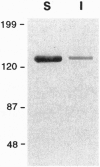
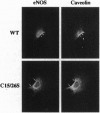
Abstract
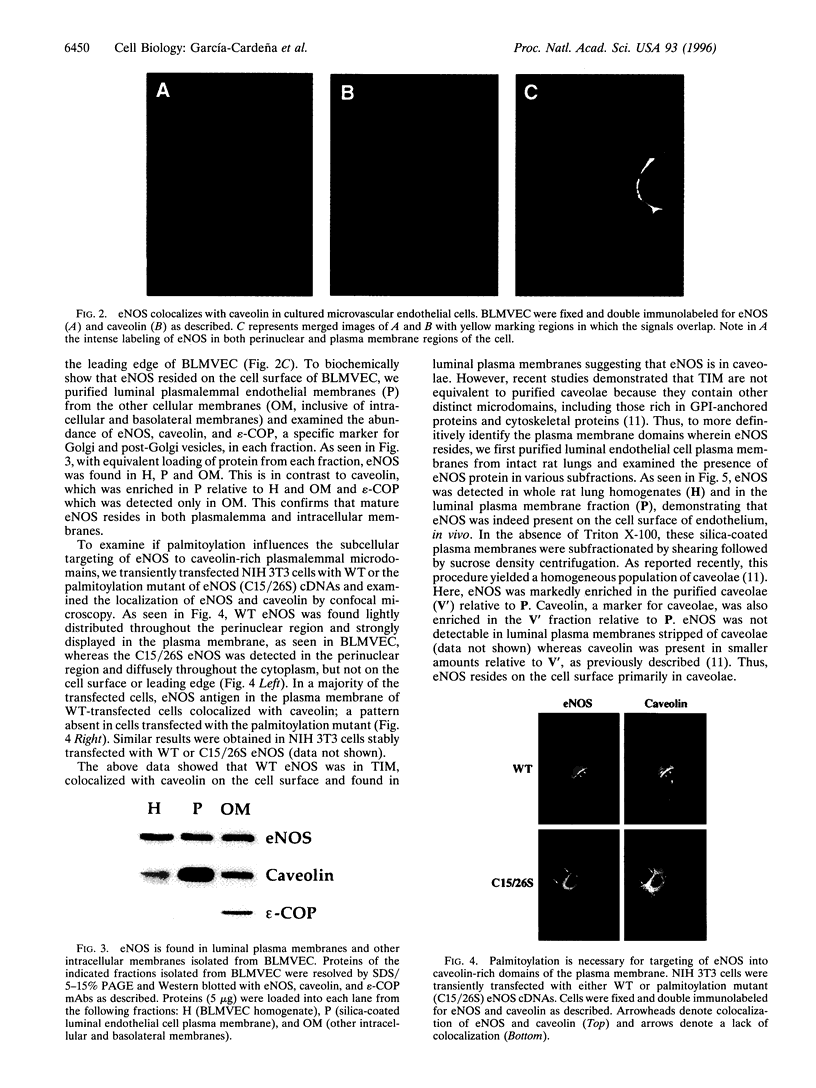
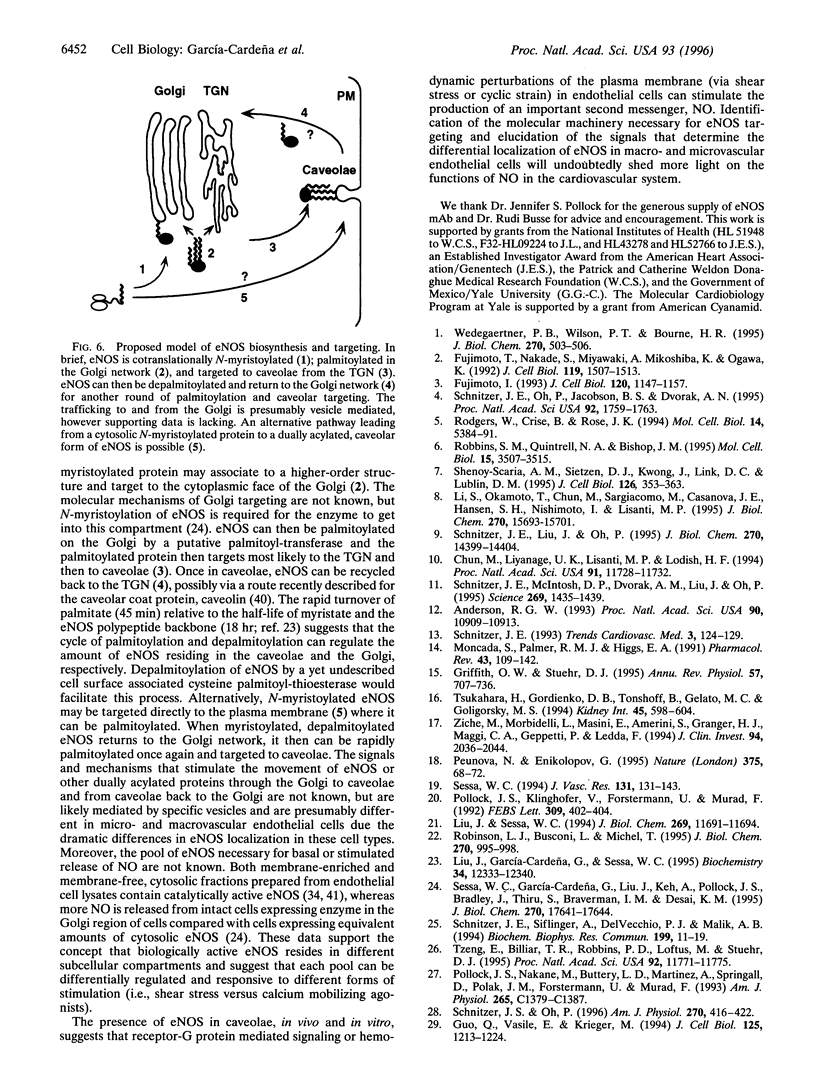
The membrane association of endothelial nitric oxide synthase (eNOS) plays an important role in the biosynthesis of nitric oxide (NO) in vascular endothelium. Previously, we have shown that in cultured endothelial cells and in intact blood vessels, eNOS is found primarily in the perinuclear region of the cells and in discrete regions of the plasma membrane, suggesting trafficking of the protein from the Golgi to specialized plasma membrane structures. Here, we show that eNOS is found in Triton X-100-insoluble membranes prepared from cultured bovine aortic endothelial cells and colocalizes with caveolin, a coat protein of caveolae, in cultured bovine lung microvascular endothelial cells as determined by confocal microscopy. To examine if eNOS is indeed in caveolae, we purified luminal endothelial cell plasma membranes and their caveolae directly from intact, perfused rat lungs. eNOS is found in the luminal plasma membranes and is markedly enriched in the purified caveolae. Because palmitoylation of eNOS does not significantly influence its membrane association, we next examined whether this modification can affect eNOS targeting to caveolae. Wild-type eNOS, but not the palmitoylation mutant form of the enzyme, colocalizes with caveolin on the cell surface in transfected NIH 3T3 cells, demonstrating that palmitoylation of eNOS is necessary for its targeting into caveolae. These data suggest that the subcellular targeting of eNOS to caveolae can restrict NO signaling to specific targets within a limited microenvironment at the cell surface and may influence signal transduction through caveolae.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. G. Caveolae: where incoming and outgoing messengers meet. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):10909–10913. doi: 10.1073/pnas.90.23.10909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Rose J. K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992 Feb 7;68(3):533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Chun M., Liyanage U. K., Lisanti M. P., Lodish H. F. Signal transduction of a G protein-coupled receptor in caveolae: colocalization of endothelin and its receptor with caveolin. Proc Natl Acad Sci U S A. 1994 Nov 22;91(24):11728–11732. doi: 10.1073/pnas.91.24.11728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad P. A., Smart E. J., Ying Y. S., Anderson R. G., Bloom G. S. Caveolin cycles between plasma membrane caveolae and the Golgi complex by microtubule-dependent and microtubule-independent steps. J Cell Biol. 1995 Dec;131(6 Pt 1):1421–1433. doi: 10.1083/jcb.131.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto T. Calcium pump of the plasma membrane is localized in caveolae. J Cell Biol. 1993 Mar;120(5):1147–1157. doi: 10.1083/jcb.120.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto T., Nakade S., Miyawaki A., Mikoshiba K., Ogawa K. Localization of inositol 1,4,5-trisphosphate receptor-like protein in plasmalemmal caveolae. J Cell Biol. 1992 Dec;119(6):1507–1513. doi: 10.1083/jcb.119.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. W., Stuehr D. J. Nitric oxide synthases: properties and catalytic mechanism. Annu Rev Physiol. 1995;57:707–736. doi: 10.1146/annurev.ph.57.030195.003423. [DOI] [PubMed] [Google Scholar]
- Guo Q., Vasile E., Krieger M. Disruptions in Golgi structure and membrane traffic in a conditional lethal mammalian cell mutant are corrected by epsilon-COP. J Cell Biol. 1994 Jun;125(6):1213–1224. doi: 10.1083/jcb.125.6.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecker M., Mülsch A., Bassenge E., Förstermann U., Busse R. Subcellular localization and characterization of nitric oxide synthase(s) in endothelial cells: physiological implications. Biochem J. 1994 Apr 1;299(Pt 1):247–252. doi: 10.1042/bj2990247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson B. R. Size and distribution of endothelial plasmalemmal vesicles in consecutive segments of the microvasculature in cat skeletal muscle. Microvasc Res. 1979 Mar;17(2):107–117. doi: 10.1016/0026-2862(79)90400-x. [DOI] [PubMed] [Google Scholar]
- Li S., Okamoto T., Chun M., Sargiacomo M., Casanova J. E., Hansen S. H., Nishimoto I., Lisanti M. P. Evidence for a regulated interaction between heterotrimeric G proteins and caveolin. J Biol Chem. 1995 Jun 30;270(26):15693–15701. doi: 10.1074/jbc.270.26.15693. [DOI] [PubMed] [Google Scholar]
- Liu J., García-Cardeña G., Sessa W. C. Biosynthesis and palmitoylation of endothelial nitric oxide synthase: mutagenesis of palmitoylation sites, cysteines-15 and/or -26, argues against depalmitoylation-induced translocation of the enzyme. Biochemistry. 1995 Sep 26;34(38):12333–12340. doi: 10.1021/bi00038a029. [DOI] [PubMed] [Google Scholar]
- Liu J., Sessa W. C. Identification of covalently bound amino-terminal myristic acid in endothelial nitric oxide synthase. J Biol Chem. 1994 Apr 22;269(16):11691–11694. [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Morin A. M., Stanboli A. Nitric oxide synthase in cultured endothelial cells of cerebrovascular origin: cytochemistry. J Neurosci Res. 1993 Oct 15;36(3):272–279. doi: 10.1002/jnr.490360305. [DOI] [PubMed] [Google Scholar]
- Niggli V., Burger M. M. Interaction of the cytoskeleton with the plasma membrane. J Membr Biol. 1987;100(2):97–121. doi: 10.1007/BF02209144. [DOI] [PubMed] [Google Scholar]
- O'Brien A. J., Young H. M., Povey J. M., Furness J. B. Nitric oxide synthase is localized predominantly in the Golgi apparatus and cytoplasmic vesicles of vascular endothelial cells. Histochem Cell Biol. 1995 Mar;103(3):221–225. doi: 10.1007/BF01454027. [DOI] [PubMed] [Google Scholar]
- Peunova N., Enikolopov G. Nitric oxide triggers a switch to growth arrest during differentiation of neuronal cells. Nature. 1995 May 4;375(6526):68–73. doi: 10.1038/375068a0. [DOI] [PubMed] [Google Scholar]
- Pollock J. S., Förstermann U., Mitchell J. A., Warner T. D., Schmidt H. H., Nakane M., Murad F. Purification and characterization of particulate endothelium-derived relaxing factor synthase from cultured and native bovine aortic endothelial cells. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10480–10484. doi: 10.1073/pnas.88.23.10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock J. S., Klinghofer V., Förstermann U., Murad F. Endothelial nitric oxide synthase is myristylated. FEBS Lett. 1992 Sep 14;309(3):402–404. doi: 10.1016/0014-5793(92)80816-y. [DOI] [PubMed] [Google Scholar]
- Pollock J. S., Nakane M., Buttery L. D., Martinez A., Springall D., Polak J. M., Förstermann U., Murad F. Characterization and localization of endothelial nitric oxide synthase using specific monoclonal antibodies. Am J Physiol. 1993 Nov;265(5 Pt 1):C1379–C1387. doi: 10.1152/ajpcell.1993.265.5.C1379. [DOI] [PubMed] [Google Scholar]
- Resh M. D. Myristylation and palmitylation of Src family members: the fats of the matter. Cell. 1994 Feb 11;76(3):411–413. doi: 10.1016/0092-8674(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Robbins S. M., Quintrell N. A., Bishop J. M. Myristoylation and differential palmitoylation of the HCK protein-tyrosine kinases govern their attachment to membranes and association with caveolae. Mol Cell Biol. 1995 Jul;15(7):3507–3515. doi: 10.1128/mcb.15.7.3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson L. J., Busconi L., Michel T. Agonist-modulated palmitoylation of endothelial nitric oxide synthase. J Biol Chem. 1995 Jan 20;270(3):995–998. doi: 10.1074/jbc.270.3.995. [DOI] [PubMed] [Google Scholar]
- Rodgers W., Crise B., Rose J. K. Signals determining protein tyrosine kinase and glycosyl-phosphatidylinositol-anchored protein targeting to a glycolipid-enriched membrane fraction. Mol Cell Biol. 1994 Aug;14(8):5384–5391. doi: 10.1128/mcb.14.8.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargiacomo M., Sudol M., Tang Z., Lisanti M. P. Signal transducing molecules and glycosyl-phosphatidylinositol-linked proteins form a caveolin-rich insoluble complex in MDCK cells. J Cell Biol. 1993 Aug;122(4):789–807. doi: 10.1083/jcb.122.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer J. E., Liu J., Oh P. Endothelial caveolae have the molecular transport machinery for vesicle budding, docking, and fusion including VAMP, NSF, SNAP, annexins, and GTPases. J Biol Chem. 1995 Jun 16;270(24):14399–14404. doi: 10.1074/jbc.270.24.14399. [DOI] [PubMed] [Google Scholar]
- Schnitzer J. E., McIntosh D. P., Dvorak A. M., Liu J., Oh P. Separation of caveolae from associated microdomains of GPI-anchored proteins. Science. 1995 Sep 8;269(5229):1435–1439. doi: 10.1126/science.7660128. [DOI] [PubMed] [Google Scholar]
- Schnitzer J. E., Oh P., Jacobson B. S., Dvorak A. M. Caveolae from luminal plasmalemma of rat lung endothelium: microdomains enriched in caveolin, Ca(2+)-ATPase, and inositol trisphosphate receptor. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1759–1763. doi: 10.1073/pnas.92.5.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer J. E., Siflinger-Birnboim A., Del Vecchio P. J., Malik A. B. Segmental differentiation of permeability, protein glycosylation, and morphology of cultured bovine lung vascular endothelium. Biochem Biophys Res Commun. 1994 Feb 28;199(1):11–19. doi: 10.1006/bbrc.1994.1185. [DOI] [PubMed] [Google Scholar]
- Sessa W. C., García-Cardeña G., Liu J., Keh A., Pollock J. S., Bradley J., Thiru S., Braverman I. M., Desai K. M. The Golgi association of endothelial nitric oxide synthase is necessary for the efficient synthesis of nitric oxide. J Biol Chem. 1995 Jul 28;270(30):17641–17644. doi: 10.1074/jbc.270.30.17641. [DOI] [PubMed] [Google Scholar]
- Sessa W. C. The nitric oxide synthase family of proteins. J Vasc Res. 1994 May-Jun;31(3):131–143. doi: 10.1159/000159039. [DOI] [PubMed] [Google Scholar]
- Shenoy-Scaria A. M., Dietzen D. J., Kwong J., Link D. C., Lublin D. M. Cysteine3 of Src family protein tyrosine kinase determines palmitoylation and localization in caveolae. J Cell Biol. 1994 Jul;126(2):353–363. doi: 10.1083/jcb.126.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart E. J., Ying Y. S., Mineo C., Anderson R. G. A detergent-free method for purifying caveolae membrane from tissue culture cells. Proc Natl Acad Sci U S A. 1995 Oct 24;92(22):10104–10108. doi: 10.1073/pnas.92.22.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukahara H., Gordienko D. V., Tonshoff B., Gelato M. C., Goligorsky M. S. Direct demonstration of insulin-like growth factor-I-induced nitric oxide production by endothelial cells. Kidney Int. 1994 Feb;45(2):598–604. doi: 10.1038/ki.1994.78. [DOI] [PubMed] [Google Scholar]
- Tzeng E., Billiar T. R., Robbins P. D., Loftus M., Stuehr D. J. Expression of human inducible nitric oxide synthase in a tetrahydrobiopterin (H4B)-deficient cell line: H4B promotes assembly of enzyme subunits into an active dimer. Proc Natl Acad Sci U S A. 1995 Dec 5;92(25):11771–11775. doi: 10.1073/pnas.92.25.11771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedegaertner P. B., Wilson P. T., Bourne H. R. Lipid modifications of trimeric G proteins. J Biol Chem. 1995 Jan 13;270(2):503–506. doi: 10.1074/jbc.270.2.503. [DOI] [PubMed] [Google Scholar]
- Ziche M., Morbidelli L., Masini E., Amerini S., Granger H. J., Maggi C. A., Geppetti P., Ledda F. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J Clin Invest. 1994 Nov;94(5):2036–2044. doi: 10.1172/JCI117557. [DOI] [PMC free article] [PubMed] [Google Scholar]