Abstract
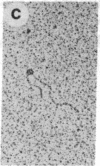
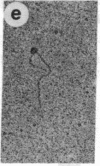
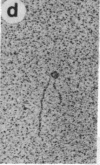
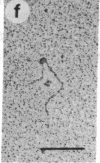
The O protein of bacteriophage lambda is required for initiation of DNA replication at the lambda replicative origin designated ori lambda. The binding sites for O protein are four direct repeats, each of which is an inverted repeat. By means of electron microscopy, we have found that phage lambda O protein utilizes these multiple binding sites to form a specific nucleoprotein structure in which the origin DNA is inferred to be folded or wound. The phage lambda O and P proteins and host DnaB protein interact at ori lambda to generate a larger structure than that formed by O protein alone; P and DnaB proteins fail to form any observable complex when O protein is excluded from the reaction mixture. We conclude that the specialized nucleoprotein structure formed by phage lambda O protein and ori lambda provides for localized initiation of DNA replication by serving as the foundation for the assembly of the initial priming structure. Specialized nucleoprotein structures may be a general means to confer exceptional accuracy on DNA transactions requiring extraordinary precision.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Better M., Lu C., Williams R. C., Echols H. Site-specific DNA condensation and pairing mediated by the int protein of bacteriophage lambda. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5837–5841. doi: 10.1073/pnas.79.19.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Better M., Wickner S., Auerbach J., Echols H. Role of the Xis protein of bacteriophage lambda in a specific reactive complex at the attR prophage attachment site. Cell. 1983 Jan;32(1):161–168. doi: 10.1016/0092-8674(83)90506-8. [DOI] [PubMed] [Google Scholar]
- Denniston-Thompson K., Moore D. D., Kruger K. E., Furth M. E., Blattner F. R. Physical structure of the replication origin of bacteriophage lambda. Science. 1977 Dec 9;198(4321):1051–1056. doi: 10.1126/science.929187. [DOI] [PubMed] [Google Scholar]
- Echols H., Dodson M., Better M., Roberts J. D., McMacken R. The role of specialized nucleoprotein structures in site-specific recombination and initiation of DNA replication. Cold Spring Harb Symp Quant Biol. 1984;49:727–733. doi: 10.1101/sqb.1984.049.01.082. [DOI] [PubMed] [Google Scholar]
- Eisen H. A., Fuerst C. R., Siminovitch L., Thomas R., Lambert L., Pereira da Silva L., Jacob F. Genetics and physiology of defective lysogeny in K12 (lambda): studies of early mutants. Virology. 1966 Oct;30(2):224–241. doi: 10.1016/0042-6822(66)90098-5. [DOI] [PubMed] [Google Scholar]
- Fiddes J. C., Barrell B. G., Godson G. N. Nucleotide sequences of the separate origins of synthesis of bacteriophage G4 viral and complementary DNA strands. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1081–1085. doi: 10.1073/pnas.75.3.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R. S., Funnell B. E., Kornberg A. The dnaA protein complex with the E. coli chromosomal replication origin (oriC) and other DNA sites. Cell. 1984 Oct;38(3):889–900. doi: 10.1016/0092-8674(84)90284-8. [DOI] [PubMed] [Google Scholar]
- Furth M. E., Blattner F. R., McLeester C., Dove W. F. Genetic structure of the replication origin of bacteriophage lambda. Science. 1977 Dec 9;198(4321):1046–1051. doi: 10.1126/science.929186. [DOI] [PubMed] [Google Scholar]
- Furth M. E., McLeester C., Dove W. F. Specificity determinants for bacteriophage lambda DNA replication. I. A chain of interactions that controls the initiation of replication. J Mol Biol. 1978 Dec 5;126(2):195–225. doi: 10.1016/0022-2836(78)90359-5. [DOI] [PubMed] [Google Scholar]
- Furth M. E., Yates J. L. Specificity determinants for bacteriophage lambda DNA replication. II. Structure of O proteins of lambda-phi80 and lambda-82 hybrid phages and of a lambda mutant defective in the origin of replication. J Mol Biol. 1978 Dec 5;126(2):227–240. doi: 10.1016/0022-2836(78)90360-1. [DOI] [PubMed] [Google Scholar]
- Hobom G., Grosschedl R., Lusky M., Scherer G., Schwarz E., Kössel H. Functional analysis of the replicator structure of lambdoid bacteriophage DNAs. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):165–178. doi: 10.1101/sqb.1979.043.01.023. [DOI] [PubMed] [Google Scholar]
- Joyner A., Isaacs L. N., Echols H., Sly W. S. DNA replication and messenger RNA production after induction of wild-type lambda bacteriophage and lambda mutants. J Mol Biol. 1966 Aug;19(1):174–186. doi: 10.1016/s0022-2836(66)80059-1. [DOI] [PubMed] [Google Scholar]
- Klein A., Lanka E., Schuster H. Isolation of a complex between the P protein of phage lambda and the dnaB protein of Escherichia coli. Eur J Biochem. 1980 Mar;105(1):1–6. doi: 10.1111/j.1432-1033.1980.tb04467.x. [DOI] [PubMed] [Google Scholar]
- LeBowitz J. H., McMacken R. The bacteriophage lambda O and P protein initiators promote the replication of single-stranded DNA. Nucleic Acids Res. 1984 Apr 11;12(7):3069–3088. doi: 10.1093/nar/12.7.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D. D., Denniston K. J., Blattner F. R. Sequence organization of the origins of DNA replication in lambdoid coliphages. Gene. 1981 Jun-Jul;14(1-2):91–101. doi: 10.1016/0378-1119(81)90151-7. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Tomizawa J. Replication of bacteriophage DNA. I. Replication of DNA of lambda phage defective in early functions. J Mol Biol. 1968 Dec 14;38(2):217–225. doi: 10.1016/0022-2836(68)90407-5. [DOI] [PubMed] [Google Scholar]
- Reha-Krantz L. J., Hurwitz J. The dnaB gene product of Escherichia coli. I. Purification, homogeneity, and physical properties. J Biol Chem. 1978 Jun 10;253(11):4043–4050. [PubMed] [Google Scholar]
- Roberts J. D., McMacken R. The bacteriophage lambda O replication protein: isolation and characterization of the amplified initiator. Nucleic Acids Res. 1983 Nov 11;11(21):7435–7452. [PMC free article] [PubMed] [Google Scholar]
- Scherer G. Nucleotide sequence of the O gene and of the origin of replication in bacteriophage lambda DNA. Nucleic Acids Res. 1978 Sep;5(9):3141–3156. doi: 10.1093/nar/5.9.3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnös M., Inman R. B. Position of branch points in replicating lambda DNA. J Mol Biol. 1970 Jul 14;51(1):61–73. doi: 10.1016/0022-2836(70)90270-6. [DOI] [PubMed] [Google Scholar]
- Thoma F., Koller T., Klug A. Involvement of histone H1 in the organization of the nucleosome and of the salt-dependent superstructures of chromatin. J Cell Biol. 1979 Nov;83(2 Pt 1):403–427. doi: 10.1083/jcb.83.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurimoto T., Matsubara K. Purification of bacteriophage lambda O protein that specifically binds to the origin of replication. Mol Gen Genet. 1981;181(3):325–331. doi: 10.1007/BF00425606. [DOI] [PubMed] [Google Scholar]
- Tsurimoto T., Matsubara K. Purified bacteriophage lambda O protein binds to four repeating sequences at the lambda replication origin. Nucleic Acids Res. 1981 Apr 24;9(8):1789–1799. doi: 10.1093/nar/9.8.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurimoto T., Matsubara K. Replication of lambda dv plasmid in vitro promoted by purified lambda O and P proteins. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7639–7643. doi: 10.1073/pnas.79.24.7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda K., McMacken R., Kornberg A. dnaB protein of Escherichia coli. Purification and role in the replication of phiX174 DNA. J Biol Chem. 1978 Jan 10;253(1):261–269. [PubMed] [Google Scholar]
- Wickner S. H. DNA replication proteins of Escherichia coli and phage lambda. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):303–310. doi: 10.1101/sqb.1979.043.01.037. [DOI] [PubMed] [Google Scholar]
- Williams R. C. Use of polylysine for adsorption of nuclei acids and enzymes to electron microscope specimen films. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2311–2315. doi: 10.1073/pnas.74.6.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold M. S., Mallory J. B., Roberts J. D., LeBowitz J. H., McMacken R. Initiation of bacteriophage lambda DNA replication in vitro with purified lambda replication proteins. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6176–6180. doi: 10.1073/pnas.79.20.6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylicz M., Gorska I., Taylor K., Georgopoulos C. Bacteriophage lambda replication proteins: formation of a mixed oligomer and binding to the origin of lambda DNA. Mol Gen Genet. 1984;196(3):401–406. doi: 10.1007/BF00436186. [DOI] [PubMed] [Google Scholar]
- Zylicz M., LeBowitz J. H., McMacken R., Georgopoulos C. The dnaK protein of Escherichia coli possesses an ATPase and autophosphorylating activity and is essential in an in vitro DNA replication system. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6431–6435. doi: 10.1073/pnas.80.21.6431. [DOI] [PMC free article] [PubMed] [Google Scholar]