the industrial fractionation of human plasma into therapeutic intravenous immunoglobulins. Clin Immunol. 2009;133:52–60. doi: 10.1016/j.clim.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Padet L, Bazin R. IVIg prevents the in vitro activation of T cells by neutralizing the T cell activators. Immunol Lett. 2013;150:54–60. doi: 10.1016/j.imlet.2012.12.011. [DOI] [PubMed] [Google Scholar]
- Dalakas MC. Advances in the diagnosis, pathogenesis and treatment of CIDP. Nat Rev Neurol. 2011;7:507–17. doi: 10.1038/nrneurol.2011.121. [DOI] [PubMed] [Google Scholar]
- Mausberg AK, Dorok M, Stettner M, et al. Recovery of the T-cell repertoire in CIDP by IV immunoglobulins. Neurology. 2013;80:296–303. doi: 10.1212/WNL.0b013e31827debad. [DOI] [PubMed] [Google Scholar]